Abstract
Antigen-presenting cells (APCs) of host origin drive graft-versus-leukemia (GVL) effects but can also trigger life-threatening graft-versus-host disease (GVHD) after hematopoietic cell transplantation (HCT) across major histocompatibility complex (MHC) barriers. We show that in vitro priming of donor lymphocytes can circumvent the need of recipient-derived APCs in vivo for mediating robust GVL effects and significantly diminishes the risk of severe GVHD. In vitro, generated and expanded T cells (ETCs) mediate anti-leukemia effects only when primed on recipient-derived APCs. Loading of APCs in vitro with leukemia cell lysate, chimerism status of the recipient, and timing of adoptive transfer after HCT are important factors determining the outcome. Delayed transfer of ETCs resulted in strong GVL effects in leukemia-bearing full chimera (FC) and mixed chimera (MC) recipients, which were comparable with the GVL/GVHD rates observed after the transfer of naive donor lymphocyte infusion (DLI). Upon early transfer, GVL effects were more pronounced with ETCs but at the expense of significant GVHD. The degree of GVHD was most severe in MCs after transfer of ETCs that had been in vitro primed either on nonpulsed recipient-derived APCs or with donor-derived APCs.
Introduction
Hematopoietic cell transplantation (HCT) from a haploidentical donor after myeloablative conditioning therapy is a potentially curative treatment option for a variety of hematopoietic malignancies in patients lacking an human leukocyte antigen (HLA)–identical donor. To a great extent, the curative potential of an allogeneic HCT is on the consequence of donor lymphocyte alloreactivity in mediating graft-versus-leukemia (GVL) effects. The GVL effect depends on the degree of major histocompatibility complex (MHC) and minor histocompatibility antigen disparities and seems to be tightly linked with the development of graft-versus-host disease (GVHD), both in the clinical setting and in preclinical models.1-3 In the clinic, HCT from haploidentical donors require rigorous T-cell depletion of the graft,4 which largely eliminates the potential of GVL effects mediated by donor-derived T cells.
The existence of a wide therapeutic window separating GVL and GVHD has been difficult to demonstrate in the clinic. However, rodent studies have shown that donor lymphocytes infusions (DLIs) into mixed hematopoietic chimeras (MCs) can trigger GVHD reactions confined to the lymphohematopoietic system.5,6 In some instances, DLI mediates stronger GVL in mixed as compared with fully allogeneic chimeras (FCs).7 MCs induced in patients by nonmyeloablative conditioning can result in remissions of advanced and refractory lymphoid malignancies.8 Using a series of clinically relevant murine transplantation models, host antigen-presenting cells (APCs) and host alloantigen expression on tumor cells have been shown to be a prerequisite for GVL. Importantly, recipient APCs origin play a predominant role in GVL responses by donor T cells contained in the initial graft used to reconstitute the recipients.9 Similar mechanisms have been shown to contribute substantially to the GVL effects after DLI. In MCs, the magnitude of DLI-mediated GVL was dependent on the level of MHC I expression on host hematopoietic cells.7
However, more extensive transfer of these promising concepts into larger clinical trials has been hampered, in part, by the difficulty to induce stable long-term MCs in humans10 and by the observation that DLI given to patients with unintentionally induced MCs after HLA-identical HCT triggered GVHD in a significant number of recipients.11 To circumvent the potent in vivo effects of host APCs on GVHD induction by DLI, we sought to determine whether in vitro priming of donor T cells derived from MHC-disparate host APCs, which had been loaded with host-type leukemia cell–lysate, would preserve the GVL mediated elimination of host leukemia cells without augmenting GVHD-induced tissue injury. For the comparison of MCs and FCs after adoptive T-cell transfer, a myeloablative conditioning regimen was used. Lethally irradiated recipients were reconstituted with T cell–depleted (TCD) MHC-disparate allogeneic bone marrow (BM) with or without recipient-type TCD marrow to produce either full MCs or FCs, respectively. Our studies demonstrate that in vitro priming of donor T cells on host-derived dendritic cells (DCs) mediates a strong GVL effect in FCs. Notably, in vitro priming of donor T cells on recipient-derived APCs was crucial for GVL effects. Moreover, the interval between HCT and transfer positively correlated with the severity of GVHD but not with the extent of GVL effects.
Methods
Animals and HCT
Animals in the experiments were used under protocols approved by the State Government of Niedersachsen, Germany. Six- to 12-week-old female C57Bl/6 (B6, H2b) mice were purchased from Charles River (Frankfurt, Germany). Donor B10.A (H2a: Kk-Ak-Ek-Dd) mice were obtained from Taconic Laboratories (Hudson, NY). The animals were housed in sterilized, individually ventilated cages at the Medizinische Hochschule Hannover animal facility and received sterilized food and water ad libidum. All manipulations were performed under aseptic conditions in a laminar flow hood.
Fully MHC-mismatched MCs and FCs were established using B10.A (H2a) → B6 (H2b) strains as previously described.7 Recipient B6 received total body irradiation of 10.5 Gy from a linear accelerator source. After 4 hours, BM was reconstituted using a mixture of TCD 15 × 106 B10.A BM cells and 3 × 106 B6 BM for MCs and TCD 15 × 106 B10.A BM cells only for FCs. TCD was performed in vitro by complement lysis of CD4 and CD8 T cells in the BM cells using monoclonal antibodies (GK1.5 and 2.43, respectively) and rabbit complement (Cedarlane, Burlington, NC).12 Hematopoietic reconstitution and chimerism analysis in the transplanted animals were monitored by flow cytometric analysis of peripheral blood leukocytes.7
Cell lines
C1498 is a B6-derived myeloid leukemia cell line expressing H2b. It was originally acquired from ATCC (Manassas, VA) and cultured short-term in serum free medium (AIMV; Gibco, Eggenstein, Germany). To induce leukemia, a 100% lethal dose (6 × 105) of C1498 cells was injected in the recipients by lateral tail vein injection.
In vitro generation of leukemia-reactive T cells
Ex vivo priming and expansion of donor T cells was performed as described previously.13 Recipient or donor-derived DCs were generated in vitro by differentiating B6 (R-DCs) or B10.A (D-DCs) BM in the presence of interleukin-4 (IL-4) and granulocyte macrophage colony-stimulating factor (GM-CSF; Sigma, Munich, Germany). The DCs were pulsed with C1498-lysate and then maturated using CpG oligodeoxynucleotides (ODN). The ODN sequence was TCG TCG TTT TTC GGT CGT TTT (CpG 10 103, 2 μg/mL; Coley Pharmaceuticals, Munich, Germany). Single cell suspensions of donor (B10.A) splenocytes were used as the source of T cells and cocultured with the maturated DCs in RPMI 1640 complete media (PAA, Pasching, Austria). After 5 days of priming, T cells were harvested and expanded using anti-CD3/anti-CD28 (BD Biosciences, Heidelberg, Germany) coated microspheres (Invitrogen, Karlsruhe, Germany) in the presence of recombinant interleukin-2 (IL-2; 20 U/mL; Amgen, Munich, Germany) and interleukin-7 (IL-7; 4 ng/mL; R&D Systems, Minneapolis, MN). Two days later, the beads were removed and the T cells expanded for 4 more days using the respective cytokines only.
In adoptive transfer (AT) experiments, the donor-derived (B10.A) ETCs or naive DLI were adoptively infused into the respective cohorts of leukemia-bearing recipients. Single cell suspensions of 40 × 106 cells per recipient were given by lateral tail vein injection 1 day after C1498 inoculation. Necropsies of the dead animals were performed to determine whether the deaths were leukemia-related or caused by GVHD. In tracking experiments, fates of transferred T cells were determined. A standard carboxyfluorescein succinimidyl ester (CFSE) staining method was used as previously published.14
Flow cytometry
The following antibodies were used: H2Dd-fluorescein isothiocyanate (FITC), CD8α-(FITC/phycoerythrin (PE)/biotin), CD4-PE, CD25-PE, CD69-PE, CD44-PE, CD62L-PE, CD45-APC, B220-PE, NK1.1-PE, CD11b-PE, GK1.1-PE, CD127-FITC, Granzyme B-PE, CD95L-APC, CXCR3-PE, α4β7-PE, and their respective isotype controls (BD Biosciences). In certain experiments, annexin V–FITC and propidium iodide (PI) were used to study cells undergoing apoptosis. Flow cytometry was performed using a BD FACSCalibur (Becton Dickinson, Heidelberg, Germany). FlowJo software (TreeStar, San Diego, CA) was used for the analysis.
Assessment of GVHD
Clinically, GVHD was assessed in a blinded fashion by a specially trained veterinary technician. We used a clinical scoring system that was described previously.15 The animals were graded based on weight loss, skin integrity, posture, fur texture, and activity. In addition, GVHD target organs (liver, lung, skin, intestines, and stomach) were harvested from surviving animals on day 100 post-AT and fixed in 10% formalin. The hemotoxylin and eosin (H&E)–stained paraffin sections of the fixed specimens were reviewed and scored, blinded to experimental groups by a pathologist (F.L.).16
In vitro cytotoxicity
Cytotoxicity assays on target C1498 cells were performed using the JAM (just another method) assay.17 [3H]thymidine (Amersham, Woburn, MA) labeled C1498 cells were co-incubated with effector ETCs or naive splenocytes in eight 2-fold dilutions of effector-target ratios starting at 100:1. After 4 hours of co-incubation, the cells were harvested and the residual cellular radioactivity determined. Percent specific killing of target C1498 cells was calculated using the following formula: % specific killing = (S − E/S) × 100 where E is the radioactivity in the presence of effector (in cpm) and S the radioactivity in the absence of effectors (spontaneous). Data are presented as the mean percent specific killing of triplicate samples (± SE) from representative experiments.
Mixed leukocyte reaction and T-cell receptor spectratype analysis
Mixed leukocyte reaction (MLR) standard techniques as described by others18 were adjusted to the specific biologic needs of prestimulated CD8+ T cells: triplicate wells containing serially diluted (4 × 105, 2 × 105, 1 × 105, 0.5 × 105) responder cells (ETCs) were cocultured for 2 days with 105 stimulators cells (host splenocytes irradiated with 3000 cGy) in the presence of IL-2 (2 U/mL). Each well was pulsed with 1 μCi of [3H]thymidine 16 hours before harvesting with an automated harvester (Perkin Elmer, Jügesheim, Germany). Incorporation of [3H]thymidine was measured by a beta plate counter. The averages of the differences in counts per minute (cpm) were determined by comparing proliferation of responders with the proliferation of stimulators alone according to the following formula: Δ cpm = average of (cpm sample − cpm stimulators). For T-cell receptor (TCR) spectratype analysis, total mRNA was transcribed into cDNA with Superscript III reverse transcriptase and oligo-dT12-18 primers (Invitrogen) cDNA was amplified by polymerase chain reaction (PCR) for 30 cycles with 21 Vβ-specific primers (Vβ1, Vβ2, Vβ3, Vβ4, Vβ5.1, Vβ5.2, Vβ6, Vβ7, Vβ8.1, Vβ8.2, Vβ8.3, Vβ9, Vβ10, Vβ11, Vβ12, Vβ13, Vβ14, Vβ15, Vβ16, Vβ17, Vβ18) and 1 Cβ1-Cβ2 primer as described previously.19 PCR products were FAM (5′-fluorescein phosphoramidite)–labeled by nested primer extension and analyzed by capillary electrophoresis as described previously (ABI 3110 DNA sequencer; Applied Biosystems, Foster City, CA).20 Analysis were performed using GeneMapper 3.7 software (Applied Biosystems).
Statistical analysis
The Kaplan-Meier product-limit method was used to calculate survival rates. Differences between the groups were determined using log-rank statistics. In other cases, statistical analyses were made by Student t test (2-tailed). P values less than or equal to .05 were considered to be significant.
Results
Generation of leukemia-reactive T cells in vitro
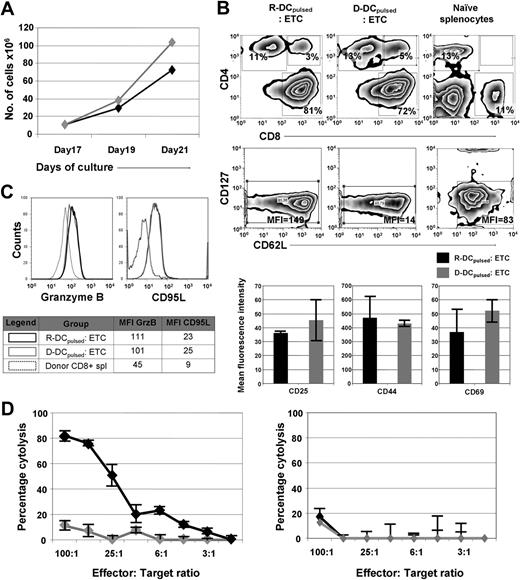
Previously, we have shown that naive T cells primed on leukemia-lysate pulsed DCs can mediate a potent GVL effect when given to syngeneic HCT recipients at the time of transplantation.13 Exposure to leukemia cell lysates pulsed DCs preferentially expanded those T cells that were reactive to a leukemia-associated antigen.21 To determine whether this approach would be efficacious in a MHC-disparate allogeneic HCT setting, donor (D)– and recipient (R)–derived DCs were generated by differentiating either B10.A- or B6-BM cells in the presence of IL-4 and GM-CSF. After 8 days of culture, DCs were pulsed with the lysate of the B6-derived leukemia cell line C1498 (henceforth referred to as DCspulsed). On day 9, the cells were maturated using a TLR9 agonist, CpG ODN. On the tenth day, splenocytes were harvested from B10.A mice and used as a source of donor T cells. For T-cell priming, splenocytes were cocultured with the DC for 5 days (culture days 10-15) and then expanded for 2 days in the presence of anti-CD3/anti-CD28 coated microspheres, IL-7 (4 ng/mL) and IL-2 (20 U/mL; days 15-17). Subsequently, the beads were removed and the cells cultured for 4 additional days in the presence of the IL-7 and IL-2 (days 17-21) during which time T cells expanded 7- to 10-fold regardless of whether R-DCspulsed or R-DCsnonpulsed were used (Figure 1A). T cells primed with R-DCspulsed or D-DCspulsed were composed of a cell population predominated by CD8+ T cells (∼ 85%) with an effector cell phenotype (low CD127 expression; Figure 1A).22 The ETCs of R-DCspulsed or D-DCspulsed had an early effector phenotype,22 consisting of the expression of CD44 (∼ 80%) and CD62L (∼ 75%) on a high frequency of T cells (Figure 1B), with a low density of CD62L (∼ 50%; data not shown). The expression of activation markers such as CD25, CD44, and CD69 also was similar. Less than 0.1% of the ETCs expressed NK1.1 (data not shown). The intracellular content of Granzyme B and CD95L expression were similarly up-regulated, suggesting comparable cytotoxic capacity after priming on either D-DCspulsed or R-DCspulsed (Figure 1C). The in vitro cytotoxicity of the ETCs against C1498 cells indicated that a strong anti-C1498 effect was observed when T cells were primed with R-DCspulsed (Figure 1D left panel). In contrast, the ETCs primed with the D-DCspulsed failed to mediate significant lysis of C1498 (P = .02). Blocking of H2-Kb and H2-Db on target cells using monoclonal antibodies (Figure 1D right panel) prevented the lytic activity of ETCs: R-DCspulsed, indicating that the ETC-mediated cytotoxicity was based on MHC recognition.
In vitro priming and expansion of donor-derived T cells result in ETCs reactive against C1498 only when they were primed with recipient-derived DCspulsed. Donor-derived ETCs primed with R- or D-DCspulsed were further characterized in vitro. (A) Expansion rates of ETCs either primed on R- DCspulsed (◆) or D-DCspulsed ( ) were determined using the trypan blue exclusion method for viability assessment. Expansion rates of up to 10-fold were obtained within 4 days. Values are shown plus or minus SE (n = 3). Similar expansion rates were achieved after priming with R-or D-DCspulsed. (B) The phenotype of ETCs and naive donor splenocytes were characterized by flow cytometric analysis. In the uppermost panels, events were gated on live cells by forward-side scatter exclusion and at least 15 000 live events were acquired. In the bottom panels, data from events gated on live CD8+ cells stained with the indicated antibodies are depicted. (C) The ETCs obtained after priming with R-DCspulsed (black continuous line) or D-DCspulsed (gray continuous line) and donor splenocytes (black dotted line) were stained to study intracellular Granzyme B and surface CD95L expression. Data from events gated on CD8+ live cells of the indicated populations are depicted. (D) Left panel: ETCs primed on R-DCspulsed (◆) demonstrated robust anti-C1498 responses in vitro, whereas D-DCspulsed (
) were determined using the trypan blue exclusion method for viability assessment. Expansion rates of up to 10-fold were obtained within 4 days. Values are shown plus or minus SE (n = 3). Similar expansion rates were achieved after priming with R-or D-DCspulsed. (B) The phenotype of ETCs and naive donor splenocytes were characterized by flow cytometric analysis. In the uppermost panels, events were gated on live cells by forward-side scatter exclusion and at least 15 000 live events were acquired. In the bottom panels, data from events gated on live CD8+ cells stained with the indicated antibodies are depicted. (C) The ETCs obtained after priming with R-DCspulsed (black continuous line) or D-DCspulsed (gray continuous line) and donor splenocytes (black dotted line) were stained to study intracellular Granzyme B and surface CD95L expression. Data from events gated on CD8+ live cells of the indicated populations are depicted. (D) Left panel: ETCs primed on R-DCspulsed (◆) demonstrated robust anti-C1498 responses in vitro, whereas D-DCspulsed ( ) failed to mediate significant cytotoxicity (P < .05). Right panel: Cytotoxicity assay performed after blocking target MHC-I (H2Db, H2Kb) led to a complete loss of the response indicating a cytotoxic activity mediated by CD8+ T cells. Values are shown plus or minus SE.
) failed to mediate significant cytotoxicity (P < .05). Right panel: Cytotoxicity assay performed after blocking target MHC-I (H2Db, H2Kb) led to a complete loss of the response indicating a cytotoxic activity mediated by CD8+ T cells. Values are shown plus or minus SE.
In vitro priming and expansion of donor-derived T cells result in ETCs reactive against C1498 only when they were primed with recipient-derived DCspulsed. Donor-derived ETCs primed with R- or D-DCspulsed were further characterized in vitro. (A) Expansion rates of ETCs either primed on R- DCspulsed (◆) or D-DCspulsed ( ) were determined using the trypan blue exclusion method for viability assessment. Expansion rates of up to 10-fold were obtained within 4 days. Values are shown plus or minus SE (n = 3). Similar expansion rates were achieved after priming with R-or D-DCspulsed. (B) The phenotype of ETCs and naive donor splenocytes were characterized by flow cytometric analysis. In the uppermost panels, events were gated on live cells by forward-side scatter exclusion and at least 15 000 live events were acquired. In the bottom panels, data from events gated on live CD8+ cells stained with the indicated antibodies are depicted. (C) The ETCs obtained after priming with R-DCspulsed (black continuous line) or D-DCspulsed (gray continuous line) and donor splenocytes (black dotted line) were stained to study intracellular Granzyme B and surface CD95L expression. Data from events gated on CD8+ live cells of the indicated populations are depicted. (D) Left panel: ETCs primed on R-DCspulsed (◆) demonstrated robust anti-C1498 responses in vitro, whereas D-DCspulsed (
) were determined using the trypan blue exclusion method for viability assessment. Expansion rates of up to 10-fold were obtained within 4 days. Values are shown plus or minus SE (n = 3). Similar expansion rates were achieved after priming with R-or D-DCspulsed. (B) The phenotype of ETCs and naive donor splenocytes were characterized by flow cytometric analysis. In the uppermost panels, events were gated on live cells by forward-side scatter exclusion and at least 15 000 live events were acquired. In the bottom panels, data from events gated on live CD8+ cells stained with the indicated antibodies are depicted. (C) The ETCs obtained after priming with R-DCspulsed (black continuous line) or D-DCspulsed (gray continuous line) and donor splenocytes (black dotted line) were stained to study intracellular Granzyme B and surface CD95L expression. Data from events gated on CD8+ live cells of the indicated populations are depicted. (D) Left panel: ETCs primed on R-DCspulsed (◆) demonstrated robust anti-C1498 responses in vitro, whereas D-DCspulsed ( ) failed to mediate significant cytotoxicity (P < .05). Right panel: Cytotoxicity assay performed after blocking target MHC-I (H2Db, H2Kb) led to a complete loss of the response indicating a cytotoxic activity mediated by CD8+ T cells. Values are shown plus or minus SE.
) failed to mediate significant cytotoxicity (P < .05). Right panel: Cytotoxicity assay performed after blocking target MHC-I (H2Db, H2Kb) led to a complete loss of the response indicating a cytotoxic activity mediated by CD8+ T cells. Values are shown plus or minus SE.
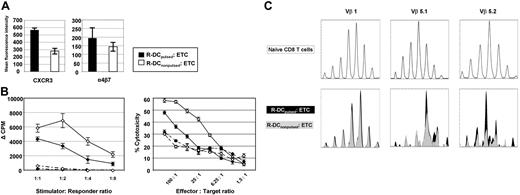
Consistent with the phenotyping data in Figure 1B, the expression of homing markers such as the effector cell-associated cytokine receptor CXCR3 and the integrin α4β5 was when T cells were primed on R-DCpulsed as compared with R-DCnonpulsed (Figure 2A). Because the priming of the naive T cells from a MHC-disparate donor on recipient-derived DCs would expose these T cells to alloantigens, donor anti–host T-cell alloresponses of ETCs were quantified in MLR consisting of irradiated host-type B6 or donor-type B10.A splenocytes (Figure 2B left panel). Because the population was mainly CD8+, alloreactivity was additionally measured in cytotoxicity assays against host targets (Figure 2B right panel). Intriguingly, the alloreactivity of the ETCs:R-DCspulsed versus ETCs:R-DCsnonpulsed was significantly lower. To determine whether the T-cell repertoire had shifted as a result of priming ETCs on pulsed or nonpulsed R-DCs, TCR spectratype analysis was performed using cDNA amplified for 21 different Vβ chains. Within Vβ 5.1 and Vβ 5.2 families, a skewed repertoire was observed in ETCs:R-DCspulsed as compared with the naive CD8 population and clonal differences were found after priming T cells on pulsed versus nonpulsed R-DCs (Figure 2C).
Pulsing the recipient-derived DCs with tumor lysates before priming affects the response to stimulation and the phenotype of the ETCs. Donor-derived ETCs primed with R-DCspulsed (solid) or R-DCsnonpulsed (hollow) were characterized in vitro. (A) The ETCs were stained to study expression of CXCR3 and α4β7. Mean fluorescent intensity of the expression on CD8+ live cells is shown. Values are shown plus or minus SE. (B) ETCs either primed on R-DCspulsed (◆) or R-DCsnonpulsed (◇) were tested for their alloreactivity either in a MLR (left panel) at decreasing stimulator/responder ratios or in a cytotoxicity assay (right panel). Either irradiated allogeneic (B6 solid lines) or syngeneic splenocytes (B10.A broken lines) were used as stimulators. For the cytotoxicity assay ConA blasts of the respective strain were used as targets. Values are shown plus or minus SE, n = 3, P < .05. (C) Differences in the TCR Vβ repertoire of CD8 T cells primed on pulsed or nonpulsed DCs. CDR3 length profile of certain Vβ populations was determined by TCR spectratype analysis. cDNA of naive (top panels), R-DCspulsed, or R-DCsnonpulsed ETCs (bottom panels) were amplified by PCR with 21 Vβ-specific primers (Vβ1, Vβ2, Vβ3, Vβ4, Vβ5.1, Vβ5.2, Vβ6, Vβ7, Vβ8.1, Vβ8.2, Vβ8.3, Vβ9, Vβ10, Vβ11, Vβ12, Vβ13, Vβ14, Vβ15, Vβ16, Vβ17, Vβ18) and 1 Cβ1-Cβ2 primer. FAM-labeled PCR products were analyzed by capillary electrophoresis. The Vβ1 is shown exemplarily for a nonaffected CDR3-profile (left panels), the Vβ5.1 and Vβ5.2 for skewed CDR3-profiles (middle and right panels). Representative results of 3 independent experiments are shown.
Pulsing the recipient-derived DCs with tumor lysates before priming affects the response to stimulation and the phenotype of the ETCs. Donor-derived ETCs primed with R-DCspulsed (solid) or R-DCsnonpulsed (hollow) were characterized in vitro. (A) The ETCs were stained to study expression of CXCR3 and α4β7. Mean fluorescent intensity of the expression on CD8+ live cells is shown. Values are shown plus or minus SE. (B) ETCs either primed on R-DCspulsed (◆) or R-DCsnonpulsed (◇) were tested for their alloreactivity either in a MLR (left panel) at decreasing stimulator/responder ratios or in a cytotoxicity assay (right panel). Either irradiated allogeneic (B6 solid lines) or syngeneic splenocytes (B10.A broken lines) were used as stimulators. For the cytotoxicity assay ConA blasts of the respective strain were used as targets. Values are shown plus or minus SE, n = 3, P < .05. (C) Differences in the TCR Vβ repertoire of CD8 T cells primed on pulsed or nonpulsed DCs. CDR3 length profile of certain Vβ populations was determined by TCR spectratype analysis. cDNA of naive (top panels), R-DCspulsed, or R-DCsnonpulsed ETCs (bottom panels) were amplified by PCR with 21 Vβ-specific primers (Vβ1, Vβ2, Vβ3, Vβ4, Vβ5.1, Vβ5.2, Vβ6, Vβ7, Vβ8.1, Vβ8.2, Vβ8.3, Vβ9, Vβ10, Vβ11, Vβ12, Vβ13, Vβ14, Vβ15, Vβ16, Vβ17, Vβ18) and 1 Cβ1-Cβ2 primer. FAM-labeled PCR products were analyzed by capillary electrophoresis. The Vβ1 is shown exemplarily for a nonaffected CDR3-profile (left panels), the Vβ5.1 and Vβ5.2 for skewed CDR3-profiles (middle and right panels). Representative results of 3 independent experiments are shown.
ETCs generated by priming on recipient-derived DCs mediate strong GVL effects after delayed AT
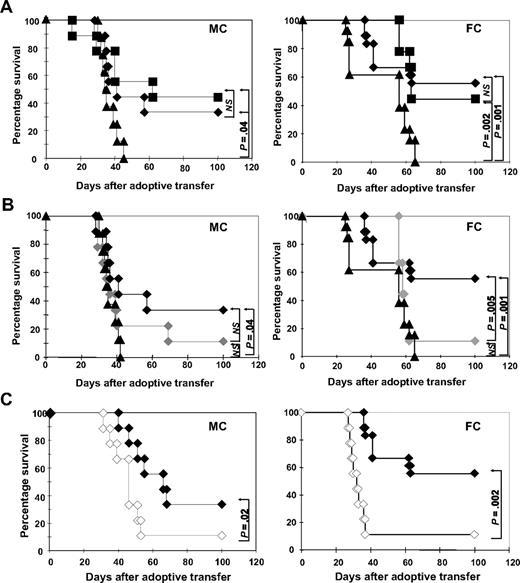
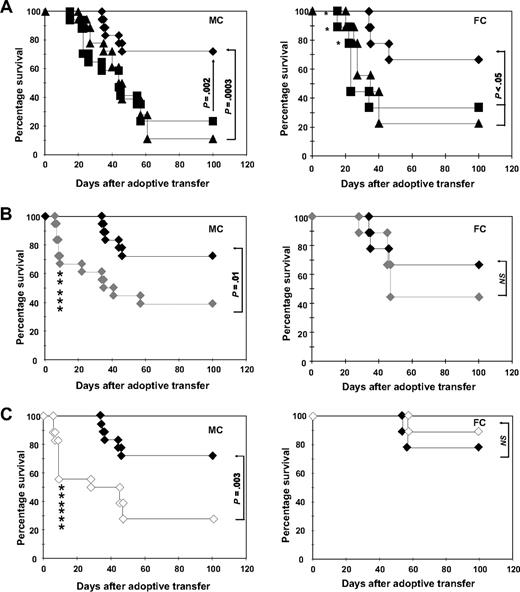
Delayed administration of DLI in hematopoietic chimeras can lead to significant GVL effects in the absence of GVHD.23 To assess the ability of ETCs to mediate GVL effects in vivo, we used a transplantation model (B10.A [H2a] [] B6 [H2b]) developed by others to elegantly examine the role of DLI in MHC-disparate MCs and FCs after HCT.7 Eight weeks after HCT, MCs and FCs were challenged with a lethal dose of leukemia cells (0.6 × 106 C1498 cells). On the following day, 40 × 106 ETCs:R-DCspulsed, ETCs:D-DCspulsed, or naive DLIs were transferred into cohorts of leukemia-bearing FCs or MCs. Recipients were monitored for survival (Figure 3). Untreated MCs and FCs (Figure 3A) controls succumbed to leukemia within 30 to 60 days of leukemia challenge. In a series of experiments, long-term (100 days postchallenge) survival rates of FCs treated with ETCs:R-DCspulsed ranged from 55% to 60%, whereas survival rates ranged from 32% to 35% in MCs (P < .05). Naive DLI rescued 50% of MCs and 40% of FCs, which was not significantly different from with the use of ETCs:R-DCspulsed.
ETCs primed with B6-DCsC1498 in vitro mediate strong GVL effects in leukemia-bearing MC and FC recipients after delayed transfer. Hematopoietic chimeras were established with a mixture of 15 × 106 TCD B10.A BM plus 5 × 106 TCD B6 BM for MCs or with 15 × 106 TCD B10.A BM alone for FCs. The recipients were injected with 0.6 × 106 C1498 cells intravenously on day 55. On day 56, the recipients were treated with 40 × 106 effector cells. Survival was monitored in MC and FC recipients for 100 days after treatment. As effectors either (A) ETCs:R-DCspulsed (-♦-, n = 9-MC, 18-FC), (B) ETCs:D-DCspulsed (-♦-, n = 9), or (C) ETCs:R-DCsnonpulsed (-◇-, n = 9) were given. Naive DLI (-■-) and PBS (-▴-) were given as controls. P greater than or equal to .05 was considered not significant (NS).
ETCs primed with B6-DCsC1498 in vitro mediate strong GVL effects in leukemia-bearing MC and FC recipients after delayed transfer. Hematopoietic chimeras were established with a mixture of 15 × 106 TCD B10.A BM plus 5 × 106 TCD B6 BM for MCs or with 15 × 106 TCD B10.A BM alone for FCs. The recipients were injected with 0.6 × 106 C1498 cells intravenously on day 55. On day 56, the recipients were treated with 40 × 106 effector cells. Survival was monitored in MC and FC recipients for 100 days after treatment. As effectors either (A) ETCs:R-DCspulsed (-♦-, n = 9-MC, 18-FC), (B) ETCs:D-DCspulsed (-♦-, n = 9), or (C) ETCs:R-DCsnonpulsed (-◇-, n = 9) were given. Naive DLI (-■-) and PBS (-▴-) were given as controls. P greater than or equal to .05 was considered not significant (NS).
Transfer of ETCs primed with D-DCspulsed or R-DCsnonpulsed failed to mediate any GVL effects (Figure 3B). These results correlated with the in vitro cytotoxicity data. Thus, only ETCs:R-DCspulsed mediated GVL effects in vivo. Remarkably, ETCs:R-DCsnonpulsed also failed to mediate significant GVL effects in spite of host-derived APCs being present in MCs, further supporting the importance of DC presentation of leukemia-associated antigens in the GVL effect (Figure 3C).
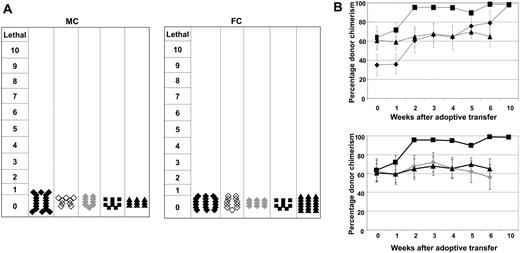
Despite the substantial GVL effects, none of the mice had clinical evidence of GVHD symptomotology, regardless as to which DC population was used for in vitro priming of ETCs (Figure 4A). These findings were confirmed on histologic sections performed on all GVHD target organs of surviving mice (data not shown). Although GVHD was not observed, MC recipients treated with ETCs:R-DCspulsed shifted hematopoietic chimerism toward full donor-derived hematopoiesis within 6 to 8 weeks after AT, indicative of in vivo donor anti-host alloreactivity with a predilection for the elimination of both nonmalignant host hematopoietic cells and host leukemia cells (Figure 4B).
Transfer of ETCs that had been primed on recipient-derived DCs mediated a shift from mixed to full hematopoietic chimerism. No GVHD was observed. (A) GVHD was monitored on a clinical 10-point scale weekly for each animal. Each symbol represents the severest degree of GVHD that was observed in a single animal during the observation period. Groups were either treated with ETCs:R-DCspulsed (-♦-, n = 18-MC, 18-FC), ETCs:D-DCspulsed (-♦-,n = 9-MC and FC), ETCs:R-DCsnonpulsed (-◇-,n = 9-MC and FC), naive DLI (-■-, n = 9 MC and FC), or PBS (-▴-, n = 9-MC, 18-FC). (B) MC were injected with 0.6 × 106 C1498 cells intravenously on day 55 and 40 × 106 effector cells on day 56. Chimerism was calculated by determining the percentage of CD45+H2Dd+ cells among the CD45+ cells. The values shown represent the mean chimerism of all mice per treatment group plus or minus SE.
Transfer of ETCs that had been primed on recipient-derived DCs mediated a shift from mixed to full hematopoietic chimerism. No GVHD was observed. (A) GVHD was monitored on a clinical 10-point scale weekly for each animal. Each symbol represents the severest degree of GVHD that was observed in a single animal during the observation period. Groups were either treated with ETCs:R-DCspulsed (-♦-, n = 18-MC, 18-FC), ETCs:D-DCspulsed (-♦-,n = 9-MC and FC), ETCs:R-DCsnonpulsed (-◇-,n = 9-MC and FC), naive DLI (-■-, n = 9 MC and FC), or PBS (-▴-, n = 9-MC, 18-FC). (B) MC were injected with 0.6 × 106 C1498 cells intravenously on day 55 and 40 × 106 effector cells on day 56. Chimerism was calculated by determining the percentage of CD45+H2Dd+ cells among the CD45+ cells. The values shown represent the mean chimerism of all mice per treatment group plus or minus SE.
ETCs trigger severe GVHD in MC recipients when transferred early after HCT
Our results indicate that the requirement for persisting recipient-derived APCs to support a potent GVL effect after HCT can be circumvented, in part, by in vitro priming of donor-derived T cells before AT. To reduce the likelihood of leukemia recurrence, AT at the time of minimal residual disease early after HCT would be more desirable than later times. Early after HCT, severe GVHD is observed with naive donor lymphocytes,23 which is dependent upon inflammatory checkpoints that regulate the recruitment of GVHD-reactive T cells into peripheral tissues.24 To determine whether preparative regimen-induced inflammation would influence GVL versus GVHD effects when ETCs were transferred before full resolution of inflammation, C1498 cells were given to MC and FC recipients early after HCT (21 days). At this time point, posttransplantation complete hematopoietic recovery is seen along with residual inflammation. The overall survival is shown in Figure 5, and the degree of GVHD observed in Figure 6. Whereas a cell dose of 0.6 × 106 C1498 cells caused 100% lethality when given 55 days after HCT (Figure 3A), 10% of MCs and 20% of FCs survived when this dose was administered on day 21 after HCT. Thus, the inflammatory environment per se appeared to reduce the observed 100% lethality when C1498 was given later after HCT in the absence of inflammation (Figure 5A).
Early adoptive transfer of ETCs is associated with improved leukemia-free survival at the expense of severe GVHD. Mixed (MC) and full chimeras (FC) were challenged with 0.6 × 106 C1498 cells intravenously on day 20. On day 21, the recipients were treated with 40 × 106 effector cells. As effectors either (A) ETCs:R-DCspulsed (-♦-, n = 9), (B) ETCs:D-DCspulsed (-♦-, n = 9), or (C) ETCs:R-DCsnonpulsed (-◇-, n = 9) were adoptively transferred by tail vein injection. Naive DLI (-■-, n = 8) and PBS (-▴-, n = 9) were used as controls. Survival was monitored in MC and FC recipients for 100 days after treatment. Deaths due to GVHD are indicated by an asterisk. Pooled data from 2 independent experiments are shown. P values are added to the figure. P less than .05 was considered to be significant.
Early adoptive transfer of ETCs is associated with improved leukemia-free survival at the expense of severe GVHD. Mixed (MC) and full chimeras (FC) were challenged with 0.6 × 106 C1498 cells intravenously on day 20. On day 21, the recipients were treated with 40 × 106 effector cells. As effectors either (A) ETCs:R-DCspulsed (-♦-, n = 9), (B) ETCs:D-DCspulsed (-♦-, n = 9), or (C) ETCs:R-DCsnonpulsed (-◇-, n = 9) were adoptively transferred by tail vein injection. Naive DLI (-■-, n = 8) and PBS (-▴-, n = 9) were used as controls. Survival was monitored in MC and FC recipients for 100 days after treatment. Deaths due to GVHD are indicated by an asterisk. Pooled data from 2 independent experiments are shown. P values are added to the figure. P less than .05 was considered to be significant.
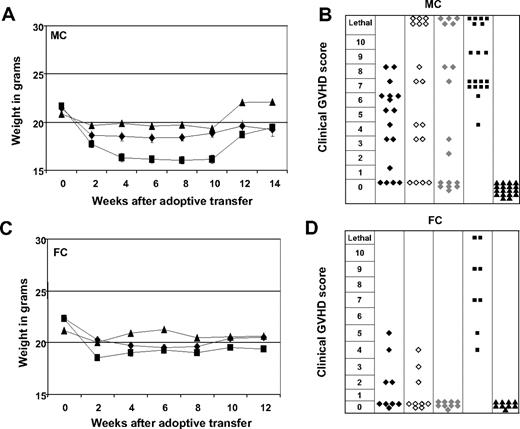
Early adoptive transfer of ETCs triggers less severe GVHD in FCs than in MCs. ETCs:R-DCspulsed (-♦- n = 18-MC, n = 9-FC), ETCs:R-DCsnonpulsed (-◇-n = 18-MC, n = 9-FC), ETCs:D-DCspulsed (-♦-, n = 18-MC, n = 9-FC), naive DLI (-■-, n = 18-MC, n = 9-FC), or PBS (-▴-, n = 18-MC, n = 9-FC) were given to either MCs (A,B) or FCs (C,D). Weight changes show more weight loss in MC animals that in FC animals. Mean weights plus or minus SE from each cohort are presented. GVHD was monitored weekly for each animal on a clinical 10-point scale. Each symbol represents the severest degree of GVHD that was observed in a single animal during the post transplant course. Mice treated with ETCs:R-DCspulsed developed less severe GVHD scores compared with all other treatment groups.
Early adoptive transfer of ETCs triggers less severe GVHD in FCs than in MCs. ETCs:R-DCspulsed (-♦- n = 18-MC, n = 9-FC), ETCs:R-DCsnonpulsed (-◇-n = 18-MC, n = 9-FC), ETCs:D-DCspulsed (-♦-, n = 18-MC, n = 9-FC), naive DLI (-■-, n = 18-MC, n = 9-FC), or PBS (-▴-, n = 18-MC, n = 9-FC) were given to either MCs (A,B) or FCs (C,D). Weight changes show more weight loss in MC animals that in FC animals. Mean weights plus or minus SE from each cohort are presented. GVHD was monitored weekly for each animal on a clinical 10-point scale. Each symbol represents the severest degree of GVHD that was observed in a single animal during the post transplant course. Mice treated with ETCs:R-DCspulsed developed less severe GVHD scores compared with all other treatment groups.
When naive DLIs were transferred into leukemia-bearing MCs, only 30% survived and lethal GVHD developed in one-third (Figures 5A, 6B) as proven by necropsy. In striking contrast, a significantly higher proportion of leukemia-bearing MCs survived when ETCs:R-DCspulsed rather than DLIs were infused, resulting in 72% long-term survival (Figure 5A left panel). Necropsy indicated that all deaths could be attributed to leukemia. When ETCs:D-DCspulsed versus ETCs:R-DCspulsed were administered to MCs, a significantly lower proportion survived resulting in only 40% leukemia-free survival (Figure 5B). Remarkably, 27% of these MC recipients died of hyperacute GVHD (Figures 5B, 6B). Transfer of ETCs:R-DCsnonpulsed rescued only 30% of leukemia-bearing MCs (Figure 5C). Again, severe lethal GVHD occurred very early after AT in 33% of the MC recipients (Figures 5C, 6B). Although GVHD was seen in all cohorts of MC mice irrespectively of the T-cell preparation infused (Figure 6A,B), the degree and the acuity of the GVHD observed were different. While the ETCs:R-DCspulsed mediated significant GVHD, the severity was less than seen after either ETCs:D-DCspulsed or ETCs:R-DCsnonpulsed infusion, both of which triggered very early onset acute GVHD with high mortality rates in MC recipients. Thus, ETCs:R-DCspulsed resulted in the highest survival of all T-cell preparations infused and caused the least severe acute GVHD.
In leukemia-bearing FC recipients, strong GVL effects were observed after ETCs:R-DCspulsed transfer early after HCT (Figure 5A right panel) with long-term survival rates of 65 to 75%. Naive DLI resulted in a significantly lower overall survival of 30% in FCs and accounted for more than 20% of deaths (Figure 5A). As compared with recipients of ETCs:R-DCspulsed, FC recipients of ETCs:D-DCspulsed had a modestly but not statistically significant lower survival rate of 45% survival. In consecutive experiments, no differences were seen in FC recipients of ETCs:R-DCspulsed versus ETCs:R-DCsnonpulsed (Figure 5B,C). The GVHD elicited in FCs was generally less severe than that elicited in the MCs (Figure 6C,D). Whereas FC recipients of naive DLIs developed severe GVHD, those treated with ETCs:R-DCspulsed or ETCs:R-DCsnonpulsed developed less severe GVHD and those given ETCs:D-DCspulsed did not develop any GVHD. We conclude that ETCs:R-DCspulsed given to FCs or MCs result in a higher leukemia-free survival rate with less severe GVHD as compared with DLIs and comparable or modestly superior survival as seen with all other ETC preparations.
Fewer in vitro primed T cells in lymphoid organs after AT correlates with decreased GVL effects in MCs
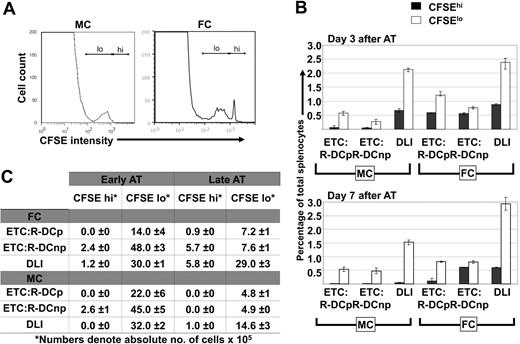
To study the in vivo fate of in vitro generated ETCs or naive DLIs administered at either 3 or 7 days after AT, donor T-cell preparations were labeled with 5 μM CFSE and transferred into MC or FC recipients. Either ETCs:R-DCspulsed, ETCs:R-DCsnonpulsed, or naive DLIs were adoptively transferred into C1498-bearing MC and FC recipients late after HCT (day 56). Single cell suspensions of live splenocytes or lymph node cells were analyzed by flow cytometric analysis for CFSE-derived signals (Figure 7). Both ETCs and DLIs proliferated to a nearly identical extent in MCs and FCs on day 3 after AT. By day 7 after AT, only a very small population of ETCs remained identifiable in the secondary lymphoid organs of MCs (Figure 7A). The transferred ETCs had undergone 3 to 4 cell divisions. compared with DLIs, fewer CFSE-labeled ETCs could be identified after AT (Figure 7B,C) that may be reflective of an impaired homing capacity, lower proliferation, or shorter survival of ETCs versus DLIs. In FCs, more ETCs were observed. Both ETCs:R-DCspulsed and naive DLIs proliferated rapidly while the ETCs:R-DCsnonpulsed underwent fewer cell divisions (Figure 7B,C). The latter was different from MC recipients that had comparable in vivo proliferation of ETCs:DCsnonpulsed and ETCs:R-DCspulsed. Chimerism was completely donor both in the blood and lymphoid organs. The higher numbers of ETCs in FCs as compared with mixed chimeras and the use of ETCs:R-DCspulsed cells correlates with the slightly increased leukemia-free survival seen in FC recipients of ETCs:R-DCspulsed.
ETCs persist longer in secondary lymphoid organs of FCs as compared with MCs. Hematopoietic chimeras received late adoptive transfer (AT) with 40 × 106 ETCs:R-DCspulsed (ETCs:R-DCp), ETCs:R-DCsnonpulsed (ETC:R-DCnp), or naive DLI. The effectors were labeled with 5 μM CFSE. Spleens of the transplanted animals were harvested 3 or 7 days later, single cell suspensions were made and analyzed for CFSE intensity. (A) Representative day 7 results from flow cytometric analysis of harvested splenocytes from MCs and FCs are shown. A CFSE high peak (nondivided ETCs) was detected in FCs only (the marker labels the CFSE high/low region). (B) Adoptively transferred ETCs clear faster from MCs than from FCs. Spleens from MCs and FCs were harvested on days 3 and 7 after delayed adoptive transfer. Data obtained from animals that received either ETCs:R-DCsp or ETC:R-DCsnp are presented separately. A DLI group was added as a control. The fraction of CFSEhi cells (indicating nondivided ETCs) and CFSElo cells (indicating proliferated ETCs) was determined by flow cytomertic analysis. (C) Both, in MCs and FCs more vigorous proliferation of ETCs is seen when adoptive transfer is performed early after transplantation (day 21). Spleens from MCs and FCs were harvested on day 7 after early or delayed AT. The absolute number of CFSEhi and CFSElo cells was calculated. Mean values are presented plus or minus SE.
ETCs persist longer in secondary lymphoid organs of FCs as compared with MCs. Hematopoietic chimeras received late adoptive transfer (AT) with 40 × 106 ETCs:R-DCspulsed (ETCs:R-DCp), ETCs:R-DCsnonpulsed (ETC:R-DCnp), or naive DLI. The effectors were labeled with 5 μM CFSE. Spleens of the transplanted animals were harvested 3 or 7 days later, single cell suspensions were made and analyzed for CFSE intensity. (A) Representative day 7 results from flow cytometric analysis of harvested splenocytes from MCs and FCs are shown. A CFSE high peak (nondivided ETCs) was detected in FCs only (the marker labels the CFSE high/low region). (B) Adoptively transferred ETCs clear faster from MCs than from FCs. Spleens from MCs and FCs were harvested on days 3 and 7 after delayed adoptive transfer. Data obtained from animals that received either ETCs:R-DCsp or ETC:R-DCsnp are presented separately. A DLI group was added as a control. The fraction of CFSEhi cells (indicating nondivided ETCs) and CFSElo cells (indicating proliferated ETCs) was determined by flow cytomertic analysis. (C) Both, in MCs and FCs more vigorous proliferation of ETCs is seen when adoptive transfer is performed early after transplantation (day 21). Spleens from MCs and FCs were harvested on day 7 after early or delayed AT. The absolute number of CFSEhi and CFSElo cells was calculated. Mean values are presented plus or minus SE.
Discussion
Residual host-derived APCs play a predominant role in mediating GVL effects after allogeneic HCT.25 It has also been shown that a loss of GVL responses after the transfer of DLIs correlates with the conversion of mixed donor-host chimerism to full donor chimerism.18 Others have confirmed and extended those observations without the possibly confounding effects of tolerance associated with MCs.9 The significance of these observations for the design of clinical HCT protocols might be substantial. However, the generation of stable long-term MC in humans remains a challenge.26 To our knowledge, our study is the first demonstration that the requirement of residual host-derived APCs for obtaining GVL responses after allogeneic HCT can in part be circumvented by in vitro priming of allogeneic donor-derived T cells on host-derived APCs before AT. We also demonstrate that the presentation of leukemia-associated antigens (of host origin) on donor-derived APCs in vitro can augment the effectiveness of GVL responses and the extent of GVHD reactions after AT.
Taking into account the strong anti-leukemic potential of DLIs derived from a MHC-disparate donor we hypothesized that in vitro priming of donor-derived T cells on APCs presenting leukemia-associated antigens would potentially limit their GVHD-evoking tissue injury while preserving their GVL capacity. Adoptive T-cell transfer was performed at an earlier (day 21) and at a later (day 56) time point after transplantation. Despite the crucial role of recipient-derived APCs in mediating GVL effects, we observed similar GVL effects in the FC and MC recipients later after HCT transfer of naive DLIs. This apparent contradiction to results reported by others7,27 could be explained by the difference in design of our experimental set up. In the prior studies, tumor cells were injected after the DLI. In this setting, naive donor-derived T cells initially would be activated and proliferate in response to allo-antigen only. Tumor cells given to these animals would encounter alloprimed T cells. In contrast to these earlier studies, we challenged the HCT recipients with a lethal dose of leukemic cells before the adoptive T-cell transfer thereby allowing T cells to experience both alloantigens and leukemia-associated antigens at the same time.
GVL effects after adoptive T-cell transfer were comparable in FCs and MCs. This was somewhat unexpected but highly reproducible. At the same time we failed to demonstrate the superior GVL effects of naive DLIs (splenocytes) in MCs as compared with FCs in this leukemia model. At different time points after the adoptive transfer of ETCs and DLIs, spleens, lymph nodes, BM, and thymi of recipients were analyzed for the presence and in vivo proliferation of transferred T cells. In vitro primed T cells were cleared faster from MCs as compared with FCs. The abundant presence of recipient-type APCs in MCs might expose ETCs to overstimulation (eg, antigen induced cell death),28 quicker clearance, and as a consequence less profound GVL effects. In contrast, transferred DLIs persisted longer in secondary lymphoid organs, both in MCs and FCs. No differences in GVL efficacy was observed between MCs and FCs. Here, the important question can be raised why significant antitumor activity is seen after the transfer of naive T cells into hosts that presumably have no residual recipient APCs. Activation of donor T cells on nonhematopoietic tissue class I MHC is known to occur. Cross-reactivity with class I MHC on leukemia cells can therefore explain GVL effects in this setting.29
The origin of APCs for in vitro priming was of great importance in our study. T cells that had been primed on APCs of host origin mediated strong GVL effects. In our MHC mismatch model, allogeneic T cells primed on syngeneic APCs neither mediated significant antileukemic effects in vitro nor caused GVL effects after AT when given late after HCT. These observations were in conformity with previously published studies,9,18,30,31 demonstrating a crucial role of the recipient-derived APCs in mediation of GVL effects. Because GVL effects in MCs were significantly reduced when nonpulsed APCs were used for in vitro priming, these data indicate that in vitro leukemia-associated antigen presented by host-type APCs is more effective in an in vitro setting. However, it needs to be acknowledged that different murine models vary in CD4 versus CD8 dependence, when GVHD and GVL are considered. Our findings were derived from a MHC-mismatched CD8-driven experimental model allowing the assessment of acute but not chronic GVHD. Elegant studies by both Falkenburg and Montagna have identified and isolated leukemia-reactive T cells in a HLA-matched transplantation setting or autologous system respectively.32-34 This indicates that GVL-specific T cells can be generated in vivo in patients with HLA-identical APCs.
Interestingly, in vitro priming to leukemia lysate-pulsed APCs resulted in reduced alloreactivity compared with naive splenocytes. After delayed AT no GVHD was seen in any of the groups, both clinically as well as confirmed on histologic sections of classic GVHD target organs (data not shown). As described by others earlier,7 a certain degree of nonspecific alloreactivity was observed against the hematopoietic system as documented by a shift of MC to FC. This effect was only seen after in vitro priming of allogeneic T cells on host-derived APCs. Allogeneic T cells primed on their syngeneic APCs did not mediate any shift of chimerism after AT.
The picture changed dramatically when the AT was performed on day 21 rather than day 56 after HCT. In contrast to later after HCT AT, the difference in GVL activity between ETCs:R-DCs and ETCs:D-DCs nearly disappeared. More importantly, GVHD became a major problem, both in MCs and FCs. Most severe GVHD was regularly observed in MCs. The presence of host APCs in MC recipients and an early transfer of ETCs:D-DCs resulted in hyperacute and lethal GVHD in up to 40% of animals. These severe GVHD scores were never reached after the transfer of naive splenocytes. ETCs:D-DCs did not lead to any observable GVHD in FC recipients bearing a limited number host APCs after late transfer. Together with previous reports,31 our data suggest that under inflammatory pressure, co-occurrence of host APCs with donor-primed T cells can lead to a heightened severity of acute GVHD. At this early time point before ETCs or DLIs, neutrophils, T cells, and B cells already had recovered to normal levels (data not shown). Therefore, a lymphopenic environment cannot account for the profound difference.
An important question has always been whether pulsing of DCs in vitro would influence biologic behavior after AT. In our system, T cells primed on pulsed versus nonpulsed DCs mediated strong GVL effects after delayed AT in FCs. Pulsing of DCs and priming T cells before early after HCT transfers also improved GVL. Priming allogeneic T cells on nonpulsed DCs resulted in a T-cell product that triggered severe GVHD, which was accentuated in MCs. Earlier reports suggested that an inflammatory milieu within nonlymphatic tissue favors trafficking of activated T cells to that site. These studies have helped to explain the paradox whereby GVHD-reactive T cell can mediate GVL without inducing GVHD in established MCs.24 Comparing GVL effects and GVHD scores after AT on day 21 or 56 day after HCT, one is tempted to hypothesize that inflammation is a prerequisite for the development of GVHD, whereas targeted GVL effects can be delivered independently of the respective inflammatory stimulators. T cells recognizing non-self MHC show exquisite specificity to MHC and its epitopes.35 It may be expected that priming of T cells with MHC-mismatched APCs presenting leukemia antigens would increase the frequencies of T cells reactive to these antigens. Indeed, a skewing of the TCRs of ETCs was observed after in vitro priming with R-DCspulsed (APCs with leukemia antigens), which is distinct from priming with R-DCsnonpulsed (APCs with no leukemia antigens). This could manifest in enhanced GVL effects exhibited by the ETCs:R-DCspulsed. Elucidating the repertoire of T cells involved in specific GVL effects and identifying key immunodominant leukemia antigens in MHC-mismatched settings might lead to increased and highly specific GVL effects.
In summary, our studies show that in vitro priming of T cells derived from MHC-disparate origin can in part overcome the need of persisting host-derived APCs in the recipient to achieve strong GVL effects after adoptive T-cell transfer. Further, we demonstrate the influence of the time after HCT on the GVL versus GVHD balance. Being aware of the important limitations, in vitro priming of allogeneic T cells might harbor significant therapeutic potential for patients having achieved full hematopoietic chimerism after transplantation from an HLA-mismatched donor.
Presented in abstract form at the Presidential Symposium of the 33rd Annual Meeting of the European Group for Blood and Marrow Transplantation, Lyon, France, March 26, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank R. Schwinzer and W. Baars for providing their radioisotope laboratory and Y. Behrmann for excellent animal husbandry.
This research was supported by grants Sa-137/1-1 (Deutsche Forschungsgemeinschaft, Bonn, Germany; M.S.) and R01 CA 73 669 (National Institutes of Health, Bethesda, MD; B.R.B.).
National Institutes of Health
Authorship
Contribution: A.G. designed research, performed experiments, analyzed and interpreted data, and drafted and edited the manuscript; W.K. performed experiments and analyzed and interpreted data; M.H., V.S., F.L., and R.B. performed experiments; C.K. and M.C. analyzed and interpreted data; K.W. designed research; B.B. analyzed and interpreted data and edited the manuscript; and M.G.S. designed research, analyzed and interpreted data, and drafted edited the manuscript.
Conflict-of-interest disclosure: The authors decalre no competing financial interests.
Correspondence: Martin G. Sauer, Department of Pediatric Hematology and Oncology, Medizinische Hochschule Hannover, OE 6780, Carl-Neuberg-Strasse 1, 30625 Hannover, Germany; e-mail: sauer.martin@mh-hannover.de.



 ) were determined using the trypan blue exclusion method for viability assessment. Expansion rates of up to 10-fold were obtained within 4 days. Values are shown plus or minus SE (n = 3). Similar expansion rates were achieved after priming with R-or D-DCspulsed. (B) The phenotype of ETCs and naive donor splenocytes were characterized by flow cytometric analysis. In the uppermost panels, events were gated on live cells by forward-side scatter exclusion and at least 15 000 live events were acquired. In the bottom panels, data from events gated on live CD8+ cells stained with the indicated antibodies are depicted. (C) The ETCs obtained after priming with R-DCspulsed (black continuous line) or D-DCspulsed (gray continuous line) and donor splenocytes (black dotted line) were stained to study intracellular Granzyme B and surface CD95L expression. Data from events gated on CD8+ live cells of the indicated populations are depicted. (D) Left panel: ETCs primed on R-DCspulsed (◆) demonstrated robust anti-C1498 responses in vitro, whereas D-DCspulsed (
) were determined using the trypan blue exclusion method for viability assessment. Expansion rates of up to 10-fold were obtained within 4 days. Values are shown plus or minus SE (n = 3). Similar expansion rates were achieved after priming with R-or D-DCspulsed. (B) The phenotype of ETCs and naive donor splenocytes were characterized by flow cytometric analysis. In the uppermost panels, events were gated on live cells by forward-side scatter exclusion and at least 15 000 live events were acquired. In the bottom panels, data from events gated on live CD8+ cells stained with the indicated antibodies are depicted. (C) The ETCs obtained after priming with R-DCspulsed (black continuous line) or D-DCspulsed (gray continuous line) and donor splenocytes (black dotted line) were stained to study intracellular Granzyme B and surface CD95L expression. Data from events gated on CD8+ live cells of the indicated populations are depicted. (D) Left panel: ETCs primed on R-DCspulsed (◆) demonstrated robust anti-C1498 responses in vitro, whereas D-DCspulsed (




