Abstract
Congenital macrothrombocytopenia is a genetically heterogeneous group of rare disorders. We identified the first TUBB1 mutation, R318W, in a patient with congenital macrothrombocytopenia. The patient was heterozygous for Q43P, but this single-nucleotide polymorphism (SNP) did not relate to macrothrombocytopenia. Although no abnormal platelet β1-tubulin localization/marginal band organization was observed, the level of β1-tubulin was decreased by approximately 50% compared with healthy controls. Large and irregular bleb protrusions observed in megakaryocytes derived from the patient's peripheral blood CD34+ cells suggested impaired megakaryocyte fragmentation and release of large platelets. In vitro transfection experiments in Chinese hamster ovary (CHO) cells demonstrated no incorporation of mutant β1-tubulin into microtubules, but the formation of punctuated insoluble aggregates. These results suggested that mutant protein is prone to aggregation but is unstable within megakaryocytes/platelets. Alternatively, mutant β1-tubulin may not be transported from the megakaryocytes into platelets. W318 β1-tubulin may interfere with normal platelet production, resulting in macrothrombocytopenia.
Introduction
Congenital macrothrombocytopenia is a genetically heterogeneous group of rare disorders.1-3 The most frequent forms include MYH9 disorders, such as May-Hegglin anomaly, and Bernard-Soulier syndrome. In approximately half of the cases the pathogenesis remains unknown; thus, a definite diagnosis is not possible. The linkage between the membrane skeleton and cytoskeletal actin filaments as well as the marginal microtubule band maintains normal platelet morphology.4,5 Defects in these systems may result in macrothrombocytopenia. The microtubules are assembled from α- and β-tubulin heterodimers. β1-Tubulin expression is restricted in the megakaryocyte/platelet lineage.6 Tubb1 knockout mice show thrombocytopenia and spherical platelets.7 TUBB1 Q43P functional polymorphism has been reported. However, it may not be relevant to macrothrombocytopenia.8 We identified the first TUBB1 mutation affecting microtubule assembly in congenital macrothrombocytopenia.
Methods
Patient
The patient was a 7-year-old boy who was incidentally found to have thrombocytopenia (platelets, 40-60 × 109/L). He was diagnosed with immune thrombocytopenic purpura. Peripheral blood smears showed the prominent appearance of giant platelets. Electron microscopy showed no other abnormalities (Figure 1A,B). There were no leukocyte inclusion bodies, confirmed by myosin IIA localization.9 The platelets aggregated normally with adenosine diphosphate (ADP), collagen, and ristocetin. Flow cytometry showed normal expression of platelet GPIb/IX. An initial bone marrow examination revealed normal megakaryocyte number and morphology. The mother of the patient also had macrothrombocytopenia. Peripheral blood samples were obtained after the mother gave informed consent in accordance with the Declaration of Helsinki for the study, which was approved by the institutional review boards (IRBs) of Nagoya Medical Center and Hokkaido University. Platelet size and TUBB1 gene frequencies were determined in 108 healthy controls and 16 consecutive, unrelated patients with macrothrombocytopenia who were enrolled in an institutional review board–approved collaborative study on congenital thrombocytopenia.
Antibodies
We raised an anti–β1-tubulin antibody (NB2301) in rabbits against a synthetic peptide corresponding to C-terminal 425-451aa of human β1-tubulin (KAVLEEDEEVTEEAEMEPEDKGH). Antiserum was collected and affinity-purified. NB2301 specifically reacted with recombinant β1-tubulin among 7 known recombinant human β-tubulin isoforms on immunoblots, and only stained platelets on blood smears (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Other antibodies used were anti-GPIIb (SZ22, Immunotech, Marseille, France), anti–α-tubulin (DM1A and RB9281, LabVision, Fremont, CA), anti–β-tubulin (TUB2.1), and anti–β5-tubulin (SAP.4G5, Abcam, Cambridge, United Kingdom).
Mutational analysis
The entire coding sequence of exons and exon-intron boundaries of TUBB1 was amplified by PCR, and the products were subjected to DNA sequence analysis (Table S1).
TUBB1 cloning, mutagenesis, and transfection
Full-length TUBB1 sequences were amplified from the patient's platelet cDNA (Table S1). TUBB1 cDNA in-frame with the C-terminal myc epitope tag was cloned into pcDNA3.1 (Invitrogen, Carlsbad, CA). We prepared 3 mutant constructs (P43, W318, and P43/W318), and used them to transfect Chinese hamster ovary (CHO) cells. At 24 hours after transfection, the cells were replated on fibronectin-coated (10 μg/mL) chamber slides, and cultured for 48 hours. For microtubule-depolymerization experiments, cells were incubated at 4°C for 4 hours or 33 mM nocodazole at 37°C for 2 hours.
Analysis of cultured megakaryocytes
Results and discussion
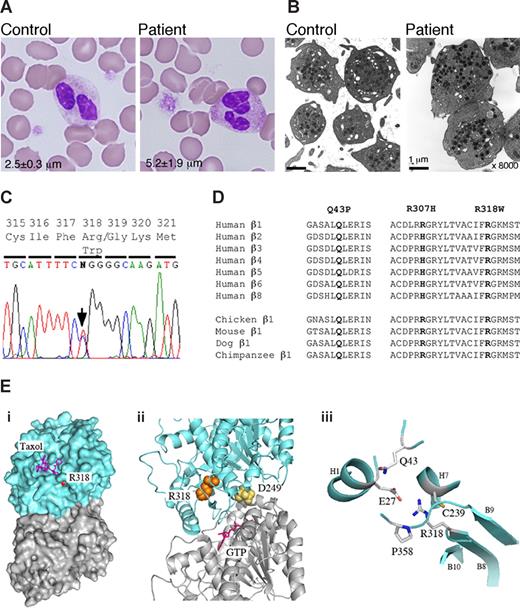
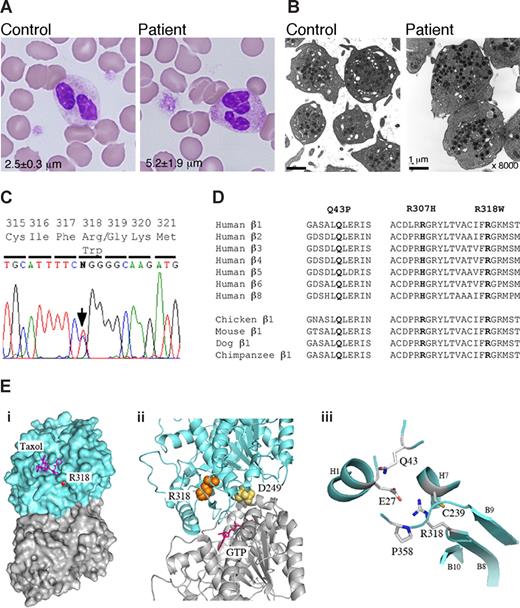
We searched for TUBB1 mutations in the patient, and found a novel conserved R318W mutation (Figure 1C,D). Restriction analysis confirmed heterozygosity in the patient and mother. The mutation was not found in 108 healthy controls or in the SNP database (http://www.ncbi.nlm.nih.gov/SNP/). In a tubulin 3D model, it is buried inside the molecule and is near the α and β intradimer interface (Figure 1E).11 The side chain is oriented toward the inside of the molecule and faces that of E27. An R318W mutation replacing the charged arginine with bulky aromatic tryptophan will disrupt the side chain interactions. Because D249N, located at the interface, has recently been reported to cause canine macrothrombocytopenia,12,13 mutations disrupting the structure of the intradimer interface may affect platelet morphology.
Platelet morphology and the TUBB1 R318W mutation. (A) Peripheral blood smears were stained with May-Grünwald Giemsa for a normal control (TUBB1 43QQ/307RR) and the patient (original magnification ×1000). The patient showed giant platelets with morphologically normal leukocytes. The number in each panel shows the mean platelet size in microns (n = 200). (B) Ultrastructure of platelets. Platelet-rich plasma prepared from acid-citrate-dextrose citrated whole blood was fixed in 2% glutaraldehyde and postfixed in 1% osmium tetroxide. The platelet samples were embedded in epoxy resin (Quetol 651; Nissin EM, Tokyo, Japan). Ultra-thin sections were cut, stained with uranyl acetate and lead citrate, and observed under a transmission electron microscope (JEM 1011, JEOL, Tokyo, Japan) Original magnification ×8000. (C) The entire coding regions of the patient's TUBB1 gene were amplified from genomic DNA by polymerase chain reaction (PCR) and amplified DNA fragments were subjected to direct cycle sequence analysis. A C-to-T transition at nucleotide 952 (952C>T), changing Arg318 to Trp (R318W), was detected. The arrow shows the position of the substitution. (D) β-Tubulin sequence alignment. Amino acid sequence alignment is shown for the 7 human β-tubulin isoforms and the known β1-tubulin from other species. R318 is conserved in other species and other human β-tubulin isoforms. The substituted amino acid is indicated in bold. (E) Structural analysis of R318 using a tubulin 3D model. (i) Position of R318 (red) in the whole tubulin molecule. The residue is almost buried beneath the taxol pocket of β-tubulin but exposed slightly. Blue indicates β-tubulin; gray, α-tubulin. (ii) R318 (orange) is located near the α and β intradimer interface. D249 (yellow) is located at the interface. GTP (magenta) bound on the N-site of α-tubulin is also shown. (iii), Residues surrounding the side chain of R318. All the figures are depicted from 1JFF with the aid of MacPyMol (DeLano Scientific, Palo Alto, CA; http://www.delanoscientific.com/).
Platelet morphology and the TUBB1 R318W mutation. (A) Peripheral blood smears were stained with May-Grünwald Giemsa for a normal control (TUBB1 43QQ/307RR) and the patient (original magnification ×1000). The patient showed giant platelets with morphologically normal leukocytes. The number in each panel shows the mean platelet size in microns (n = 200). (B) Ultrastructure of platelets. Platelet-rich plasma prepared from acid-citrate-dextrose citrated whole blood was fixed in 2% glutaraldehyde and postfixed in 1% osmium tetroxide. The platelet samples were embedded in epoxy resin (Quetol 651; Nissin EM, Tokyo, Japan). Ultra-thin sections were cut, stained with uranyl acetate and lead citrate, and observed under a transmission electron microscope (JEM 1011, JEOL, Tokyo, Japan) Original magnification ×8000. (C) The entire coding regions of the patient's TUBB1 gene were amplified from genomic DNA by polymerase chain reaction (PCR) and amplified DNA fragments were subjected to direct cycle sequence analysis. A C-to-T transition at nucleotide 952 (952C>T), changing Arg318 to Trp (R318W), was detected. The arrow shows the position of the substitution. (D) β-Tubulin sequence alignment. Amino acid sequence alignment is shown for the 7 human β-tubulin isoforms and the known β1-tubulin from other species. R318 is conserved in other species and other human β-tubulin isoforms. The substituted amino acid is indicated in bold. (E) Structural analysis of R318 using a tubulin 3D model. (i) Position of R318 (red) in the whole tubulin molecule. The residue is almost buried beneath the taxol pocket of β-tubulin but exposed slightly. Blue indicates β-tubulin; gray, α-tubulin. (ii) R318 (orange) is located near the α and β intradimer interface. D249 (yellow) is located at the interface. GTP (magenta) bound on the N-site of α-tubulin is also shown. (iii), Residues surrounding the side chain of R318. All the figures are depicted from 1JFF with the aid of MacPyMol (DeLano Scientific, Palo Alto, CA; http://www.delanoscientific.com/).
Two other nonsynonymous substitutions, Q43P and R307H, were identified. The former was recently described as a functional polymorphism.8,14 The prevalence of the P43 allele was higher in patients with congenital macrothrombocytopenia (0.12) than controls (0.06).8 We also found a similar gene frequency of 0.16 and 0.06 in our patients and controls, respectively (Table S2). Although R307 is conserved, this position contains basic histidine in other human β-tubulins, and the gene frequency was the same in patients and controls. Control individuals with Q43P or R307H even in their homozygous state had normal-sized platelets (Table S3). We therefore suggested that both substitutions are not related to macrothrombocytopenia.
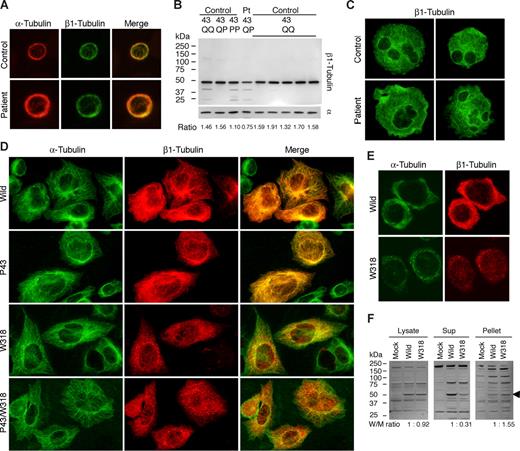
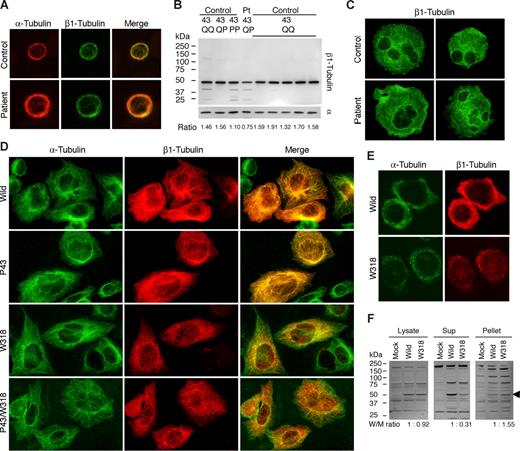
The expression and localization of platelet β1-tubulin were investigated using the newly produced NB2301antibody. β1-Tubulin was normally localized in the marginal microtubule band in resting patient platelets (Figure 2A). Immnunoblotting showed the normal electrophoretic mobility. However, the expression level was decreased: the β1-tubulin/α-tubulin ratio was decreased by approximately 50% compared with controls (Figure 2B). Q43P is reportedly associated with the reduced platelet β1-tubulin.8 We found in a control P43 homozygote that the β1-tubulin/α-tubulin ratio was not decreased (Figure 2B). Thus, the decreased expression of β1-tubulin in the patient is likely to be due to R318W and not Q43P. We examined megakaryocytes cultured from CD34+ peripheral blood cells. Cultured mature megakaryocytes frequently extend blebs, similar to demarcation membrane system-like structure.15,16 We observed large and irregular bleb protrusions in the patient (Figure 2C). This suggested impaired megakaryocyte fragmentation and release of large platelets. The plasma glycocalicin concentration, a proteolytic fragment of GPIbα (molecular marker of platelet production/destruction),17 was below the normal limit (1.02 μg/mL; normal values, 1.40 ± 0.25 μg/mL), indicating that thrombocytopenia is not due to underproduction of platelets but peripheral destruction.
Biochemical analysis of β1-tubulin in platelets and transfected cells. (A) Immunofluorescence analysis of platelets. After being fixed in methanol and permeabilized in acetone, the peripheral blood smears were hydrated with PBS and blocked with normal goat serum. Slides were concomitantly incubated with DM1A and NB2301, and then reacted with Alexa 555-labeled anti–mouse IgG and Alexa 488-labeled anti–rabbit IgG (Invitrogen). The stained cells were examined under a BX50 fluorescence microscope with a 100×/1.35 numeric aperture oil objective (Olympus, Tokyo, Japan). Image slides were taken with a DP70 digital camera using DP Manager software (Olympus). (B) Immunoblot analysis of platelets. Whole platelet proteins were isolated using a NucleoSpin RNA/Protein kit (Macherey-Nagel, Düren, Germany), separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 4% to 12% gradient acrylamide slab gels (Invitrogen), and electroblotted onto polyvinylidine difluoride membranes. The blots were incubated with RB9281 and NB2301, and reacted with horseradish peroxidase-conjugated secondary antibody. The bound antibodies were visualized using an enhanced chemiluminescent substrate. Densitometric analysis of blots performed using ImageQuant software (Molecular Dynamics, Sunnyvale, CA) showed that the β1-tubulin/α-tubulin ratio was reduced by 50% in the patient compared with controls (0.75 vs 1.57 ± 0.11; n = 7). (C) Megakaryocytes derived from the peripheral blood CD34+ cells of a normal control and the patient were doubly stained with anti-GPIIb mouse monoclonal antibody SZ22 (not shown) and NB2301 (green). Although the distribution of β1-tubulin in the patient's megakaryocytes was normal, irregular and large bleb protrusions were observed in the patient. (D) Immunofluorescence analysis of transfected CHO cells. TUBB1-myc transfected CHO cells grown on chamber slides were doubly stained with anti-myc tag antibody and DM1A, and reacted with Alexa 488-labeled anti–rabbit IgG and Alexa 555-labeled anti–mouse IgG. Note that W318 mutants were only localized as small punctuate structures. (E) TUBB1-myc transfected CHO cells grown on chamber slides were exposed to 4°C for 4 hours to facilitate the depolymerization of microtubules, and then fixed and stained. Aggregates of β1-tubulin W318 did not dissociate under conditions of microtubule depolymerization. (F) Low temperature-exposed TUBB1-myc transfected CHO cells were lysed in 10 mM Pipes, pH 6.8, 1 mM MgCl2, 1 mM EGTA, 10% dimethyl sulfoxide, 0.5% Nonidet P-40, and Complete Protease Inhibitor Cocktail (Roche Diagnostics, Mannheim, Germany), and incubated for 30 minutes at 37°C. Cell lysates were centrifuged at 1000g for 10 minutes at 37°C before supernatants and pellets were collected for immunoblot analysis with anti-myc tag antibody. Densitometric analysis of the blots showed that a large proportion of β1-tubulin W318 was recovered in the pellets. The arrowhead indicates recombinant myc-tagged β1-tubulin.
Biochemical analysis of β1-tubulin in platelets and transfected cells. (A) Immunofluorescence analysis of platelets. After being fixed in methanol and permeabilized in acetone, the peripheral blood smears were hydrated with PBS and blocked with normal goat serum. Slides were concomitantly incubated with DM1A and NB2301, and then reacted with Alexa 555-labeled anti–mouse IgG and Alexa 488-labeled anti–rabbit IgG (Invitrogen). The stained cells were examined under a BX50 fluorescence microscope with a 100×/1.35 numeric aperture oil objective (Olympus, Tokyo, Japan). Image slides were taken with a DP70 digital camera using DP Manager software (Olympus). (B) Immunoblot analysis of platelets. Whole platelet proteins were isolated using a NucleoSpin RNA/Protein kit (Macherey-Nagel, Düren, Germany), separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 4% to 12% gradient acrylamide slab gels (Invitrogen), and electroblotted onto polyvinylidine difluoride membranes. The blots were incubated with RB9281 and NB2301, and reacted with horseradish peroxidase-conjugated secondary antibody. The bound antibodies were visualized using an enhanced chemiluminescent substrate. Densitometric analysis of blots performed using ImageQuant software (Molecular Dynamics, Sunnyvale, CA) showed that the β1-tubulin/α-tubulin ratio was reduced by 50% in the patient compared with controls (0.75 vs 1.57 ± 0.11; n = 7). (C) Megakaryocytes derived from the peripheral blood CD34+ cells of a normal control and the patient were doubly stained with anti-GPIIb mouse monoclonal antibody SZ22 (not shown) and NB2301 (green). Although the distribution of β1-tubulin in the patient's megakaryocytes was normal, irregular and large bleb protrusions were observed in the patient. (D) Immunofluorescence analysis of transfected CHO cells. TUBB1-myc transfected CHO cells grown on chamber slides were doubly stained with anti-myc tag antibody and DM1A, and reacted with Alexa 488-labeled anti–rabbit IgG and Alexa 555-labeled anti–mouse IgG. Note that W318 mutants were only localized as small punctuate structures. (E) TUBB1-myc transfected CHO cells grown on chamber slides were exposed to 4°C for 4 hours to facilitate the depolymerization of microtubules, and then fixed and stained. Aggregates of β1-tubulin W318 did not dissociate under conditions of microtubule depolymerization. (F) Low temperature-exposed TUBB1-myc transfected CHO cells were lysed in 10 mM Pipes, pH 6.8, 1 mM MgCl2, 1 mM EGTA, 10% dimethyl sulfoxide, 0.5% Nonidet P-40, and Complete Protease Inhibitor Cocktail (Roche Diagnostics, Mannheim, Germany), and incubated for 30 minutes at 37°C. Cell lysates were centrifuged at 1000g for 10 minutes at 37°C before supernatants and pellets were collected for immunoblot analysis with anti-myc tag antibody. Densitometric analysis of the blots showed that a large proportion of β1-tubulin W318 was recovered in the pellets. The arrowhead indicates recombinant myc-tagged β1-tubulin.
To determine the functional and structural consequences of the R318W mutation on microtubule assembly, we monitored mutant β1-tubulins after their transfection into CHO cells. The wild-type and P43 mutant myc-tagged β1-tubulin were localized as fine filamentous cytoplasmic networks, indicating normal incorporation of recombinant β1-tubulin into microtubules with endogenous α-tubulin. In contrast, W318 and P43/W318 mutants accumulated in punctuate structures in the cytoplasm (Figure 2D). Under conditions of microtubule depolymerization by low temperature (Figure 2E,F) or nocodazole treatment, aggregates of W318 mutants did not dissociate.
Our results suggested that the net effect of the TUBB1 R318W mutation in platelets is the instability of mutant protein. These data seem to contradict those obtained by in vitro expression experiments, whereby β1-tubulin W318 formed insoluble aggregates. One important difference is that the latter employs an expression vector with strong cytomegalovirus (CMV) promoter. Thus, the unstable but aggregate-prone W318 mutant was detected as insoluble aggregates. The coordinated production of α- and β-tubulins necessary for proper heterodimer assembly are regulated both transcriptionally and translationally and, when tubulin is overexpressed in cells, synthesis of the endogenous form is strongly inhibited.18-20 In Tubb1 knockout mice, up-regulation of β2- and β5-tubulins partially compensates for the null expression of β1-tubulin.7 Because heterozygous Tubb1 knockout mice do not exhibit giant platelets, simple haploinsufficiency alone cannot explain the pathogenesis of macrothrombocytopenia. Mutant β1-tubulin may dominantly affect microtubule assembly in some manner. Normal expression of mutant TUBB1 mRNA (not shown) and decreased β1-tubulin in platelets lead to an alternative hypothesis that mutant β1-tubulin is not transported from the megakaryocyte into platelets.
Elucidating the precise molecular mechanisms will not only advance our classification and diagnosis of congenital macrothrombocytopenias, but also our comprehensive understanding of megakaryopoiesis and platelet genesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Yoshimi Ito for her skillful technical assistance.
This work was supported by grants to S.K. from the Japan Society for the Promotion of Science (18591094 and 20591161), the Ministry of Health, Labor and Welfare (Grant for Child Health and Development 19C-2), Charitable Trust Laboratory Medicine Foundation of Japan, and National Hospital Organization (network research grant for congenital thrombocytopenia).
Authorship
Contributions: S.K. designed and performed research, analyzed data, and wrote the paper; R.K. contributed patient samples and clinical data; T.J.I. designed the tubulin experiments and wrote the paper; and M.H. and H.S. supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shinji Kunishima, Department of Advanced Diagnosis, Clinical Research Center, National Hospital Organization Nagoya Medical Center 4-1-1 Sannomaru, Naka-ku, Nagoya 4600001, Japan. e-mail: kunishis@nnh.hosp.go.jp.