Abstract
Toll-like receptors orchestrate rapid local protective innate-immune responses to invading pathogens and optimize leukocyte priming of subsequent adaptive responses. Paradoxically, systemic excess of the TLR2 ligand, bacterial lipoprotein (BLP), suppresses peripheral inflammatory responses. Here, we demonstrate that this phenomenon is regulated via the TLR2-dependent, cell-autonomous down-regulation of inflammatory chemokine receptor expression on a variety of leukocyte subsets. Remarkably, BLP mediated no effect on constitutive chemokine receptor expression. By tracking adoptively transferred wild-type and TLR2−/− leukocytes in vivo, we observed that BLP mediated chemokine receptor switching directed leukocytes away from inflamed sites toward secondary lymphoid organs. These data highlight a novel role for TLR ligands, such as BLP, in regulating leukocyte retention and migration away from innate immune lesions via discrete constitutive and inflammatory chemokine receptor regulation.
Introduction
Toll-like receptors (TLRs) represent the primary sentinels of microbial infection and have evolved to detect, and respond to, a variety of pathogen-associated molecular patterns (PAMPs).1 TLR signaling,1,2 after PAMP binding in leukocytes, and in cells endogenous to the site of infection such as epithelial and endothelial cells, triggers the rapid synthesis and secretion of inflammatory cytokines and chemokines. In particular, the expression of chemokines after PAMP exposure is necessary for the efficient recruitment of inflammatory leukocytes to the tissue.3 Chemokines are characterized by their common ability to direct leukocyte migration and can be defined as either inflammatory or homeostatic, according to the contexts in which they function.3,4 TLR signaling specifically culminates in the production of inflammatory chemokines that orchestrate the recruitment of inflammatory leukocytes into infected or damaged tissues. Interactions between the TLR and chemokine arms of the innate immune reaction therefore represent a key axis regulating host responses to microbial infection. Consequently, elucidating the regulatory and functional relationship between the TLR and chemokine receptor families is of fundamental importance.
The proinflammatory nature of TLR signaling is logical in the context of mounting inflammatory responses to localized microbial infection. However, several studies have reported, paradoxically, that systemic exposure to some TLR ligands (including bacterial lipoprotein [BLP] and lipopolysaccharide [LPS], ligands for TLRs 2 and 4, respectively) is anti-inflammatory and can suppress subsequent leukocyte accumulation in response to a variety of locally applied inflammatory stimuli.5-8 We have previously shown that although local PAMP administration can increase ovalbumin (OVA)–induced lung inflammation, such inflammation can be substantially ameliorated by the systemic administration of PAMPs such as the TLR2 ligand BLP.7 Appropriately harnessed, such TLR-mediated anti-inflammatory pathways may offer therapeutic potential in the context of harmful peripheral inflammatory lesions.
Here, we define the mechanism explaining the inflammation-suppressing effects of BLP. We demonstrate TLR2-dependent down-regulation of inflammatory chemokine receptor expression on numerous inflammatory leukocyte subtypes and provide in vivo evidence to show that the consequence of this action is a redirection of leukocyte migration away from inflamed sites toward secondary lymphoid organs. Thus, systemic PAMPs can suppress peripheral inflammatory responses by transcriptionally down-regulating inflammatory chemokine receptor expression, thereby preventing leukocyte accumulation at inflamed sites. These data provide substantial and novel insight to the complex interactions between TLR and chemokine-dependent arms of the innate immune response and provide a hitherto-unrecognized immune modulatory mechanism with therapeutic potential.
Methods
Cell culture and reagents
Macrophages were grown from murine bone-marrow precursors for 6 days in complete RPMI 1640 medium with L929 conditioned media. Nonadherent cells were removed and replaced with fresh media on day 3 of culture. Dendritic cells were grown in a similar manner except that GM-CSF (10 ng/mL) was used in place of L929 conditioned medium. CD4+ T cells were derived from spleens from WT or TLR2−/− mice9 with negative selection kits (Miltenyi Biotec, Bergisch Gladbach, Germany) and purity analyzed by fluorescence-activated cell sorting (FACS). The cells (2 × 106/mL) were cultured in complete RPMI in 24-well plates for 24 hours in the presence of immobilized anti-CD3 antibody (1 μg/mL; BD Bioscience, San Jose, CA) to induce TLR2 expression.9 Activated CD4+ T cells were then cultured with immobilized anti-CD3 antibody with or without the TLR2 ligand BLP (Pam3CSK4; EMC Microcollections, Tuebingen, Germany) at 1 μg/mL for 4 hours. Neutrophils were isolated from bone marrow using anti–Ly-6G microbeads (Miltenyi Biotec) per manufacturer's instructions (purity of 98% determined by cytospin) and were cultured in complete RPMI. NF-κB blocker BAY11-7085 was supplied by Sigma-Aldrich (St Louis, MO). All other treatments of cells were conducted in complete RPMI with L929 conditioned media (20% final vol/vol) included for macrophage cultures.
Induction of cutaneous inflammation
Wild-type (WT) and D6-deficient female mice (on a 129-C57BL/6 background) between 6 and 10 weeks of age were used in all experiments.10 All animal-related procedures reported in this study were approved by the Glasgow University local ethics review committee and are supported by a United Kingdom Government Home Office Project License. Cutaneous inflammation was induced by application of 12-O-tetradecanoyl phorbol-13-acetate (TPA) as described previously.10 In brief, 150 μL of a 50 μmol/L solution of TPA in acetone was applied to shaved dorsal skin on 3 consecutive days and the animals killed 4 days after this treatment. Where BLP was administered, it was by intraperitoneal injection, and 100 μg of BLP was used per treatment per mouse. Chemokine concentrations were measured by the use of enzyme-linked immunosorbent assay (ELISA) in homogenates of back skin. All animal experiments were conducted under license from the United Kingdom Home office.
All histologic and immunocytochemical analyses were conducted as described previously,10 slides were visualized with an Axioscope A microscope (Carl Zeiss, Welwyn Garden City, United Kingdom), and images were captured with Axiovision 3 software (Zeiss). Cells were counted (blinded) in sections as the total number of cells per 20 random fields.
Reverse transcriptase polymerase chain reaction
RNA was extracted and genomic DNA removed with the use of RNeasy columns (QIAGEN, Germantown, MD) for cell lysates. A total of 3 μg total RNA was reverse-transcribed with AffinityScript (Stratagene, La Jolla, CA) and gene transcripts levels normalized to GAPDH. Quantitative polymerase chain reaction (qPCR) was undertaken with the use of Power-SYBR-green master mix on a prism 7900HT (Applied Biosystems, Foster City, CA). Primers were designed using primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), as previously described.11 Primer sequences are listed in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Migration assay
Mouse T cells, stimulated as described previously, and human monocytes stimulated with BLP (100 ng/mL) for 4 hours in complete RPMI were placed in a transwell system. CCL3 for T-cell migration and CCL2 for monocyte migration (both from R&D Systems, Minneapolis, MN) were added at various concentrations to the lower chamber of the appropriate transwells and incubated at 37°C for 3 hours. The total numbers of cells that had migrated to lower chamber were counted and expressed as a migration index, with 1 equaling the level of random cell migration independent of chemokine and each integer representing a fold increase in cell numbers.
Adoptive transfer of labeled cells to TPA-painted mice
Bone marrow-derived macrophages, or spleen-derived anti-CD3–treated T cells, were labeled with either carboxyfluorescein succinimidyl ester (CFSE) for WT cells or 5(6)-carboxy tetramethylrhodamine [5(6)-TAMRA] for TLR2-deficient cells. T cells (1.5 × 107 of each type) were mixed and administered intravenously to mice with TPA-induced cutaneous inflammation and BLP or phosphate-buffered saline (PBS) administered intraperitoneally 2 hours later. Labeled macrophages were mixed together (1.5 × 107 of each type) and similarly given to another group of TPA-painted mice with or without intraperitoneal BLP. Twenty-four hours later peripheral lymph nodes were removed, and numbers of labeled cells determined by FACS. Skin sections were frozen with OCT and mounted with DAPI. Numbers of WT (CFSE/green) and TLR2-deficient (TAMRA/red) cells were counted with the use of confocal microscopy (Carl Zeiss). For each mouse, multiple (>10) frozen skin sections (representing the full length of the TPA-treated skin) were used for cell counting.
Interferon-γ treatment of cells
To induce CCR5 expression in macrophages, bone marrow–derived macrophages were prepared with the use of WT mouse bone marrow as described previously. These cells were then treated with 10 ng/mL of interferon (IFN)-γ for 24 hours after which they were either left unaltered or were exposed to 100 ng/mL of BLP for a further 4 hours. RNA was then prepared from the cells and CCR5 expression levels measured by qPCR as described previously.
Fluorescently labeled chemokine binding and flow cytometry
Uptake assays were performed with the use of isolated CD3-treated T cells and bone marrow–derived cultures of macrophages and dendritic cells in complete RPMI supplemented with 20 mmol/L HEPES to buffer chemokine binding. Cells were cultured for 30 minutes to re-establish cell metabolism at 37°C. Cells were exposed to either 25 ng/mL labeled chemokine CCL2-Alexa Fluor 647 (Almac Sciences, Elvingston, United Kingdom) or a similar concentration of phycoerythrin (PE)–conjugated biotinylated CCL19 (R&D Systems). Cells were incubated with chemokine for 80 minutes, washed in PBS, and analyzed for fluorescence in either a FACSCalibur or a Miltenyi Biotec MacsQuant Flow Cytometer.
Cell viability measurements
Leukocytes were incubated with BLP at 100 ng/mL for either 4 hours or 16 hours and cell viability determined by adding viaprobe (BD Biosciences). Stained cells were analyzed by FACS to determine the percentage of dying and dead cells.
Detection of plasma chemokine levels
Mice were administered with an intraperitoneal injection of either saline or 100 μg of BLP. After either 6 hours or 24 hours (n = 3 for all groups), mice were killed by CO2 and blood removed with a 25-gauge needle and syringe from the vena cava into 100-μL citrate-dextrose solution (Sigma C3821) to prevent clotting. Samples were spun for 10 minutes at 600g to remove all cells, and the clear red-blood cell free plasma fraction was removed for analysis. Plasma samples were analyzed for chemokine protein using the Luminex (Biosource/Invitrogen, Carlsbad, CA) mouse chemokine 5-plex on a Luminex 200 analyzer per manufacturer's instructions.
Results
Systemic BLP administration blocks cutaneous inflammatory responses
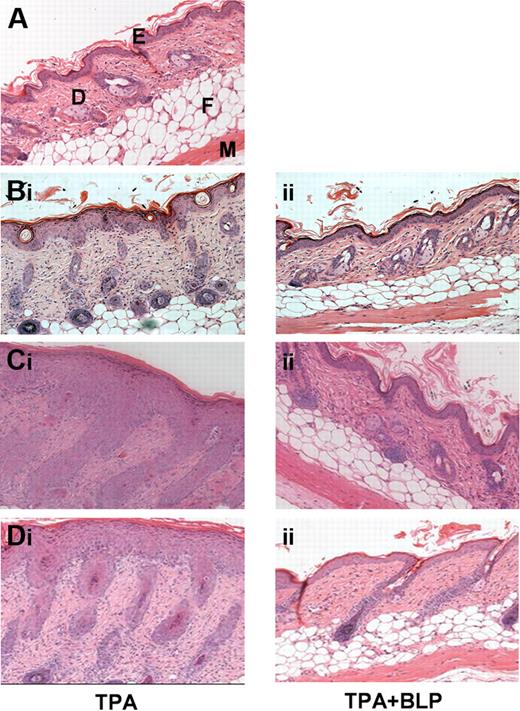
We previously demonstrated that the systemic administration of the TLR2 ligand, BLP, modulates peripheral inflammatory responses associated with allergen-driven murine airways hypersensitivity and inflammation.7 To test whether such effects are observed in nonantigen/allergen-driven inflammatory models, we examined the effects of systemic BLP on the cutaneous inflammatory response to phorbol ester. Compared with normal untreated murine skin (Figure 1A), application of the phorbol ester TPA to shaved WT murine dorsal skin induced a transient, mild inflammatory response characterized by epidermal hyperplasia, skin thickening, and infiltration of leukocytes.10 Mice were given daily, prophylactic, intraperitoneal doses of either saline or BLP, and TPA was applied daily for 3 days. Systemic BLP significantly suppressed the TPA-induced cutaneous inflammation in this model. This finding was evident qualitatively by histomorphometry (Figure 1B) and quantitatively (Figure S1A) with the use of skin thickening (epidermal and dermal) as an index of cutaneous inflammation. These data indicate that the anti-inflammatory properties of systemic BLP are not restricted to pulmonary inflammatory models but also can be seen in models of transient, nonantigen-specific, cutaneous inflammatory responses.
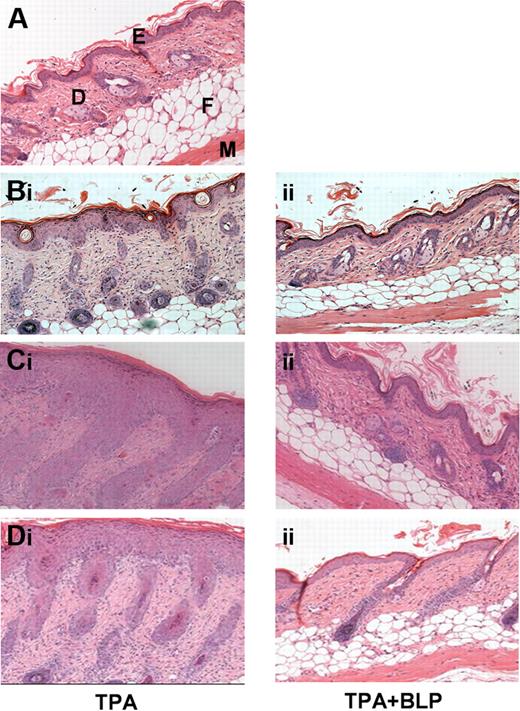
Systemically administered BLP ameliorates cutaneous inflammation. (A) Hematoxylin and eosin (H&E) staining of untreated murine dorsal skin. E, epidermis; D, dermis; F, fatty layer; M, muscle layer. (B) WT mice were given daily intraperitoneal prophylactic doses of either (i) saline or (ii) 100 μg BLP, followed by 3 days of TPA painting to shaved dorsal skin (150 μL of a 50-μmol/L solution in acetone). Two days later mice were culled and skin sections stained by H&E. (C) D6-deficient mice were given daily intraperitoneal prophylactic doses of either (i) saline or (ii) BLP and subjected to TPA painting, as for WT mice. Four days after the TPA painting, mice were culled and skin sections stained by H&E. (D) D6-deficient mice were painted with TPA daily for 3 days and given (i) therapeutic saline or (ii) 100 μg BLP 48 hours after final TPA paint. All results are representative of 3 separate experiments with 5 mice per group per experiment.
Systemically administered BLP ameliorates cutaneous inflammation. (A) Hematoxylin and eosin (H&E) staining of untreated murine dorsal skin. E, epidermis; D, dermis; F, fatty layer; M, muscle layer. (B) WT mice were given daily intraperitoneal prophylactic doses of either (i) saline or (ii) 100 μg BLP, followed by 3 days of TPA painting to shaved dorsal skin (150 μL of a 50-μmol/L solution in acetone). Two days later mice were culled and skin sections stained by H&E. (C) D6-deficient mice were given daily intraperitoneal prophylactic doses of either (i) saline or (ii) BLP and subjected to TPA painting, as for WT mice. Four days after the TPA painting, mice were culled and skin sections stained by H&E. (D) D6-deficient mice were painted with TPA daily for 3 days and given (i) therapeutic saline or (ii) 100 μg BLP 48 hours after final TPA paint. All results are representative of 3 separate experiments with 5 mice per group per experiment.
We, and others, recently showed that mice with a homozygous deletion in the D6 chemokine decoy receptor display exaggerated, and prolonged, cutaneous inflammatory responses compared with wild-type mice.10,12,13 In this model, TPA induces an extremely marked program of epidermal hyperproliferation, and leukocyte influx, thus representing an exaggerated inflammatory response. This is associated with excessive accumulation of cutaneous inflammatory chemokines after TPA administration and results in greater numbers of infiltrating leukocytes and the development of a psoriasis-like inflammatory pathology.10,14 To determine whether systemic BLP could also ameliorate the inflammatory response in this pathologic model of severe cutaneous inflammation, we examined the cutaneous inflammatory response of D6-deficient mice to TPA in the presence, or absence, of systemically administered BLP. TPA application caused an exaggerated inflammatory reaction in dorsal skin of control D6-deficient mice (Figure 1Ci) that was substantially, and significantly, suppressed by systemic BLP delivery as shown by both histologic (Figure 1Cii) and quantitative (Figure S1B) analyses and by a reduction in the numbers of epidermal T cells and dermal mast cells (Figure S1C, D) in the inflamed skin. These data show that systemic BLP administration blocked both transient self-resolving, and pathologic, cutaneous inflammatory responses in murine skin.
To determine whether BLP also was capable of acting “therapeutically” in this model, mice were given a dose of systemic BLP just before the maximal point of the inflammatory response, that is, at 2 days after the TPA application, and were culled 2 days later. This therapeutic BLP significantly reduced cutaneous inflammation. This was manifest histologically (Figure 1Di,ii) and by quantitative analyses (Figure S1E) that revealed significant reduction in skin thickness after BLP administration. Thus, systemic BLP was effective at preventing, or suppressing, ongoing cutaneous inflammatory responses.
BLP does not alter cutaneous inflammatory mediator expression
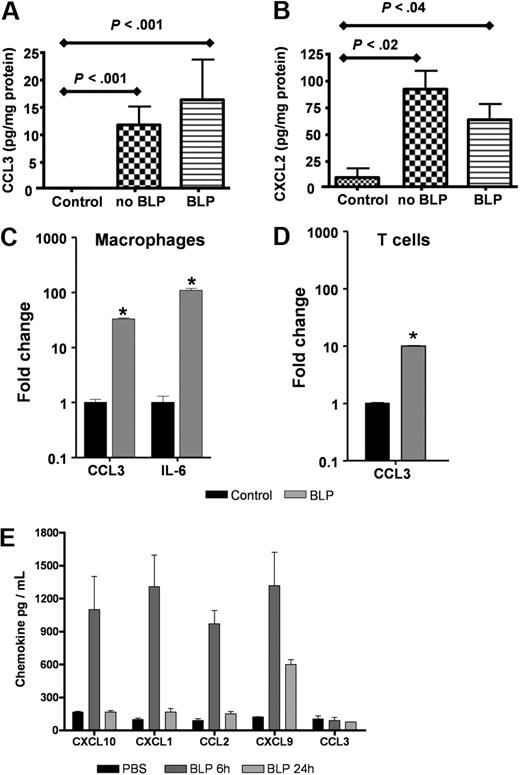
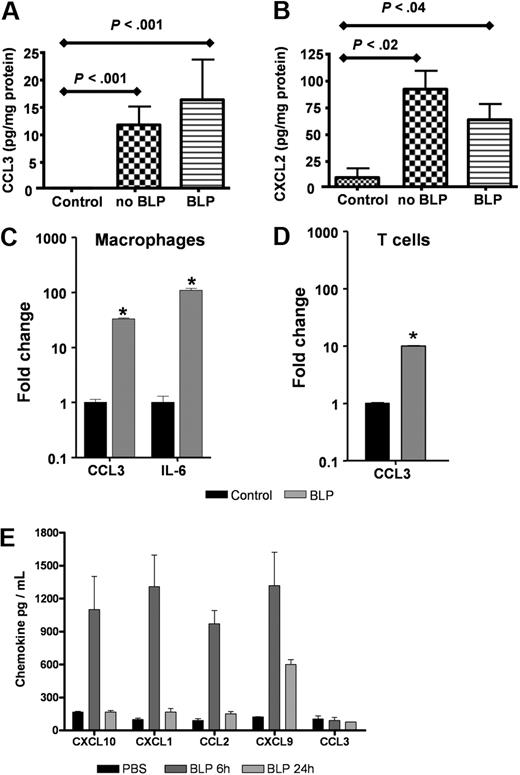
To examine the possibility that systemic BLP may mediate its anti-inflammatory effects by down-regulating the expression of inflammatory mediators at inflamed sites, we measured levels of the inflammatory chemokines MIP-1α/CCL3 and MIP2/CXCL2 in homogenates of TPA-treated skin from D6-deficient mice treated with, or without, systemic BLP. As shown in Figure 2A and B, neither CCL3 nor CXCL2 were detected at appreciable levels in untreated mouse skin. However, TPA treatment induced a marked and significant induction of both these chemokines, and these levels of induction were unaltered by systemic administration of BLP. Indeed, and in agreement with previous reports,15 BLP induced high levels of inflammatory agents when applied directly to leukocytes. Specifically, BLP stimulation of primary macrophages (Figure 2C) and T cells (Figure 2D) in vitro resulted in significant, transcriptional up-regulation of inflammatory chemokines (eg, CCL3) and cytokines, (eg, interleukin [IL]-6; Figure 2C,D). In addition, and as predicted, systemic administration of BLP resulted in up-regulation of circulating inflammatory chemokine levels (Figure 2E). Interestingly, this induction of chemokine protein was transient, peaking at 6 hours for the majority of chemokines and, with the exception of CXCL9, returning to baseline levels by 24 hours. Curiously, levels of circulating MIP-1α/CCL3 were not seen to be induced by systemic BLP administration although overall, these data again confirm the essentially proinflammatory nature of BLP. Thus, the inflammatory-suppressive effects of systemic BLP are not mediated at the level of either local or systemic suppression of inflammatory mediator production.
BLP does not alter inflammatory chemokine production in the skin. (A,B) D6-deficient mice were given intraperitoneal BLP (100 μg) or vehicle injections, TPA was applied to skin and mice culled 24 hours later. Skins were removed, lysed, and levels of CCL3 (A) and CXCL2 (B) were measured by the use of ELISA and compared with levels detectable in untreated control mouse skin. These data are representative of 2 separate experiments with 3 to 5 mice per group. (C,D) Primary cultures of bone marrow–derived macrophages (C) and anti-CD3 primed CD4+ T cells from D6-deficient mice (D) were stimulated with 100 ng/mL BLP for 4 hours in complete RPMI, RNA extracted, and transcripts for CCL3 and IL-6 quantified by qPCR and expressed as fold change relative to unstimulated cultures. Bars show the mean of 4 replicates plus or minus SEM. Marked bars are statistically different from control unstimulated cells (Student t test; *P < .01). (E) BLP (100 μg) was administered intraperitoneally to D6-deficient mice, and blood was collected and plasma prepared 6 and 24 hours later. Chemokine levels in the plasma were assayed by Luminex. These data are mean average measurements from 3 mice per point ± SD.
BLP does not alter inflammatory chemokine production in the skin. (A,B) D6-deficient mice were given intraperitoneal BLP (100 μg) or vehicle injections, TPA was applied to skin and mice culled 24 hours later. Skins were removed, lysed, and levels of CCL3 (A) and CXCL2 (B) were measured by the use of ELISA and compared with levels detectable in untreated control mouse skin. These data are representative of 2 separate experiments with 3 to 5 mice per group. (C,D) Primary cultures of bone marrow–derived macrophages (C) and anti-CD3 primed CD4+ T cells from D6-deficient mice (D) were stimulated with 100 ng/mL BLP for 4 hours in complete RPMI, RNA extracted, and transcripts for CCL3 and IL-6 quantified by qPCR and expressed as fold change relative to unstimulated cultures. Bars show the mean of 4 replicates plus or minus SEM. Marked bars are statistically different from control unstimulated cells (Student t test; *P < .01). (E) BLP (100 μg) was administered intraperitoneally to D6-deficient mice, and blood was collected and plasma prepared 6 and 24 hours later. Chemokine levels in the plasma were assayed by Luminex. These data are mean average measurements from 3 mice per point ± SD.
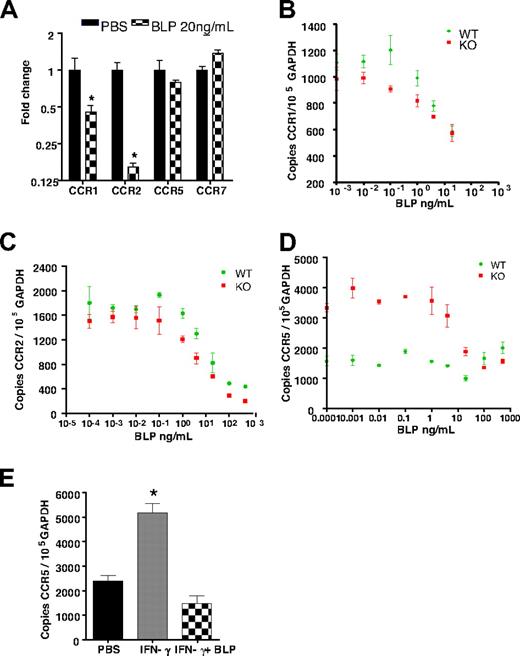
BLP selectively down-regulates inflammatory chemokine receptor expression
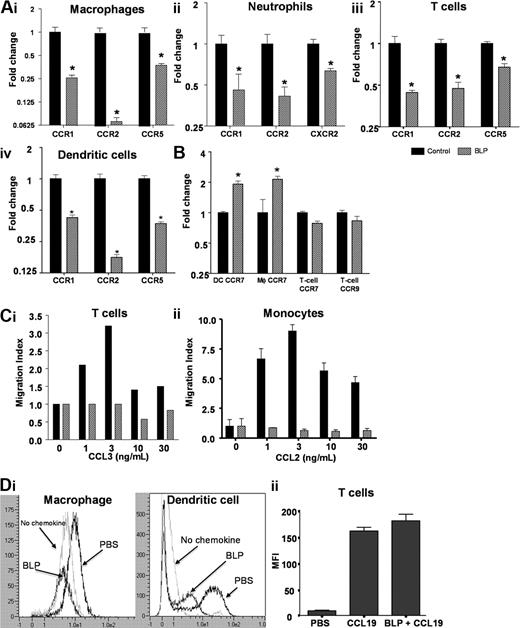
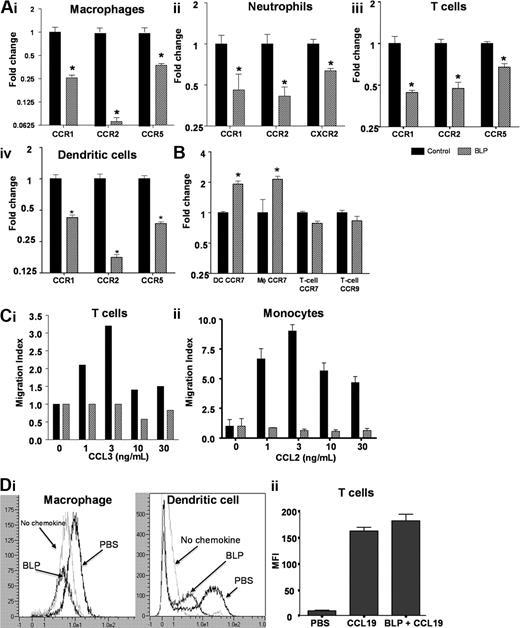
We next hypothesized that altered leukocyte trafficking in the D6-deficient mice may underpin the anti-inflammatory effects of systemic BLP and therefore investigated the impact of BLP on inflammatory chemokine receptor expression in leukocytes from these mice. Bone marrow–derived macrophages expressed the inflammatory receptors CCRs1, 2 and 5, with CCR2 being most highly expressed. Significant and substantial down-regulation of CCR1, CCR5, and particularly CCR2, transcripts occurred within 4 hours of exposure to BLP (Figure 3Ai). Murine neutrophils expressed CCR1, CCR2 (at low levels), and CXCR2, all of which were down-regulated after exposure to BLP (Figure 3Aii). As shown in Figures 3Aiii and 3Aiv, BLP similarly induced down-regulation of CCRs1, 2, and 5 in T cells and dendritic cells (DCs). Analysis of the BLP response in bone marrow–derived macrophages showed that the down-regulation of inflammatory chemokine receptor transcripts occurred concurrent with TNF transcript up-regulation (Figure S2A,B) and, as shown for CCR2, was dependent on BLP dose with half maximal effect at 4.3 ng/mL BLP (Figure S2A). Thus BLP exposure results in down-regulation of expression of inflammatory chemokine receptors in a variety of leukocyte subtypes.
BLP down-regulates inflammatory chemokine receptor expression. (A) Macrophages (i), neutrophils (ii), CD4+ T cells (iii), and dendritic cells (iv) from D6-deficient mice were stimulated with BLP at 50 ng/mL (macrophages, neutrophils, DCs) or 1 μg/mL (T-cells) for 4 hours and inflammatory chemokine receptor expression determined by qPCR. (B) Transcripts for homeostatic chemokine receptors were also determined by qPCR. (C) Untreated or BLP-treated (i) mouse CD4+ T cells and (ii) human monocytes were tested for migration toward CCL3 and CCL2, respectively, in transwell assays. The data represent the alterations in the measured migration index and are representative of 3 experiments each. SEMs are indicated for the monocyte migration assays. (Di) Surface CCR2 levels were assessed after treatment of macrophages and DCs for 16 hours with 100 ng/mL BLP by analysis of binding of the Alexa Fluor–labeled CCR2 ligand, CCL2. Binding of this labeled ligand was quantified using flow cytometry. (Dii) BLP does not alter cell surface expression of CCR7 as measured by PE-labeled CCL19 binding and internalization. All bars for qPCR data show mean fold change of 4 replicates ± SEM compared with cells treated with vehicle. Marked bars are statistically different from unstimulated cells (Student t test; *P < .01).
BLP down-regulates inflammatory chemokine receptor expression. (A) Macrophages (i), neutrophils (ii), CD4+ T cells (iii), and dendritic cells (iv) from D6-deficient mice were stimulated with BLP at 50 ng/mL (macrophages, neutrophils, DCs) or 1 μg/mL (T-cells) for 4 hours and inflammatory chemokine receptor expression determined by qPCR. (B) Transcripts for homeostatic chemokine receptors were also determined by qPCR. (C) Untreated or BLP-treated (i) mouse CD4+ T cells and (ii) human monocytes were tested for migration toward CCL3 and CCL2, respectively, in transwell assays. The data represent the alterations in the measured migration index and are representative of 3 experiments each. SEMs are indicated for the monocyte migration assays. (Di) Surface CCR2 levels were assessed after treatment of macrophages and DCs for 16 hours with 100 ng/mL BLP by analysis of binding of the Alexa Fluor–labeled CCR2 ligand, CCL2. Binding of this labeled ligand was quantified using flow cytometry. (Dii) BLP does not alter cell surface expression of CCR7 as measured by PE-labeled CCL19 binding and internalization. All bars for qPCR data show mean fold change of 4 replicates ± SEM compared with cells treated with vehicle. Marked bars are statistically different from unstimulated cells (Student t test; *P < .01).
Importantly, and as shown for T cells, DCs, and macrophages in Figure S2C, this reduction in inflammatory chemokine receptor expression was not associated with any alteration in cell viability, as measured with Viaprobe (and annexin staining; data not shown), after the treatment of cells with BLP. In contrast to the effects on inflammatory receptors, BLP did not affect the expression levels of the constitutive chemokine receptors CCR7 and CCR9 in T cells, whereas CCR7 expression in DCs and macrophages was significantly increased (Figure 3B). Thus, in a range of leukocytes, BLP induced selective reduction in inflammatory chemokine receptor transcript levels, suggesting that this may be the basis for the anti-inflammatory effects of systemic BLP.
To determine whether the decrease in receptor transcript levels was associated with a decrease in chemokine responsiveness, leukocytes were either left unstimulated or stimulated with BLP as before and their ability to migrate toward JE/CCL2 or MIP-1α/CCL3 determined. Untreated murine CD4+ T cells exhibited a “bell-shaped” dose response to CCL3 with maximum migration occurring at 3 ng/mL. Conversely BLP treated cells failed to migrate at levels greater than background (Figure 3Ci). Similarly, data were obtained by examining human monocyte responses to CCL2 (Figure 3Cii). These data demonstrate a functional impairment of the BLP-treated cells in migration assays. To examine whether decreased receptor protein expression on the cells is associated with this functional impairment, we performed ligand binding assays with the use of Alexa Fluor–labeled CCL2. The results showed a reduction in cell surface expression of CCR2 in murine bone marrow–derived macrophages and DCs after 4 (data not shown) and 16 hours of BLP treatment (Figure 3Di).
Similar analysis of BLP-treated macrophages demonstrated a similar effect of BLP on CCR2 levels in these cells (data not shown). Thus, the BLP-dependent transcriptional down-regulation of inflammatory chemokine receptors leads to a reduction in cell surface expression of inflammatory chemokine receptors and an inability of the treated leukocytes to respond to inflammatory chemokines. Importantly, and in keeping with the lack of reduction in expression of RNA for the constitutive chemokine receptors, such as CCR7, the binding, and internalization, of PE-labeled CCL19 in T cells showed CCR7 protein levels to be unchanged compared with untreated cells (Figure 3Dii). Thus, after BLP treatment, CCR7 protein levels remain unchanged, and the receptor is functional, as judged by its ability to bind and internalize ligand.
Nuclear factor (NF)κB may be involved in chemokine receptor down-regulation by BLP
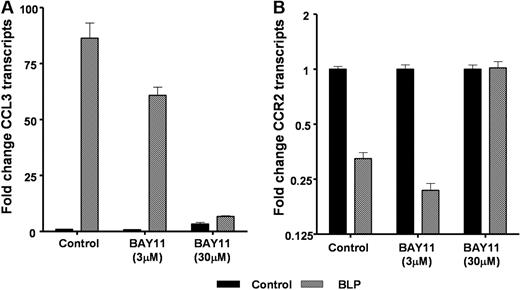
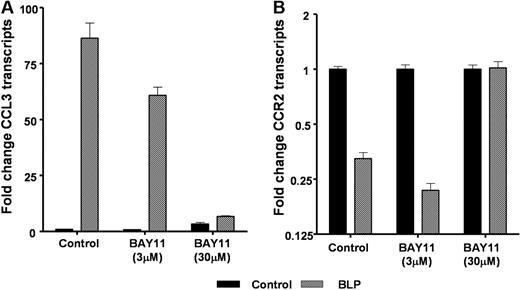
Evidence to date suggests that BLP binding to TLR2 culminates in the classical proinflammatory NF-κB signaling pathway.16 To examine the role for NF-κB signaling in the effects of BLP on inflammatory chemokine receptor transcript levels, we treated D6-deficient macrophages with a blocker of NF-κB (BAY-11)17 during the BLP treatment phase. BAY-11 blocks NFκB activity by suppressing the phosphorylation of IκB-α and has been previously used in several studies to demonstrate NFκB involvement in a range of inflammatory contexts.18-22 When tested at 3 and 30 μmol/L, it was apparent that BAY-11 was an efficient blocker of NF-κB signaling, at the higher concentration, as measured by its ability to inhibit BLP-induced CCL3 expression (Figure 4A). In addition, 30 μmol/L BAY-11 blocked the reduction in expression of CCR2 (Figure 4B) as well as CCRs 1 and 5 (data not shown). Therefore, although these data have been generated using only a single blocker of NFκB, they are suggestive of a role for IκB-α/NF-κB signaling in the BLP-mediated down-regulation of inflammatory chemokine receptor expression.
Chemokine receptor down-regulation and NF-κB. Macrophages from D6-deficient mice were either left untreated (■) or were stimulated with 100 ng/mL BLP ( ) in the presence or absence of the NF-κB blocker BAY-11 at both 3 and 30 μmol/L for 4 hours and CCL3 (A) and CCR2 (B) transcription determined by qPCR.
) in the presence or absence of the NF-κB blocker BAY-11 at both 3 and 30 μmol/L for 4 hours and CCL3 (A) and CCR2 (B) transcription determined by qPCR.
Chemokine receptor down-regulation and NF-κB. Macrophages from D6-deficient mice were either left untreated (■) or were stimulated with 100 ng/mL BLP ( ) in the presence or absence of the NF-κB blocker BAY-11 at both 3 and 30 μmol/L for 4 hours and CCL3 (A) and CCR2 (B) transcription determined by qPCR.
) in the presence or absence of the NF-κB blocker BAY-11 at both 3 and 30 μmol/L for 4 hours and CCL3 (A) and CCR2 (B) transcription determined by qPCR.
Leukocyte activation status impacts on TLR responsiveness
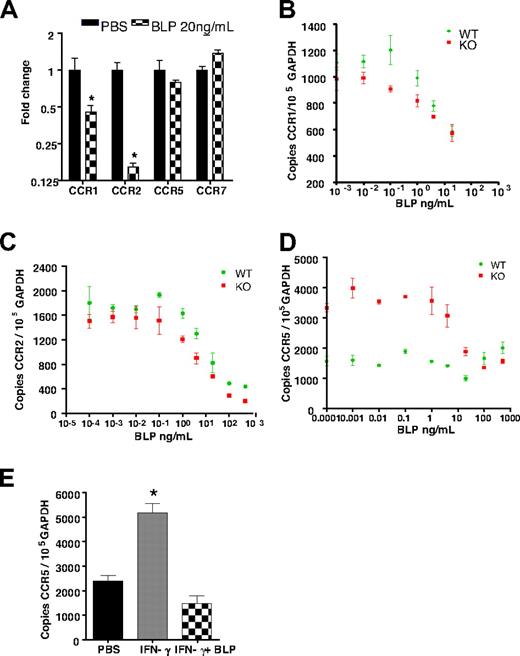
The aforementioned analyses were conducted with the use of cells from D6-deficient mice. Interestingly, when cells from WT mice were studied, CCR1 and CCR2 showed a significant reduction in transcript levels in response to BLP; however, no alterations in CCR5 expression were observed (Figure 5A). CCR1 and CCR2 levels were indistinguishable in WT and D6-deficient macrophages and displayed identical dose–response relationships in the BLP-mediated transcriptional down-regulation of their expression levels (Figures 5B,C). In contrast, we noted that D6-null macrophages expressed significantly greater levels of CCR5 than their WT counterparts and that BLP reduced this expression level to approximately that found in WT macrophages (Figure 5D). This finding suggested that it was the induced levels of CCR5 seen in the D6-null macrophages that were being reduced and that the low basal levels seen in WT macrophages were not amenable to similar BLP-mediated reduction. Thus, we tested the hypothesis that activation status of the macrophages can impact on BLP responsiveness. Specifically we treated WT macrophages with IFNγ23 for 24 hours to induce CCR5 expression (Figure 5E). Importantly, although BLP did not reduce CCR5 levels in resting WT macrophages, it did reduce the levels observed after IFNγ treatment (Figure 5E), strengthening the notion that the activation status of cells in WT mice, in the context of peripheral or systemic inflammation, is likely to contribute to the ability of BLP to down-regulate chemokine receptor expression and thus suppress inflammation.
Activation is required for BLP-mediated reduction of CCR5 transcript levels. (A) Bone marrow-derived macrophages from WT mice were stimulated with 20 ng/mL BLP and CCR transcript levels determined. CCR1 (B) and CCR2 (C) transcript reduction was similar and dose-dependent in both WT and D6-deficient macrophages. (D) CCR5 transcripts were greater in resting D6-deficient macrophages and BLP stimulation reset these levels to those seen in WT macrophages. (E) Treatment with 10 ng/mL of IFN-γ for 24 hours induced CCR5 up-regulation in WT macrophages was reset by 4 hours of BLP stimulation to the lower level observed in resting WT cells. Marked bars are statistically different from unstimulated cells (Student t test; *P = .02).
Activation is required for BLP-mediated reduction of CCR5 transcript levels. (A) Bone marrow-derived macrophages from WT mice were stimulated with 20 ng/mL BLP and CCR transcript levels determined. CCR1 (B) and CCR2 (C) transcript reduction was similar and dose-dependent in both WT and D6-deficient macrophages. (D) CCR5 transcripts were greater in resting D6-deficient macrophages and BLP stimulation reset these levels to those seen in WT macrophages. (E) Treatment with 10 ng/mL of IFN-γ for 24 hours induced CCR5 up-regulation in WT macrophages was reset by 4 hours of BLP stimulation to the lower level observed in resting WT cells. Marked bars are statistically different from unstimulated cells (Student t test; *P = .02).
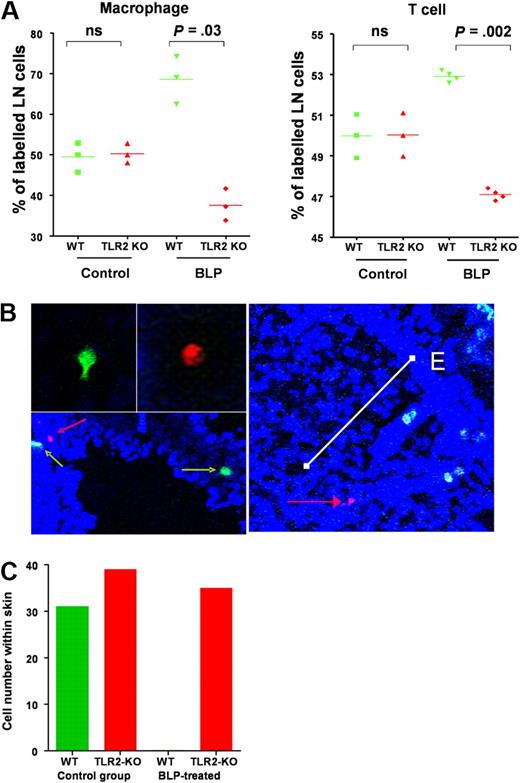
Systemic BLP orchestrates redistribution of inflammatory leukocytes in vivo
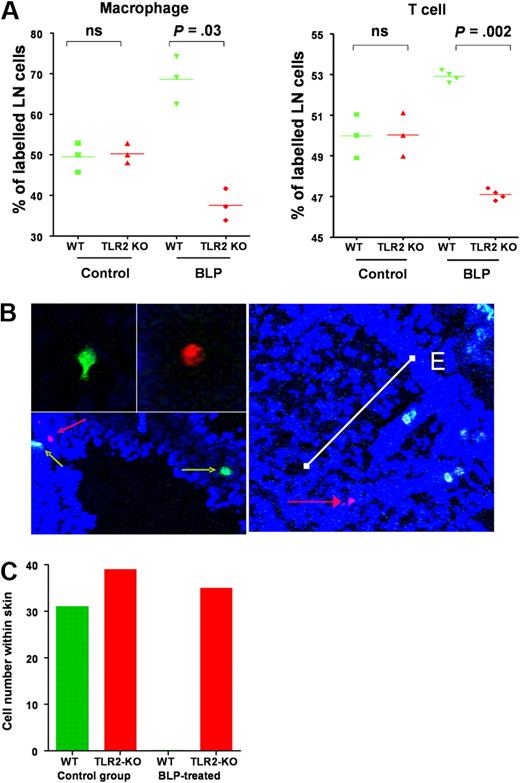
The aforementioned data suggest that the in vivo anti-inflammatory effects of systemic BLP are likely to be the consequence of chemokine receptor switching on a broad range of inflammatory leukocytes. To test this, we compared the impact of systemic BLP on the in vivo migration of WT and TLR2-deficient leukocytes in the D6-deficient mouse model of cutaneous inflammation. WT mice painted with TPA (2 paintings, each 24 hours apart) to induce a mild inflammatory reaction, received a mix of differentially dye-labeled macrophages from WT and TLR2-deficient donors with or without an intraperitoneal administration of BLP. At 24 hours later, mice were culled and lymph nodes and skin collected. Flow cytometric analysis of lymph node cell populations indicated that, in non-BLP–treated mice, equal numbers of dye-labeled TLR2-deficient and WT macrophages migrated to lymph nodes. However, BLP administration caused a preferential redistribution of WT cells to lymph nodes that was not found with TLR2-deficient cells (Figure 6A). This finding is in keeping with the TLR2-dependent down-regulation of inflammatory chemokine receptor levels on WT cells with the remaining constitutive receptors driving lymph node homing. The fact that TLR2-deficient cells did not show preferential lymph node homing confirmed that BLP redirects leukocyte migration on a cell-autonomous basis. Similar data were obtained when dye-labeled T cells were transferred instead of macrophages (Figure 6A) and thus such mechanisms are likely to impact on the in vivo migration of a range of inflammatory leukocytes. Thus, the selective down-regulation of inflammatory chemokine receptor transcript levels in the WT cells after BLP administration resulted in a preferential redistribution of these cells, in vivo, to peripheral lymph nodes. In conjunction with the data in Figure 3Dii, this further supports the contention that CCR7 expression and function remain unaltered after BLP treatment of either macrophages or T cells.
systemic BLP redirects leukocyte migration in vivo. (A) TLR2-null and WT macrophages (1.5 × 107 of each) labeled with spectrally different fluorescent makers were administered intravenously to TPA-painted D6-deficient mice (red TLR2-deficient cells and green WT cells). Concurrently, mice were intraperitoneally administered either BLP or saline and culled 24 hours later. LN were removed and cells examined by FACS to specifically determine numbers of dye-labeled macrophages that had migrated to the LN. Values shown represent the percentage of dye-labeled macrophages that were either WT or TLR2-null for individual mice. CD4 T cells were isolated from TLR2-deficient and WT mice and similarly labeled and injected (numbers as above) into TPA-treated mice that received either saline or BLP intraperitoneally and percentage of labeled cells migrating to LN determined by FACS. Statistical probability were determined with the Student t test (ns, not significant). (B) Identification of WT (green cells, green arrows) and TLR2-deficient cells (red cells, red arrows) in TPA-inflamed skin. The white bar on the right panel shows the positioning of a TLR2-deficient cell (red arrow) at an approximate distance of 200 μm from the epidermal surface (E). (C) Quantification of labeled macrophage numbers within TPA-painted skin from control and BLP-treated mice. These data are representative of data obtained from 3 separate experiments with 3 mice per group. Cell counts were from multiple (> 10) full-length skin sections per mouse.
systemic BLP redirects leukocyte migration in vivo. (A) TLR2-null and WT macrophages (1.5 × 107 of each) labeled with spectrally different fluorescent makers were administered intravenously to TPA-painted D6-deficient mice (red TLR2-deficient cells and green WT cells). Concurrently, mice were intraperitoneally administered either BLP or saline and culled 24 hours later. LN were removed and cells examined by FACS to specifically determine numbers of dye-labeled macrophages that had migrated to the LN. Values shown represent the percentage of dye-labeled macrophages that were either WT or TLR2-null for individual mice. CD4 T cells were isolated from TLR2-deficient and WT mice and similarly labeled and injected (numbers as above) into TPA-treated mice that received either saline or BLP intraperitoneally and percentage of labeled cells migrating to LN determined by FACS. Statistical probability were determined with the Student t test (ns, not significant). (B) Identification of WT (green cells, green arrows) and TLR2-deficient cells (red cells, red arrows) in TPA-inflamed skin. The white bar on the right panel shows the positioning of a TLR2-deficient cell (red arrow) at an approximate distance of 200 μm from the epidermal surface (E). (C) Quantification of labeled macrophage numbers within TPA-painted skin from control and BLP-treated mice. These data are representative of data obtained from 3 separate experiments with 3 mice per group. Cell counts were from multiple (> 10) full-length skin sections per mouse.
Confocal imaging of TPA-painted skin revealed equivalent accumulation of WT and TLR2-deficient cells that had migrated to the inflamed site in the absence of BLP treatment (Figure 6B). Cells entered the skin at a mean depth of approximately 200 μm from the epidermal surface (data not shown) although labeled cells were found throughout the dermis and epidermis. However, and in marked contrast to the lymph node leukocyte accumulation, analysis of multiple sections from BLP treated mice revealed only TLR2 deficient macrophages in the inflamed skin (Figure 6C) indicating that such cells had eluded BLP-mediated inflammatory chemokine receptor down-regulation and thus continued to be recruited to the inflamed tissue site.
Discussion
Despite being proinflammatory in nature, systemically administered TLR ligands can be highly effective at suppressing allergen-induced inflammation at peripheral sites. We have now investigated this phenomenon in a model of antigen/allergen-independent innate immune responses. Systemic BLP was effective either prophylactically or therapeutically in suppressing peripheral inflammation after application of TPA to mouse skin. We observed a consistent switch in chemokine receptor expression that made cells nonresponsive to inflammatory chemokines, and instead encouraged migration to lymph nodes. Thus, we propose that TLR-ligand induced chemokine receptor switching in a variety of leukocytes explains the anti-inflammatory properties of these agents when systemically administered. As we clearly show in Figure 2E that systemic BLP leads to increased circulating inflammatory chemokine levels, this may also contribute to the blunting of inflammatory leukocyte migration by functionally down-regulating the inflammatory receptors on circulating leukocytes. However, it should be noted that the induction of the circulating chemokines is transient and may thus play only a limited role in this context.
Importantly, although we have demonstrated reduction in inflammatory chemokine receptors by down-regulation of transcript levels, previous studies have reported regulation of inflammatory chemokine receptor expression by ligands for TLR 2 and TLR 4 through a variety of other mechanisms.24-30 Thus, there may be several ways in which TLR ligands mediate, and sustain, suppression of inflammatory chemokine receptor function. Interestingly some reports have suggested that expression of some chemokine receptors such as CCR5 may not be regulated by TLR ligands at the transcriptional level26,31 thus apparently conflicting with data presented in this article. As demonstrated in Figure 5, we now show that WT leukocytes require activation of expression of CCR5 to “supernumerary” levels before significant transcriptional down-regulation by BLP is observed. In the previous studies26,31 there was no previous enhancement of CCR5 levels, thus accounting for the reported lack of effect of TLR ligands on transcript levels. In contrast to CCR5, CCR1 and CCR2 levels are down-regulated regardless of the activation status of the cells, suggesting that levels of these receptors are regulated differently to those of CCR5. Finally our data suggest that the D6-null mice have greater transcript levels for CCR5 on their leukocytes and this may reflect an enhanced systemic inflammatory activation state in these mice.
Thus, the question arises as to what role such counterintuitive receptor switching has in the more physiologic setting of localized host response to pathogens. Previous studies have shown that LPS activation via TLR4 induces a switch of chemokine receptor expression from inflammatory receptors on immature DCs to homeostatic receptors, such as CCR7, on mature DCs.31 The overall effect of this receptor switching is to enhance the capacity of mature DCs to present antigen by migrating to draining lymph nodes (LNs) under control of CCR7 and its ligands. Our data expand on these observations by suggesting the following: (1) induction of chemokine receptor switching is not a TLR4-specific phenomenon; (2) TLR2, and potentially other TLRs, can display similar regulatory properties; and (3) the effect of TLRs is mediated not only on tissue antigen-presenting cells (APCs), such as DCs, but on a broad range of leukocytes that invade inflamed sites. We therefore propose a model in which leukocyte encounter with TLR ligands, in an infected peripheral site, induces local inflammatory chemokine production that facilitates inflammatory chemokine receptor-dependent accumulation of leukocytes at the infected sites. The leukocytes then sample the PAMP-rich environment and undergo chemokine receptor switching.
Interestingly, although monocytes/ macrophages and neutrophils have been detected in lymph32 and draining LNs,32-34 and may be involved in antigen presentation, the mechanisms regulating their movement from peripheral tissues have not been elucidated. Our model would predict that, in the context of microbial infection, this process is mediated, in large part, by TLR control of chemokine receptor switching on these cells. Furthermore, this TLR-dependent receptor switching may contribute to the control of effector/memory T-cell entry and CCR7-dependent exit from peripheral tissues.35,36 In addition this receptor-switching is likely to be a key regulator of the clearance of inflammatory cells from inflamed sites and thus in the effective resolution of inflammatory responses.
These results inform our general understanding of the coordination of the TLR and chemokine receptor mediated responses to inflammatory insults. In addition, however, our data also suggest new TLR-ligand based therapies for chronic inflammatory diseases that act at the level of influencing cell recruitment. Highly specific chemokine receptor blockade has proven disappointing in clinical practice thus far and broader chemokine receptor regulation appears necessary to achieve efficacious effects.37 Our data suggest that appropriately dosed, and administered, TLR ligands may be useful as anti-inflammatory agents in this regard. Further clinical implications of our data relate to the “hygiene hypothesis,” which suggests that decreased exposure to pathogens in developed countries results in an alteration in the regulation of immune and allergic responses.38,39 Curiously, there is evidence that several pathogens, such as Mycobacterium tuberculosis, when systemically administered, can suppress subsequent allergic and inflammatory responses in peripheral tissues.38-42 Although there may be several contributing explanations for this, it is entirely consistent with our data that TLR triggering by these agents in the systemic context may suppress high level recruitment of inflammatory leukocytes to peripheral sites and thus protect against exaggerated inflammatory or allergic responses. In this way, our current data may help inform our understanding of the molecular mechanisms underlying the “hygiene hypothesis.”
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Dr Chris Hansell's assistance with the flow cytometry experiments is gratefully acknowledged.
This study was supported by grants from the Medical Research Council (London, United Kingdom), The European Union INNOCHEM program, Novartis Institutes for Biomedical Research (Vienna, Austria), Cancer Research UK (London, United Kingdom), and TENOVUS Scotland (Glasgow, United Kingdom).
Authorship
Contribution: C.S.M. performed the majority of the experiments reported in the paper; M.M., N.I.P., and C.B. were responsible for the in vivo cell distribution experiments; T.J. was responsible for the cutaneous inflammatory response experiments; D.X. assisted with cell isolation; A.R.F. performed much of the flow cytometric analysis in the paper; I.B.M., F.Y.L., and R.J.B. helped with the design and interpretation of experiments; and G.J.G. devised the study and was responsible, with C.S.M., for much of the data interpretation and for the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerard J. Graham, Division of Immunology, Infection and Inflammation, University of Glasgow, Level 3, Glasgow Biomedical Research Centre, 120 University Pl, Glasgow, G12 8TA, United Kingdom; e-mail: g.graham@clinmed.gla.ac.uk.


 ) in the presence or absence of the NF-κB blocker BAY-11 at both 3 and 30 μmol/L for 4 hours and CCL3 (A) and CCR2 (B) transcription determined by qPCR.
) in the presence or absence of the NF-κB blocker BAY-11 at both 3 and 30 μmol/L for 4 hours and CCL3 (A) and CCR2 (B) transcription determined by qPCR.