Abstract
Pregnancy loss and intrauterine growth restriction (IUGR) are serious pregnancy complications, and the triggers and mediators of placental and fetal damage are not completely understood. Using a mouse model of recurrent spontaneous miscarriages (DBA/2-mated CBA/J mice) that shares features with human recurrent miscarriage and fetal growth restriction, we identified tissue factor (TF) as an essential participating factor in placental and fetal injury. We have previously shown that C5a releases antiangiogenic molecule sFlt-1 in monocytes that causes defective placental development and fetal death in DBA/2-mated CBA/J mice. In this study, we found that TF not only activates the coagulation pathway, but it also mediates sFlt-1 release in monocytes causing defective placental development and fetal death. Blockade of TF with a monoclonal antibody inhibited sFlt-1 release, prevented the pathological activation of the coagulation pathway, restored placental blood flow, prevented placental oxidative stress, and rescued pregnancies. We also demonstrated that pravastatin, by down-regulating TF expression on monocytes and trophoblasts, prevented placental damage and protected pregnancies in DBA/2-mated CBA/J mice. These studies indicate that TF is an important mediator in fetal death and growth restriction and that statins may be a good treatment for women with recurrent miscarriages and IUGR.
Introduction
Recurrent pregnancy loss affects 1% to 3% of couples. Women who experience repeated miscarriages may undergo expensive and lengthy tests to try to identify a cause, but often get no answers. Intrauterine growth restriction (IUGR) is another pregnancy complication that occurs in up to 10% of infants, making fetal growth restriction the second leading cause of perinatal morbidity and mortality, following prematurity.1 Fetuses with IUGR are at high risk for poor short- and long-term outcomes.2,3 Despite aggressive attempts to understand the triggers and mediators of placental and fetal damage underlying fetal death and growth restriction, their incidence has remained unchanged over the past 30 years. Furthermore, in 50% to 60% of cases the well-established genetic, anatomic, endocrine, and infectious causes of fetal damage are not demonstrable. Experimental observations suggest a relationship between pregnancy complications and aberrant angiogenesis and thrombosis.4-6
DBA/2-mated CBA/J mice (CBA/J × DBA/2) are a well-studied model of pregnancy loss that shares features with human recurrent miscarriage.7,8 Embryos derived from mating CBA/J females with DBA/2 males showed an increased frequency of resorption compared with control matings (CBA/J × BALB/c) and surviving fetuses show consistent and significant growth restriction.
We previously demonstrated that generation of complement component C5a causes defective angiogenesis, abnormal placental development, fetal death, and IUGR in this model.9,10 Other authors showed that high frequency of abortion in the CBA/J × DBA/2 model may be explained by exposure to bacterial lipopolysaccharides (LPSs) at the time of mating8,11,12 or increased absorption of LPS from intestinal flora.7,11-13 A variety of inflammatory stimuli, including bacterial cell products and components of the complement system, are known to promote the expression of tissue factor (TF) on the surface of endothelial cells, monocytes, and neutrophils.14-16 In view of previous studies showing that aberrant TF expression may be responsible for thrombosis, we considered the possibility that increased TF expression induces pathological activation of the coagulation pathway, placental failure, fetal death, and growth restriction in this model of spontaneous abortion.
We found that TF not only activates the coagulation cascade but it also mediates the release of soluble receptor for vascular endothelial growth factor 1 (VEGF-1) (sVEGFR-1 or sFlt-1), which sequesters VEGF-inducing placental abnormalities and pregnancy complications in CBA/J × DBA/2 mice. These studies provide the first evidence linking TF, an initiator of the coagulation cascade, to defective angiogenesis and placental dysfunction, and identify a new effector of pregnancy complications. In addition, antibodies that block TF and pravastatin, which down-regulates TF expression, protected pregnancies in CBA/J × DBA/2 mice, identifying TF as a novel and important target for prevention of miscarriages and IUGR.
Methods
Mice
Inbred CBA/J (H-2k), DBA/2 (H-2d), and BALB/c (H-2d) mice from The Jackson Laboratory (Bar Harbor, ME) were used. Eight- to 10-week-old virgin female CBA/J mice were mated with 8- to 14-week-old BALB/c or DBA/2 males. Pregnant females were killed at day 7 and at day 15. The frequency of fetal resorption was calculated on day 15 as previously described.9 Fetal weights were also determined. To block TF, a group of mice were treated with intraperitoneal injections of anti-mTF antibody 1H1 (provided by Daniel Kirchhofer, Genentech, South San Francisco, CA) (0.5 mg)16 or an isotype-matched control antibody (rat IgG2a; 0.5 mg; Zymed Laboratories, South San Francisco, CA) on days 4, 7, and 10 of pregnancy. A group of mice were treated with hirudin (Sigma-Aldrich, St Louis, MO; 8 μg/twice a day subcutaneously) or fondaparinux sodium (Sanofi-Synthelabo, Cambridge, MA; 10 μg/day subcutaneously) from days 4 to 15 of pregnancy. To deplete monocytes/macrophages, mice were treated on day 4 of pregnancy with intravenous injections of dichloromethylene diphosphonate (Cl2MDP) clodronate liposomes17 (1 mL/100 g body weight), which has been shown to induce the complete depletion of macrophages within 24 hours.17 Repeat injections of 0.5 mL/100 g Cl2MDP clodronate liposome suspension were made on days 8 and 12 of pregnancy. Monocyte depletion in peripheral blood and decidual tissue persisted until the end of the studies. Mice receiving phosphate-buffered saline (PBS)–containing liposomes served as controls. Another group of mice was treated with pravastatin (Sigma-Aldrich; 5 μg/mouse, intraperitoneally) from days 4 to 15 of pregnancy. Pravastatin was directly dissolved in sterile PBS. Blood samples were obtained from pregnant females from days 1 to 15 of pregnancy from the submandibular vein. Thrombin anti–thrombin III complex (TAT) was measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Enzygnost TAT; Dade Behring, Deerfield, IL). Nitric oxide (NO) was measured by a colorimetric method (R&D Systems, Minneapolis, MN). Plasma sVEGFR-1 was measured with a commercial ELISA kit (R&D Systems). Some mice from each experimental group were studied until delivery and the litter sizes were recorded at birth.
Mice with a selective deletion of the TF gene in myeloid cells (TFfloxed/floxed/LysM-Cre mice),16 were used for isolation of monocytes to study sVEGFR-1 production in vitro.
Procedures that involved mice were approved by the Institutional Animal Care and Use Committee of the Hospital for Special Surgery (New York, NY) and were conducted in strict accordance with guidelines for the care and use of laboratory research animals promulgated by the National Institutes of Health (NIH, Bethesda, MD).
Human placental samples
Placentas from normal pregnancies and from intrauterine growth–restricted neonates were harvested after Cesarean sections at Southern Illinois University (SIU) School of Medicine. The Institutional Review Boards of SIU approved the collection and use of samples for research purposes. Three placentas from women with normal pregnancy and 3 placentas from pregnancies complicated by IUGR were studied. Women with normal pregnancies showed no medical, obstetric, or surgical complications at the time of the study and delivered a term infant, appropriate for gestational age, without complications. Placentas from 3 women who gave birth to neonates with birth weight below the 10th percentile were studied in the IUGR group.18 The placentas from neonates with IUGR were obtained from patients who developed preeclampsia (PE). Preeclampsia was defined in the presence of hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on at least 2 occasions, 4 hours to 1 week apart) and proteinuria (≥ 300 mg in a 24-hour urine collection or one dipstick measurement ≥ 2+).
Immunohistochemistry
For immunohistochemical studies in mice, frozen sections of deciduas from day 7 of pregnancy and placentas from day-15 surviving fetuses were stained for mouse TF with 1H1 antibody and for fibrin with a polyclonal rabbit antihuman fibrinogen/fibrin antibody that cross-reacts with mouse fibrin (DakoCytomation, Carpinteria, CA). An HRP-labeled secondary antibody and DAB as substrate were used to develop the reaction. Human placentas samples were fixed in formaldehyde and embedded in paraffin prior to sectioning for immunohistochemical studies. Antigen retrieval with boiling citrate solution, pH 6, for 20 minutes was performed prior to TF immunostaining. TF was detected using FITC-conjugated mouse anti–human TF (American Diagnostica, Greenwich, CT). Nonimmune rabbit serum or nonspecific mouse immunoglobulin was used as negative control for rabbit and mouse primary antibodies, respectively. Images were acquired using a Eclipse E400 microscope fitted with a Digital Camera DXM 1200 (Nikon, Tokyo, Japan).
Determination of TF functional activity in deciduas
The deciduas collected at day 7 and the placentas from surviving fetuses at day 15 were homogenized and cells were lysed by repeated freeze-thaw cycles and the TF was extracted as previously described.16 TF procoagulant activity was assayed with one-stage clotting time using mouse plasma as described.19 TF activity was calculated by reference to a standard curve performed with recombinant soluble mouse TF(1-219) (also provided by Daniel Kirchhofer, Genentech).
Placental perfusion studies
Placental perfusion was examined by injecting pregnant females with 100 μL of 25 mg/mL FITC-labeled dextran (MW 2 000 000; Sigma-Aldrich, St Louis, MO) via the retro-orbital vein, at day 15 of pregnancy. After 15 minutes, the mice were killed and the placentas removed and flash frozen. Serial frozen sections were examined and photographed under a Microphot-FXA microscope (Nikon).
TF expression on mouse monocytes
TF expression on peripheral blood monocytes was analyzed by 2-color fluorescence-activated cell sorting (FACS). Heparinized whole blood obtained at day 7 from DBA/2 or BALB/c mated CBA/J females was stained with fluorescein isothiocyanate (FITC)–labeled antimouse F4/80 for FL1 (BD Biosciences Pharmingen, San Jose, CA) to identify monocytes and biotinylated rat anti-mTF antibody 1H1 and streptavidin-PerCP (BD Biosciences Pharmingen) as the FL3 fluorochrome. Red cells were lysed with ACK buffer.16 Preparations were then incubated with SA-PerCP and analyzed by FACS using a FACscan (BD Biosciences Pharmingen).
8-Isoprostane measurements
Isoprostane 8-iso-prostaglandin F2a (STAT-8) was measured as a marker for oxidative stress. Placental tissue was harvested at day 15 of pregnancy and homogenized in 9 volumes of 0.1 M Tris (pH 7.4) containing 1 mM EDTA and 10 μM indomethacin and stored at −80°C in the presence of 0.005% BHT before being assayed for free 8-isoprostane using a STAT-8-Isoprostane EIA kit (Cayman Chemical, Ann Arbor, MI).
Assessment of superoxide production in decidual tissue by dihydroethidium fluorescence
In situ superoxide (O2−) levels were assessed using the fluorescent probe dihydroethidium (DHE).16,20 Day-15 placentas were frozen in OCT compound, cut into 10-μm sections, and incubated with DHE (10 μM) for 30 minutes at 37°C. Subsequently, the sections were washed and fluorescence images were obtained (λEx: 520, Em: 605 nm). To exclude an influence of the embedding procedure on fluorescence, a placenta from each treatment group was embedded in the same block and analyzed simultaneously.
Production of sVEGFR-1 by monocytes in vitro
Splenocytes from nonpregnant CBA/J female mice were incubated for 4 hours in culture medium9 supplemented with 10% inactivated fetal bovine serum (FBS). Nonadherent cells were removed and adherent cells (> 95% peroxidase positive) were incubated in culture medium with the addition of the following stimuli: 10 nM mouse recombinant C5a, 10 nM recombinant C5a plus anti-TF 1H1 antibody (0.5 mg/mL). A group of adherent monocytes was treated for 3 hours with pravastatin (5 μg/mL) prior to incubation with C5a. After 4 hours of incubation with C5a, culture supernatants were collected and analyzed for sVEGFR-1 by ELISA (R&D Systems).
Trophoblast cells culture
SM9-1 trophoblast cells, derived from a gestational day-9 Swiss-Webster mouse placenta (SM9-1),21 were incubated with the supernatants from monocytes incubated with C5a containing high levels of sFlt-1.9 Some SM9-1 cells were incubated in the presence of increasing amounts of mouse sFlt-1 (R&D Systems; 1000, 2000, 4000, and 8000 pg/mL). After 72 hours, cell proliferation was evaluated by light microscopy. Some SM9-1 cells were incubated with pravastatin (5 μg/mL) for 3 hours prior to incubation with sFlt-1. Mouse VEGF (500 pg/mL; R&D Systems) was added to some SM9-1 incubated with sFlt-1 or monocyte supernatants. Immunohistochemical identification of TF with 1H1 and superoxide production by DHE were performed on the SM9-1 cells after 72 hours of culture.
Statistical analysis
Data are expressed as mean plus or minus standard deviation. After confirming that the data were normally distributed (Kolomogorov-Smirnov test of normalcy), statistical analyses were conducted using Student t test to compare differences in means. Associations were considered to be statistically significant if the value of P was less than .05. Data were processed using SigmaStat, version 3.1 (Systat, Point Richmond, CA), statistical program for Windows.
Results
Increased TF staining in deciduas and placentas from CBA/J × DBA/2 matings
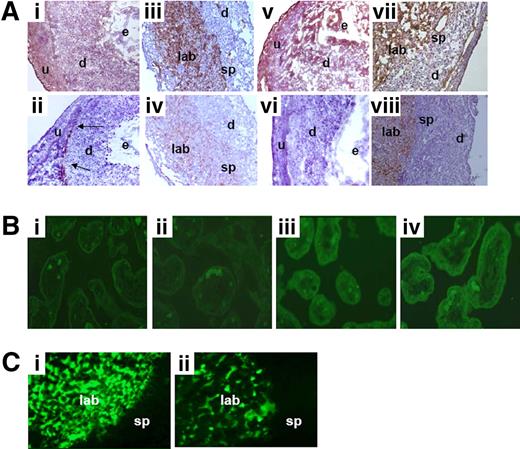
In abortion-prone CBA/J × DBA/2 matings, approximately 30% of the conceptuses showed robust TF staining throughout the deciduas (d) and injured embryos at 7 of pregnancy (Figure 1Ai). In contrast, the rest of the embryos displayed weak TF staining, comparable with embryos from control CBA/J × BALB/c matings (Figure 1Aii). In conceptuses from control CBA/J × BALB/c matings, minimal TF staining was found in the mesometrial area (Figure 1Aii). In addition, increased TF staining was observed in the labyrinth and on the spongiotrophoblasts (sp) in day-15 placentas from the surviving growth-restricted fetuses from CBA/J × DBA/2 matings (Figure 1Aiii) compared with placentas from fetuses in control matings (Figure 1Aiv). The increased TF expression observed in CBA/J × DBA/2 matings was associated with increased fibrin staining at day 7 of pregnancy (Figure 1Av), whereas minimal fibrin deposition was observed in deciduas from CBA/J × BALB/c matings (Figure 1Avi). In addition, robust fibrin deposition was observed in the labyrinth and the spongiotrophoblast area in placentas from surviving fetuses in CBA/J × DBA/2 matings (Figure 1Avii). Moreover, increased TF procoagulant activity was also observed in the placentas from surviving fetuses in CBA/J × DBA/2 matings compared with CBA/J × BALB/c mice (2.1 ± 0.3 vs 1.2 ± 0.3 μg TF, respectively, P < .001). Despite the increased TF and fibrin expression in decidual and placental tissue in CBA/J × DBA/2 matings, no visible thrombi were found. However, thrombi can form transiently and can be rapidly lysed.
Expression of TF in decidual tissue and placentas from CBA/J × DBA/2 mice. (A) Deciduas sections from CBA/J × DBA/2 and CBA/J × BALB/c mice were harvested at day 7 of pregnancy, cut, and stained with anti–mouse TF. In CBA/J × DBA/2 mice (i), in approximately 30% of the conceptuses there was extensive TF staining in deciduas (d). In contrast, minimal TF staining in the mesometrial area (arrows) was observed in all the conceptuses in CBA/J × BALB/c mice (ii). Placentas collected at day 15 of pregnancy from CBA/J × DBA/2 mice (iii) showed massive TF staining (brown color) in the labyrinth (lab) and spongiotrophoblasts (sp) area, whereas placentas from control CBA/J × BALB/c matings showed minimal TF staining (iv). Deciduas from day-7 pregnant CBA/J × DBA/2 mice showed increased fibrin deposition (v) compared with CBA/J × BALB/c mating (vi). In CBA/J × DBA/2 mice, day-15 placentas showed robust fibrin deposition (brown color) in the labyrinth (lab) and spongiotrophoblasts (sp) area (vii), whereas placentas from control CBA/J × BALB/c matings showed minimal fibrin deposition (viii). Original magnification ×40. (B) Tissue factor staining in human placentas. Robust TF staining (green fluorescence) was observed in the villous trophoblast cells and basement membranes in placentas from IUGR neonates (iii,iv). In contrast, minimal TF staining was observed in placentas from uncomplicated pregnancies (i,ii). Original magnification ×10. (C) Placental blood perfusion was measured in CBA/J × DBA/2 and CBA/J × BALB/c mice after FITC-dextran (MW 2 000 000) injection in the maternal circulation. In control CBA/J × BALB/c matings with normal pregnancies, the fluorescent tracer accumulated in the placental labyrinth (i). Less blood perfusion was observed in the labyrinth of CBA/J × DBA/2 matings (ii). n = 6-8 mice/each experimental group. Four to 5 deciduas or placentas were studied in each experimental group.
Expression of TF in decidual tissue and placentas from CBA/J × DBA/2 mice. (A) Deciduas sections from CBA/J × DBA/2 and CBA/J × BALB/c mice were harvested at day 7 of pregnancy, cut, and stained with anti–mouse TF. In CBA/J × DBA/2 mice (i), in approximately 30% of the conceptuses there was extensive TF staining in deciduas (d). In contrast, minimal TF staining in the mesometrial area (arrows) was observed in all the conceptuses in CBA/J × BALB/c mice (ii). Placentas collected at day 15 of pregnancy from CBA/J × DBA/2 mice (iii) showed massive TF staining (brown color) in the labyrinth (lab) and spongiotrophoblasts (sp) area, whereas placentas from control CBA/J × BALB/c matings showed minimal TF staining (iv). Deciduas from day-7 pregnant CBA/J × DBA/2 mice showed increased fibrin deposition (v) compared with CBA/J × BALB/c mating (vi). In CBA/J × DBA/2 mice, day-15 placentas showed robust fibrin deposition (brown color) in the labyrinth (lab) and spongiotrophoblasts (sp) area (vii), whereas placentas from control CBA/J × BALB/c matings showed minimal fibrin deposition (viii). Original magnification ×40. (B) Tissue factor staining in human placentas. Robust TF staining (green fluorescence) was observed in the villous trophoblast cells and basement membranes in placentas from IUGR neonates (iii,iv). In contrast, minimal TF staining was observed in placentas from uncomplicated pregnancies (i,ii). Original magnification ×10. (C) Placental blood perfusion was measured in CBA/J × DBA/2 and CBA/J × BALB/c mice after FITC-dextran (MW 2 000 000) injection in the maternal circulation. In control CBA/J × BALB/c matings with normal pregnancies, the fluorescent tracer accumulated in the placental labyrinth (i). Less blood perfusion was observed in the labyrinth of CBA/J × DBA/2 matings (ii). n = 6-8 mice/each experimental group. Four to 5 deciduas or placentas were studied in each experimental group.
Increased TF staining in human placenta from growth-restricted neonates
To translate our findings in mice into humans, we investigated the presence of TF in human placentas. Increased TF staining was observed in the villous trophoblast cells and basement membranes in placentas from IUGR neonates (Figure 1Biii,iv) compared with placentas from neonates with normal body weight (Figure 1Bi,ii).
Impaired blood supply in placentas from CBA/J × DBA/2 mice
To detect blood perfusion defects secondary to potential thrombi in placental vessels in CBA/J × DBA/2 mice, FITC-dextran was injected in the maternal circulation at day 15 of pregnancy. In control CBA/J × BALB/c matings with normal pregnancies, the fluorescent tracer accumulated in the placental labyrinth (Figure 1Ci). In contrast, diminished blood perfusion was observed in the labyrinth of CBA/J × DBA/2 matings (Figure 1Cii). This observation is in accordance with the increased fibrin deposition observed in this abortion-prone CBA/J × DBA/2 mating. These data support the notion that fetal loss and growth restriction in this mouse model can be attributed to pathological activation of the coagulation cascade in placental vessels and impaired perfusion of the placenta.
Increased plasma thrombin anti–thrombin III complex levels in CBA/J × DBA/2 pregnancies
Plasma thrombin anti–thrombin III complex (TAT) levels, representing a functional state of clotting system, were studied in abortion-prone and control matings. TAT plasma levels increased throughout pregnancy in CBA/J × DBA/2 mice and by day 15, CBA/J × DBA/2 mice showed TAT levels 3 times greater than those seen in the control strain combination (89 ± 12 ng/mL in CBA/J × DBA/2 vs 31 ± 17 ng/mL in CBA/J × BALB/c, P < .001).
Anticoagulation protects pregnancies in CBA/J × DBA/2 mice
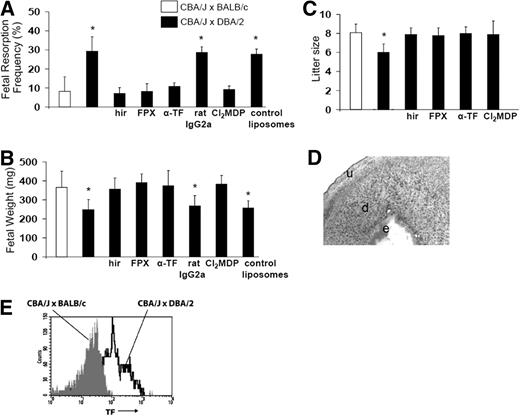
Increased coagulation seems to play a crucial role in the development of pregnancy complications in CBA/J × DBA/2 mice. To substantiate this finding, we examined the effects of anticoagulants in CBA/J × DBA/2 mice pregnancy outcomes at day 15. As increased thrombin generation was observed in CBA/J × DBA/2 mice, we first studied the effects of direct thrombin inhibitor hirudin. As expected, treatment with hirudin prevented fetal resorptions and growth restriction in CBA/J × DBA/2 mice at day 15 of pregnancy (Figure 2A,B). Fondaparinox, direct inhibitor of factor Xa, also prevented fetal loss and growth restriction in abortion-prone CBA/J × DBA/2 mice (Figure 2A,B). A group of mice was followed until the end of the pregnancy. In accordance with the data obtained at day 15 of pregnancy, DBA/2-mated CBA/J mice that showed increased fetal resorption rate at day 15 showed smaller litter sizes at birth compared with BALB/c-mated CBA/J mice (Figure 2C). Hirudin and fondaparinox rescued pregnancies in CBA/J × DBA/2 mice increasing the litter size (Figure 2C). That anticoagulation prevents pregnancy loss and IUGR in this model emphasizes that pathological activation of the coagulation pathway plays a role in fetal injury in the CBA/J × DBA/2 model.
Blockade of TF, anticoagulation, or monocyte depletion prevents fetal death and IUGR in CBA/J × DBA/2 mice. CBA/J × BALB/c mice, CBA/J × DBA/2 mice, and CBA/J × DBA/2 mice that received different treatments were killed on day 15 of pregnancy, uteri were dissected, and fetal resorption rates (A) and fetal weight were recorded (B). (C) Litter size at birth. (D) Immunohistochemical analysis for TF in a section of decidua from CBA/J × DBA/2 mice depleted of monocytes with Cl2MDP. Minimal amounts of TF and intact embryo (e) were observed in deciduas (d) of CBA/J × DBA/2 mice depleted of monocytes. Original magnification ×40. (E) FACS analysis of TF expression on whole blood monocytes from BALB/c- and DBA/2-mated CBA/J mice. The number of TF-positive monocytes increased in CBA/J × DBA/2 mice in comparison with CBA/J × BALB/c mice (P < .001). n = 5-8 mice/each experimental group. Four to 5 placentas per mouse were studied. Data are mean values plus or minus SD.
Blockade of TF, anticoagulation, or monocyte depletion prevents fetal death and IUGR in CBA/J × DBA/2 mice. CBA/J × BALB/c mice, CBA/J × DBA/2 mice, and CBA/J × DBA/2 mice that received different treatments were killed on day 15 of pregnancy, uteri were dissected, and fetal resorption rates (A) and fetal weight were recorded (B). (C) Litter size at birth. (D) Immunohistochemical analysis for TF in a section of decidua from CBA/J × DBA/2 mice depleted of monocytes with Cl2MDP. Minimal amounts of TF and intact embryo (e) were observed in deciduas (d) of CBA/J × DBA/2 mice depleted of monocytes. Original magnification ×40. (E) FACS analysis of TF expression on whole blood monocytes from BALB/c- and DBA/2-mated CBA/J mice. The number of TF-positive monocytes increased in CBA/J × DBA/2 mice in comparison with CBA/J × BALB/c mice (P < .001). n = 5-8 mice/each experimental group. Four to 5 placentas per mouse were studied. Data are mean values plus or minus SD.
Blockade of TF prevents fetal injury in DBA/2-mated CBA/J mice
To assess the importance of TF in fetal death and growth restriction in CBA/J × DBA/2 mice, we inhibited TF with a rat monoclonal anti-mTF antibody 1H1.16 Administration of 1H1 was associated with a significant decrease in fetal resorption frequency (Figure 2A) and prevention of IUGR at day 15 of pregnancy (Figure 2B). Control antibody rat IgG2a did not affect pregnancy outcomes in CBA/J × DBA/2 mice (Figure 2A-B). A significant survival benefit for the fetuses of DBA/2-mated CBA/J mice treated with 1H1 was also observed at birth (Figure 2C). Litter sizes in CBA/J × DBA/2 mice treated with 1H1 were not different from CBA/J × BALB/c control matings (Figure 2C). The increased survival observed in CBA/J × DBA/2 mice treated with anti-mTF antibody 1H1 demonstrates that TF is a crucial effector in pregnancy loss in this model.
Increased TF expression on monocytes from CBA × DBA matings
Because monocytes have shown to be important mediators of fetal damage in this model 9,22 and these cells have been shown to express TF in response to C5a,23 we investigated whether monocytes contribute to the generation of decidual TF in CBA/J × DBA/2. Monocyte depletion was achieved using Cl2MDP clodronate liposomes.17 Neither pregnancy complications (Figure 2A-B) nor increase in decidual TF expression was observed in CBA/J × DBA/2 mice lacking monocytes (Figure 2D). CBA/J × DBA/2 mice treated with Cl2MDP clodronate showed minimal TF staining in deciduas at day 7 of pregnancy (Figure 2D). Macrophage depletion also increased the number of surviving fetuses as indicated by the bigger litter sizes at birth (Figure 2C).
Given that monocytes are critical effectors of fetal death, and that TF is required for pregnancy complications in CBA/J × DBA/2 mice, we tested the hypothesis that increased TF expression is present in monocytes from CBA/J × DBA/2 mice at day 7 of pregnancy. Monocytes from CBA/J × DBA/2 mice were 32% plus or minus 9% positive for TF (Figure 2E) compared with 6% plus or minus 3% of the monocytes from CBA/J × BALB/c mice (P < .005). Less than 1% of the monocytes showed nonspecific binding when incubated with isotype control antibody rat IgG2a.
Increased placental oxidative stress in CBA/J × DBA/2 matings
There is mounting evidence that oxidative stress in placental tissue plays a pivotal role in the development of placental-related diseases such as fetal death and IUGR.24 Isoprostane 8-iso-prostaglandin F2a (STAT-8) is a marker for oxidative stress in vivo. STAT-8 is also a potent vasoconstrictor, activates platelets, induces derangement of endothelial cells,25 and reduces trophoblast invasion in vitro.26 We observed increased STAT-8 levels in day-15 placentas from DBA/2-mated CBA/J females compared with uneventful pregnancies in control matings (Figure 3A). These data are in accordance with studies showing increased STAT-8 levels in women with pregnancy complications.27,28 In addition to the increased STAT-8 levels, placentas from CBA/J × DBA/2 matings exhibited increased superoxide production measured as dihydroethidium (DHE)–derived fluorescence at microscopy (Figure 3Bi) compared with CBA/J × BALB/c mice (Figure 3Bii). These results indicate there is a correlation between pregnancy complications and placental oxidative stress injury.
Increased placental oxidative stress in CBA/J × DBA/2 mice. Increased STAT-8 levels were observed in day-15 placentas from DBA/2-mated CBA/J mice compared with control matings (*P < .001). Treatment with hirudin (hir) or fondaparinox (FPX) prevented STAT-8 increase in CBA/J × DBA/2 mice (n = 6-8 mice/experimental group). Treatment with anti-TF 1H1 antibody or with Cl2MDP clodronate also prevented placental STAT-8 increase (n = 6-8 mice/experimental group). (B) Increased superoxide (O2−) production (DHE red staining) was observed in CBA/J × DBA/2 mice (i). O2− production was attenuated in CBA/J × DBA/2 mice treated with anti-TF (iii) or with Cl2MDP (iv). Minimal O2− production was observed in placentas from control matings (ii). (C) Macrophages from nonpregnant CBA/J female mice were stimulated with C5a, LPS, anti-TF, anti-TF + C5a, or control medium. Blockade of TF or genetic deletion of TF (TFfloxed/floxed/LysM-Cre mice) prevented C5a-induced release of sFlt-1 (n = 4-6 experiments/group; *P < .005 vs control). (D) Trophoblast proliferation assays. SM9-1 cells were incubated in RPMI-1640 as described before.21 Incubation with supernatants of monocytes incubated with C5a inhibited cell proliferation (ii). Incubation with increasing doses of sFlt-1 showed a dose-response inhibitory effect on SM9-1 cell proliferation ([iii] 2000 pg/mL, [iv] 4000 pg/mL, [v] 8000 pg/mL). Increased proliferation was observed when VEGF was added to the supernatant of C5a-treated monocytes (vi). (E) Superoxide (O2−) production in SM9-1 cells. Abundant SM9-1 cells with weak DHE-positive staining were observed in SM9-1 incubated with media (i). Fewer cells but strongly positive with DHE staining were observed in SM9-1 cells incubated with monocyte supernatants (ii) or sFlt-1 (4000 pg/mL [iii] and 8000 pg/mL [iv]). Decreased O2− production was observed when VEGF was added to the supernatant of C5a-treated monocytes (v). (F) Immunocytochemical analysis of TF. A large number of confluent SM9-1 cells with weak TF staining was observed after incubation with medium (i). Diminished cell proliferation and strong positive TF staining were observed in SM9-1 cells incubated with supernatants from C5a-treated monocytes (ii), sFlt-1 4000 pg/mL (iii), or sFlt-1 8000 pg/mL (iv). Increased proliferation and decreased TF were observed when VEGF was added to the supernatant of C5a-treated monocytes (v). Data are mean values plus or minus SD.
Increased placental oxidative stress in CBA/J × DBA/2 mice. Increased STAT-8 levels were observed in day-15 placentas from DBA/2-mated CBA/J mice compared with control matings (*P < .001). Treatment with hirudin (hir) or fondaparinox (FPX) prevented STAT-8 increase in CBA/J × DBA/2 mice (n = 6-8 mice/experimental group). Treatment with anti-TF 1H1 antibody or with Cl2MDP clodronate also prevented placental STAT-8 increase (n = 6-8 mice/experimental group). (B) Increased superoxide (O2−) production (DHE red staining) was observed in CBA/J × DBA/2 mice (i). O2− production was attenuated in CBA/J × DBA/2 mice treated with anti-TF (iii) or with Cl2MDP (iv). Minimal O2− production was observed in placentas from control matings (ii). (C) Macrophages from nonpregnant CBA/J female mice were stimulated with C5a, LPS, anti-TF, anti-TF + C5a, or control medium. Blockade of TF or genetic deletion of TF (TFfloxed/floxed/LysM-Cre mice) prevented C5a-induced release of sFlt-1 (n = 4-6 experiments/group; *P < .005 vs control). (D) Trophoblast proliferation assays. SM9-1 cells were incubated in RPMI-1640 as described before.21 Incubation with supernatants of monocytes incubated with C5a inhibited cell proliferation (ii). Incubation with increasing doses of sFlt-1 showed a dose-response inhibitory effect on SM9-1 cell proliferation ([iii] 2000 pg/mL, [iv] 4000 pg/mL, [v] 8000 pg/mL). Increased proliferation was observed when VEGF was added to the supernatant of C5a-treated monocytes (vi). (E) Superoxide (O2−) production in SM9-1 cells. Abundant SM9-1 cells with weak DHE-positive staining were observed in SM9-1 incubated with media (i). Fewer cells but strongly positive with DHE staining were observed in SM9-1 cells incubated with monocyte supernatants (ii) or sFlt-1 (4000 pg/mL [iii] and 8000 pg/mL [iv]). Decreased O2− production was observed when VEGF was added to the supernatant of C5a-treated monocytes (v). (F) Immunocytochemical analysis of TF. A large number of confluent SM9-1 cells with weak TF staining was observed after incubation with medium (i). Diminished cell proliferation and strong positive TF staining were observed in SM9-1 cells incubated with supernatants from C5a-treated monocytes (ii), sFlt-1 4000 pg/mL (iii), or sFlt-1 8000 pg/mL (iv). Increased proliferation and decreased TF were observed when VEGF was added to the supernatant of C5a-treated monocytes (v). Data are mean values plus or minus SD.
Treatment with antibody anti-TF 1H1 prevented STAT-8 increase (Figure 3A) and superoxide production in CBA/J × DBA/2 mice (Figure 3Biii). Anticoagulation with hirudin or fondaparinox also prevented STAT-8 increase in CBA/J × DBA/2 mice (Figure 3A). In addition, monocyte depletion with CL2MDP clodronate liposomes, which protected pregnancies, also prevented placental STAT-8 increase (Figure 3A) and placental superoxide production in CBA/J × DBA/2 mice (Figure 3Biv). These data suggest that TF and monocytes are essential participating factors in placental oxidative damage and pregnancy complications in this model.
Monocytes do not release sFlt-1 in response to C5a in the absence of TF
We previously described that complement component C5a triggers release of sFlt-1 by monocytes in vitro.9 LPS also stimulates sFlt-1 release from monocytes (Figure 3C). Blockade of TF with antibody 1H1 or genetic deletion of TF on monocytes (TFfloxed/floxed LysM-Cre mice) prevented sFlt-1 release in response to C5a (Figure 3C), suggesting that TF expression on monocytes is required for sFlt-1 release. In addition, in vivo experiments show that TF blockade diminished plasma sFlt-1 levels in CBA/J × DBA/2 mice at day 7 of pregnancy (sFlt-1 [pg/mL]: 16 890 ± 6745 in CBA/J × DBA/2 + 1H1 [n = 7] vs 29 780 ± 8760 in untreated DBA/2 mated CBA/J mice [n = 10], P < .01).
Reduced cell proliferation, increased oxidative injury, and increased TF expression in trophoblasts incubated with sFlt-1
sFlt-1 is significantly increased in plasma from DBA/2-mated CBA/J mice and is released by monocytes incubated with C5a.9 Knowing that sFlt-1 is a potent antiangiogenic molecule that sequesters circulating VEGF,9,29,30 we studied whether sFlt-1 affects trophoblast proliferation in vitro. SM9-1 cells incubated with monocyte supernatants (Figure 3Dii) or sFlt-1 (Figure 3Diii-v) showed diminished cell proliferation compared with cells incubated with culture media only (Figure 3Di). The antiproliferative effect of sFlt-1 on trophoblasts was dose responsive (Figure 3Diii,iv). Addition of VEGF to the trophoblasts incubated with monocyte supernatants or sFlt-1 restored cell proliferation (Figure 3Dvi). These data indicate that sFlt-1 by quenching VEGF causes abnormal proliferation of trophoblasts and may be the cause for abnormal placentation and poor pregnancy outcomes in CBA/J × DBA/2.
In addition to inhibition of proliferation, trophoblast cells incubated with supernatants of monocytes incubated with C5a (Figure 3Eii) and sFlt-1 (Figure 3Eiii,iv) exhibited increased superoxide production measured by DHE. Addition of VEGF restored cell growth and prevented oxidative damage in trophoblasts (Figure 3Ev). Trophoblasts incubated with media only showed abundant cells and weak DHE fluorescence (Figure 3Ei). We also found increased TF staining in SM9-1 trophoblasts incubated with supernatants from monocytes or with sFlt-1 (Figure 3Fiii,iv). Addition of VEGF prevented TF expression (Figure 4Ev). These data suggest that the inhibition of trophoblast proliferation by sFlt-1 is mediated by VEGF and is associated with increased oxidative stress and increased TF expression.
Pravastatin prevents pregnancy loss in CBA/J × DBA/2 mice. (A,B) CBA/J × DBA/2 and CBA/J × BALB/c mice were treated with pravastatin (P) (n = 5-7). Mice were killed on day 15 of pregnancy, uteri were dissected, and fetal resorption frequency was calculated. (A) Treatment with P prevented fetal loss (*P < .005, CBA/J × DBA/2 versus CBA/J × DBA/2 plus P). (B) P also prevented IUGR in CBA/J × DBA/2 mice (*P < .01, CBA/J × DBA/2 versus CBA/J × DBA/2 plus P). (C) Litter size at birth. P increased the litter size in CBA/J × DBA/2 mice. (D) Superoxide generation, and TF and fibrin staining in day-15 placentas. Increased free radical–mediated lipid peroxidation was observed in placentas from CBA/J × DBA/2 mice (i). No signs of oxidative damage were observed in placentas of CBA/J × DBA/2 mice that received P (ii). Extensive TF (iii) and fibrin (v) staining (brown color) was found in placentas from CBA/J × DBA/2 mice. In contrast, CBA/J × DBA/2 mice treated with P showed minimal and diffuse TF (iv) and fibrin staining (vi) in the placentas. Original magnification ×100. (E) Serum total nitric oxide (NO) production. In control CBA/J × BALB/c matings, NO levels increase along pregnancy. In contrast, in CBA/J × DBA/2 mice NO levels diminish as pregnancy progresses. P treatment restores NO levels to values comparable with those observed in CBA/J × BALB/c. (F) Placental perfusion studies. In placentas from CBA/J × DBA/2 mice treated with P, as in control matings (Figure 1Ci) with normal pregnancies, the fluorescent tracer accumulated in the placental labyrinth showing an adequate blood perfusion (n = 5-6). (G) FACS analysis of TF in peripheral blood monocytes. Increased TF-positive monocytes were observed in CBA/J × DBA/2 matings. P inhibited increased TF expression on monocytes. (H) TF staining and superoxide production in SM9-1 trophoblasts. Increased TF staining (i) and superoxide production (iii) were observed in SM9-1 cells incubated with 8000 pg/mL sFlt-1. In contrast, addition of P 3 hours prior to sFlt-1 incubation prevented TF expression (ii) and superoxide generation (iv) (n = 5-7 experiments/group). Data are mean values plus or minus SD.
Pravastatin prevents pregnancy loss in CBA/J × DBA/2 mice. (A,B) CBA/J × DBA/2 and CBA/J × BALB/c mice were treated with pravastatin (P) (n = 5-7). Mice were killed on day 15 of pregnancy, uteri were dissected, and fetal resorption frequency was calculated. (A) Treatment with P prevented fetal loss (*P < .005, CBA/J × DBA/2 versus CBA/J × DBA/2 plus P). (B) P also prevented IUGR in CBA/J × DBA/2 mice (*P < .01, CBA/J × DBA/2 versus CBA/J × DBA/2 plus P). (C) Litter size at birth. P increased the litter size in CBA/J × DBA/2 mice. (D) Superoxide generation, and TF and fibrin staining in day-15 placentas. Increased free radical–mediated lipid peroxidation was observed in placentas from CBA/J × DBA/2 mice (i). No signs of oxidative damage were observed in placentas of CBA/J × DBA/2 mice that received P (ii). Extensive TF (iii) and fibrin (v) staining (brown color) was found in placentas from CBA/J × DBA/2 mice. In contrast, CBA/J × DBA/2 mice treated with P showed minimal and diffuse TF (iv) and fibrin staining (vi) in the placentas. Original magnification ×100. (E) Serum total nitric oxide (NO) production. In control CBA/J × BALB/c matings, NO levels increase along pregnancy. In contrast, in CBA/J × DBA/2 mice NO levels diminish as pregnancy progresses. P treatment restores NO levels to values comparable with those observed in CBA/J × BALB/c. (F) Placental perfusion studies. In placentas from CBA/J × DBA/2 mice treated with P, as in control matings (Figure 1Ci) with normal pregnancies, the fluorescent tracer accumulated in the placental labyrinth showing an adequate blood perfusion (n = 5-6). (G) FACS analysis of TF in peripheral blood monocytes. Increased TF-positive monocytes were observed in CBA/J × DBA/2 matings. P inhibited increased TF expression on monocytes. (H) TF staining and superoxide production in SM9-1 trophoblasts. Increased TF staining (i) and superoxide production (iii) were observed in SM9-1 cells incubated with 8000 pg/mL sFlt-1. In contrast, addition of P 3 hours prior to sFlt-1 incubation prevented TF expression (ii) and superoxide generation (iv) (n = 5-7 experiments/group). Data are mean values plus or minus SD.
Pravastatin prevented pregnancy loss and placental oxidative stress in CBA/J × DBA/2 mice
Statins are postulated to inhibit inflammation and coagulation31,32 and to reduce TF expression and activity in blood monocytes and neutrophils.20,33,34 Knowing that TF is a crucial mediator in pregnancy complications observed in CBA/J × DBA/2 mice, we sought to investigate whether pravastatin can prevent pregnancy loss in this model of spontaneous miscarriages. In accordance with our hypothesis, pravastatin prevented fetal loss (Figure 4A) and IUGR (Figure 4B) in DBA/2-mated CBA/J mice at day 15 of pregnancy. The beneficial effects of pravastatin on pregnancy outcomes in CBA/J × DBA/2 mice were also found at birth (Figure 4C). Pravastatin also prevented oxidative stress (Figure 4Dii) and decreased TF expression (Figure 4Div) and fibrin deposition (Figure 4Dvi) in placentas from CBA/J × DBA/2 mice compared with untreated CBA/J × DBA/2 mice (Figure 4Di-iv).
Pravastatin restored NO levels in abortion-prone mice
In normal CBA/J × BALB/s mating, plasma NO levels increase gradually during pregnancy (Figure 4E). In contrast, NO levels do not increase in CBA/J × DBA/2 mice (Figure 4E). Plasma NO levels in CBA/J × DBA/2 mice treated with pravastatin were not different from control matings with good pregnancy outcomes (Figure 4E). Consistent with the finding that pravastatin prevented plasma NO diminution and inhibited placental fibrin deposition, pravastatin restored placental blood flow in CBA/J × DBA/2 mice (Figure 4F). By preventing the diminution of vasodilator molecule NO and inhibiting fibrin deposition, pravastatin restores placental blood flow and rescues pregnancies in CBA/J × DBA/2 mice.
Pravastatin prevented sFlt-1 release and TF expression by monocytes
We previously showed that in response to C5a, monocytes release high amounts of sFlt-1 (Figure 3C). Monocytes preincubated with pravastatin release small amounts of sFlt-1 in response to C5a (sFlt-1 [pg/mL]:C5a [n = 5]: 6749 ± 1927 vs 387 ± 78 in C5a + pravastatin [n = 4], P < .01). Immunocytochemical staining revealed minimal TF staining in monocytes treated with C5a that have been previously incubated with pravastatin (data not shown). In addition, FACS analysis showed that pravastatin prevented TF expression on monocytes in CBA/J × DBA/2 mice (% TF positive monocytes: 32.5 ± 9 in CBA/J × DBA/2 mice vs 10 ± 4 in CBA/J × DBA/2 + pravastatin, P < .01, n = 5-6; Figure 4G). These data reinforce the idea that TF expression on monocytes is an essential factor in the pathogenesis of fetal injury in CBA/J × DBA/2 mice. In addition, pravastatin treatment diminished plasma sFlt-1 levels in CBA/J × DBA/2 mice at day 7 of pregnancy (sFlt-1 [pg/mL]: 18 650 ± 5680 in CBA/J × DBA/2 + pravastatin [n = 6] vs 29 780 ± 8760 in untreated DBA/2 mated CBA/J mice [n = 10], P < .01).
Pravastatin prevented oxidative stress and TF expression in mouse trophoblasts
As we showed before, mouse trophoblasts incubated with sFlt-1 exhibited growth inhibition, increased TF expression, and increased superoxide production. Incubation of trophoblasts with pravastatin prior to sFlt-1 incubation restored cell growth, prevented TF expression (Figure 4Hii), and prevented oxidative damage (Figure 4Hiv).
Discussion
On average, 50% to 70% of all human conceptions fail. Pregnancy loss and growth restriction are serious pregnancy complications and the triggers and mediators of placental and fetal damage are not completely understood. Using a mouse model of recurrent spontaneous miscarriages (CBA/J × DBA/2 mice) that shares features with human recurrent miscarriage and fetal growth restriction,9 we identified tissue factor (TF) as an essential participating factor in placental and fetal injury. We found that by inhibiting TF with monoclonal antibodies or with pravastatin, release of antiangiogenic factor sFlt-1 is inhibited, trophoblast proliferation and placental flow are restored, placental oxidative damage is prevented, and pregnancies are rescued.
Thrombi in the placental circulation can impair placental blood supply and thus affect fetal nutrition resulting in fetal death and/or IUGR.35-37 TF is best known as the primary cellular initiator of blood coagulation.38 Although TF is required for uterine hemostasis and maintenance of the placental labyrinth during gestation,39 increased placental TF expression can result in pregnancy complications such as preeclampsia (PE).40,41 We previously demonstrated that pregnancy loss induced by antiphospholipid antibodies in mice is associated with increased TF expression.16,20 In this study, we found increased TF expression and activity in placentas from CBA/J × DBA/2 matings compared with control matings with normal pregnancies. Interestingly, we were able to translate the results obtained in mice in humans. Increased TF staining was observed in human placentas from growth-restricted neonates compared with placentas from neonates with appropriate weight for gestational age. These data are in accordance with other studies showing aberrant TF expression in decidual endothelium of pregnancies complicated by IUGR and/or fetal loss.4 Increased plasma TF levels were also described in patients with PE.40 The patients who presented growth-restricted neonates also presented PE symptoms (increased blood pressure and proteinuria). We recognize that increased TF expression in placentas from patients with PE can be caused by the maternal vascular disease observed in PE. However, increased levels of sFlt-1, thrombin, and STAT-8 were found in plasma from abortion-prone CBA/J × DBA/2 matings, similarly to patients with PE.29,30
We demonstrated that blockade of TF has protective effect in pregnancy loss and IUGR in CBA/J × DBA/2 mice. Blockade of TF not only rescued pregnancies but also diminished placental STAT-8 generation and superoxide production, suggesting that TF is a crucial effector of placental oxidative damage in CBA/J × DBA/2 mice. Increased placental TF expression, fibrin deposition, and procoagulant activity were observed in DBA/2-mated CBA/J mice, suggesting that TF-dependent pathological activation of the coagulation pathway mediates demise of embryos and growth restriction in this model. However, we did not find any visible placental thrombi in these mice. It is possible that thrombi form transiently in the placentas and are rapidly lysed. Despite the absence of visible thrombi, the increased fibrin deposition and diminished blood flow observed in placentas from DBA/2-mated CBA/J females indicate that cross-linked fibrin is being deposited and is occluding the placental blood vessels and compromising the oxygen and nutrient supply for the fetus, resulting in trophoblast injury and fetal growth restriction. In addition, a robust thrombin generation was also observed in the abortion-prone matings, reinforcing the concept that activation of the coagulation cascade is required for pregnancy complications. The protective effect of hirudin emphasizes that active thrombin is required for pregnancy loss and IUGR in DBA/2-mated CBA/J females. Clark et al demonstrated up-regulation of prothrombinase Fgl2 associated with increased fibrin staining in trophoblasts from CBA/J × CBA/2 mice.42 They also reported that anticoagulation with hirudin significantly protected against abortions in this model.42 Lockwood et al demonstrated that thrombin enhanced sFlt-1 mRNA and levels of secreted sFlt-1 protein in early pregnancy decidual cells.43 Besides its anticoagulant effect, hirudin can protect pregnancies in CBA/J × DBA/2 mice by inhibiting sFlt-1 release from decidual cells.
Monocytes are crucial cellular effectors of placental and fetal damage in this model.9 Indeed, we demonstrated that monocyte depletion prevented pregnancy loss and IUGR in the CBA/J × DBA/2 matings. We demonstrated increased TF expression on monocytes from CBA/J × DBA/2 mice compared with CBA/J × BALB/c mice. Blockade of TF with specific monoclonal antibodies prevented sFlt-1 release from monocytes, suggesting that TF is required for sFlt-1 release. In addition, studies performed in monocytes with a genetic deletion of TF gene emphasize the role of TF in sFlt-1 release. Moreover, blockade of TF prevented increased sFlt-1 plasma levels in CBA/J × DBA/2 mice. Plasma sFlt-1 levels in CBA/J × DBA/2 mice treated with anti-TF were not different from control CBA/J × BALB/c mice with normal pregnancies.
We demonstrated that TF is required for the release of sFlt-1 from monocytes. In addition, trophoblasts incubated with sFlt-1 or supernatants from monocytes incubated with C5a with high levels of sFlt-1 showed diminished cell proliferation, increased oxidative stress, and increased TF expression compared with trophoblasts incubated with only media. Based on these in vitro results, we postulate that TF on monocytes increases the release of antiangiogenic factor sFlt-1 that sequesters VEGF, thus inducing inadequate trophoblast proliferation and up-regulating TF expression. Increased TF expression on trophoblasts can then perpetuate the coagulation cascade activation, leading to diminished placental flow and fetal death. That minimal decidual TF expression and surviving embryos were observed in CBA/J × DBA/2 mice depleted of monocytes emphasizes the role of monocytes on trophoblast TF expression.
Increased fibrin deposition, decreased placental perfusion, and increased placental oxidative stress were observed in CBA/J × DBA/2 mice. Knowing that reactive oxygen species can consume nitric oxide, it was not surprising to find decreased plasma NO levels in CBA/J × DBA/2 mice. Reduced levels of this anti-inflammatory and vasorelaxant molecule can also account for the placental perfusion abnormalities observed in this model of recurrent miscarriages. Decreased NO levels and increased oxidative stress were also observed in patients with preeclampsia, and this may promote the endothelial dysfunction observed in these patients.44
TF can be expressed on monocytes in response to C5a and/or lipopolysaccharide (LPS),14,15 and a synergism between C5a and LPS was demonstrated in human monocytes and mouse peritoneal macrophage activity.45,46 Thus, we need to consider a possible synergistic effect of LPS and C5a. It is possible that a small amount of LPS can amplify the effects of C5a, inducing the release of bigger amounts of sFlt-1. Monocytes incubated with LPS up-regulated TF expression and released high amounts of sFlt-1.
Statins reduce TF synthesis, expression, and activity in many different cells as blood monocytes, neutrophils, endothelial cells, and breast carcinoma cells.20,47-49 In abortion-prone CBA/J × DBA/2 mice, statins by down-regulating TF expression, prevented sFlt-1 release from monocytes and restored the levels of angiogenic factors necessary for trophoblast proliferation, placental vessel formation, and good pregnancy outcomes. Moreover, statins prevented oxidative stress in trophoblasts incubated with sFlt-1. It is interesting to note that other authors reported that treatment of endothelial cells with statins up-regulated heme oxygenase-1 HO-1 and inhibited the release of sFlt-1.50 Furthermore, these authors reported that simvastatin decreased sFlt-1 release from normal term placental villous explants.50 It has been also demonstrated that statins such as simvastatin induce mRNA expression and protein secretion of VEGF in endothelial cells.51 Besides decreasing sFlt-1 release from monocytes, statins may also have beneficial actions on placental development and pregnancy outcomes in CBA/J × DBA/2 mice by increasing VEGF levels in trophoblasts. That pravastatin diminishes sFlt-1 levels and increases VEGF secretion suggests that it may be a good treatment for women with preeclampsia.
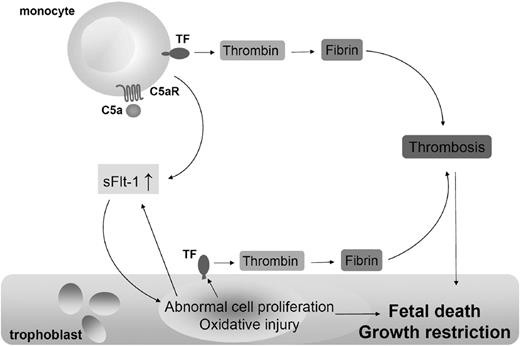
Collectively, our study demonstrates that TF expression on monocytes is essential in fetal death and growth restriction observed in CBA/J × DBA/2 matings. We propose that as a consequence of C5a-C5aR interaction on monocytes, TF expression increases. TF expression on monocytes enhances the release of antiangiogenic molecule sFlt-1. sFlt-1 impairs trophoblast proliferation, diminishes placental flow, and induces oxidative stress and TF expression resulting in untoward pregnancy outcomes (Figure 5). Although the triggers of immune dysregulation and complement activation in CBA/2-mated CBA/J mice remain unknown, the finding that TF is required for placental and fetal injury in this model identifies a potential target for interventions to prevent recurrent pregnancy complications in patients. TF inhibition with monoclonal antibodies or statins prevents sFlt-1 release, reverses angiogenic factors imbalance, restores placental blood flow, prevents placental oxidative stress, and rescues pregnancies in abortion-prone CBA/J × DBA/2 matings.
Mechanism of trophoblast injury and fetal death in CBA/J × DBA/2 mice. As a result of C5a-C5aR interaction on monocytes, TF expression increases. TF expression on monocytes enhances the release of antiangiogenic molecule sFlt-1. sFlt-1 impairs trophoblasts proliferation, reduces placental blood flow, induces oxidative stress, and increased TF expression on trophoblasts leading to fetal death and IUGR. Thrombin can cleave C5, producing more C5a.
Mechanism of trophoblast injury and fetal death in CBA/J × DBA/2 mice. As a result of C5a-C5aR interaction on monocytes, TF expression increases. TF expression on monocytes enhances the release of antiangiogenic molecule sFlt-1. sFlt-1 impairs trophoblasts proliferation, reduces placental blood flow, induces oxidative stress, and increased TF expression on trophoblasts leading to fetal death and IUGR. Thrombin can cleave C5, producing more C5a.
Considering the beneficial effects of pravastatin in our animal studies and that epidemiologic studies showed no increased teratogenicity following gestational exposure to pravastatin in women,52 we postulate that pravastatin may be a good therapy to prevent recurrent miscarriages and intrauterine growth restriction in humans. Moreover, as pravastatin diminishes sFlt-1 levels and increases VEGF it can also be a good treatment for preeclamptic women. We recognize that these studies were performed in mice and that more epidemiologic data should be collected to test the safety of statins in pregnant women.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Joan S. Hunt (Department of Anatomy and Cell Biology, University of Kansas Medical Center, Kansas City, KS) for making SM9-1 trophoblast cells available for our studies. We are grateful to Dr Daniel Kirchhofer (Genentech) for generously providing 1H1 anti–mouse TF antibody and recombinant mouse TF, to Dr Steven B. Doty (Hospital for Special Surgery) for technical assistant with fluorescence microscopy, and to Dr Nigel Mackman (University of North Carolina at Chapel Hill, NC) for providing the TFfloxed/floxed/LysM-Cre mice.
This research was supported by grants from the Mary Kirkland Center for Lupus Research at Hospital for Special Surgery (New York, NY; G.G.).
Authorship
Contribution: P.R. performed research and contributed to experimental design; N.v.R. contributed the clodronate liposomes and administration protocol; D.T. contributed the human placental samples; and G.G. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guillermina Girardi, Hospital for Special Surgery, 535 East 70th St, New York, NY 10021; e-mail: girardig@hss.edu.

![Figure 3. Increased placental oxidative stress in CBA/J × DBA/2 mice. Increased STAT-8 levels were observed in day-15 placentas from DBA/2-mated CBA/J mice compared with control matings (*P < .001). Treatment with hirudin (hir) or fondaparinox (FPX) prevented STAT-8 increase in CBA/J × DBA/2 mice (n = 6-8 mice/experimental group). Treatment with anti-TF 1H1 antibody or with Cl2MDP clodronate also prevented placental STAT-8 increase (n = 6-8 mice/experimental group). (B) Increased superoxide (O2−) production (DHE red staining) was observed in CBA/J × DBA/2 mice (i). O2− production was attenuated in CBA/J × DBA/2 mice treated with anti-TF (iii) or with Cl2MDP (iv). Minimal O2− production was observed in placentas from control matings (ii). (C) Macrophages from nonpregnant CBA/J female mice were stimulated with C5a, LPS, anti-TF, anti-TF + C5a, or control medium. Blockade of TF or genetic deletion of TF (TFfloxed/floxed/LysM-Cre mice) prevented C5a-induced release of sFlt-1 (n = 4-6 experiments/group; *P < .005 vs control). (D) Trophoblast proliferation assays. SM9-1 cells were incubated in RPMI-1640 as described before.21 Incubation with supernatants of monocytes incubated with C5a inhibited cell proliferation (ii). Incubation with increasing doses of sFlt-1 showed a dose-response inhibitory effect on SM9-1 cell proliferation ([iii] 2000 pg/mL, [iv] 4000 pg/mL, [v] 8000 pg/mL). Increased proliferation was observed when VEGF was added to the supernatant of C5a-treated monocytes (vi). (E) Superoxide (O2−) production in SM9-1 cells. Abundant SM9-1 cells with weak DHE-positive staining were observed in SM9-1 incubated with media (i). Fewer cells but strongly positive with DHE staining were observed in SM9-1 cells incubated with monocyte supernatants (ii) or sFlt-1 (4000 pg/mL [iii] and 8000 pg/mL [iv]). Decreased O2− production was observed when VEGF was added to the supernatant of C5a-treated monocytes (v). (F) Immunocytochemical analysis of TF. A large number of confluent SM9-1 cells with weak TF staining was observed after incubation with medium (i). Diminished cell proliferation and strong positive TF staining were observed in SM9-1 cells incubated with supernatants from C5a-treated monocytes (ii), sFlt-1 4000 pg/mL (iii), or sFlt-1 8000 pg/mL (iv). Increased proliferation and decreased TF were observed when VEGF was added to the supernatant of C5a-treated monocytes (v). Data are mean values plus or minus SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/17/10.1182_blood-2008-12-194258/4/m_zh80200934880003.jpeg?Expires=1765913083&Signature=PwfJc4x0nV6Y6yInkEaGDhGok6jHpQLpedcuQTLC~Nko6KbE6FO5mDtsXEhqG8ZGXQwivIPswLWH8obZs75TaYsgDyaBa93J2RepiGPT2NiNlL2mBrg1p1YIvp-7Sy27Kuci9fP8RLypkyy-vVJVLR3hzGLoPrfATNP2M~0p0rS9kXb8uF18bs3BZ4HVYe-RahBpFIWI1OuvsuKLE-6IY938o-sKs5S35LMJ6P2QaQOa8gZojpplkibJXPUWsZ5SyNiv~BmzqNOOawJn03gHiDZcB8MXhtLs3Cy49PZ93WnxTvWcHbfsWalrFkwC0pm0PqAJwot6o1vSJ7nf9sc8og__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal