Abstract
Platelets play a fundamental role in hemostasis and thrombosis. They are also involved in pathologic conditions resulting from blocked blood vessels, including myocardial infarction and ischemic stroke. Platelet adhesion, activation, and aggregation at sites of vascular injury are regulated by a diverse repertoire of tyrosine kinase–linked and G protein–coupled receptors. Src family kinases (SFKs) play a central role in initiating and propagating signaling from several platelet surface receptors; however, the underlying mechanism of how SFK activity is regulated in platelets remains unclear. CD148 is the only receptor-like protein tyrosine phosphatase identified in platelets to date. In the present study, we show that mutant mice lacking CD148 exhibited a bleeding tendency and defective arterial thrombosis. Basal SFK activity was found to be markedly reduced in CD148-deficient platelets, resulting in a global hyporesponsiveness to agonists that signal through SFKs, including collagen and fibrinogen. G protein–coupled receptor responses to thrombin and other agonists were also marginally reduced. These results highlight CD148 as a global regulator of platelet activation and a novel antithrombotic drug target.
Introduction
The primary physiologic function of platelets is to stop bleeding from sites of vascular injury. In addition, it is becoming increasingly recognized that they are involved in other physiologic processes, including angiogenesis, inflammation, and immunity. Platelets can also have deleterious effects on health, as in the case of atherothrombosis, which can lead to myocardial infarction and ischemic stroke, 2 of the leading causes of mortality in the Western world. Platelets prevent excessive blood loss from sites of vascular injury by adhering to exposed extracellular matrix proteins and forming aggregates that plug damaged blood vessels. Further, they regulate vascular tone through release of secondary mediators, including serotonin, adenosine diphosphate (ADP), and thromboxane A2 (TxA2). Platelet-derived ADP and TxA2 act in a positive feedback loop to amplify the initiating stimulatory signal. The surface of activated platelets serves as a platform on which clotting factors assemble into complexes that accelerate the localized generation of thrombin. Thrombin directly activates platelets and converts fibrinogen into fibrin that consolidates the platelet aggregate, making it less susceptible to the mechanical forces of flowing blood.
Thrombus formation and stability are regulated by the coordinated action of tyrosine kinase–linked and G protein–coupled receptors. Two of the major tyrosine kinase-linked receptors on platelets are the collagen receptor glycoprotein VI (GPVI), which signals through the immunoreceptor tyrosine-based activation motif (ITAM)–containing FcR γ-chain, and the integrin αIIbβ3, which binds to several matrix proteins including fibrinogen and is essential for platelet aggregation. Although there are many similarities between the GPVI and integrin αIIbβ3 signaling cascades, with critical roles for Src and Syk tyrosine kinases and the downstream targets SLP-76, Vav, and PLCγ2, only GPVI uses the FcR γ-chain to recruit and activate Syk.1 In contrast, the integrin αIIbβ3 is believed to activate Syk directly through the β3 integrin cytoplasmic tail independent of an ITAM, although this model has recently been questioned.2–4
The earliest identified GPVI signaling event is activation of Src family kinases (SFKs). Previous studies using mutant mouse models and transfected cell lines have shown that the SFKs Lyn and Fyn are constitutively associated with the proline-rich region of GPVI via their SH3 domains and are essential for initiating and propagating the GPVI signaling cascade.5,6 Similarly, Src is constitutively associated with the C-terminal region of the β3 integrin cytoplasmic tail and is activated after fibrinogen binding to αIIbβ3.1,7,8 Interestingly, the cytosolic protein tyrosine phosphatase (PTP) PTP-1B lies upstream of Src and is essential for αIIbβ3-mediated Src activation, but is not required to activate Lyn and Fyn downstream of GPVI.9
The activity of SFKs is tightly regulated by tyrosine phosphorylation and intramolecular interactions. SFKs are maintained in an inactive conformation by 2 intramolecular interactions, one of which is between the SH3 domain and the polyproline sequence in the linker region (between the SH2 and kinase domain), and the other between the SH2 domain and the inhibitory tyrosine residue in the C-terminal tail.10,11 Maximal activation of SFKs requires uncoupling of the intramolecular SH2 and SH3 interactions and trans-autophosphorylation of the activation loop tyrosine residue, thereby relieving the steric hindrance that impedes substrate binding.10,12 Phosphorylation of the C-terminal tail inhibitory tyrosine residue by Csk and the related kinase Ctk/Chk maintains the molecule in an inactive conformation, whereas dephosphorylation of this site allows the SFK to adopt an active conformation.13–15 In the case of the B- and T-cell receptors this action is achieved primarily through the receptor-like protein tyrosine phosphatase (RPTP) CD45, with the structurally distinct RPTP, CD148, also playing a minor role in B cells.16–20 In macrophages, CD45 and CD148 play redundant roles in Fc receptor–mediated SFK activation.16
We recently identified CD148 as the only RPTP expressed on the human platelet surface using a global membrane proteomics approach.21 This led us to investigate whether CD148 plays a role in regulating platelet function using CD148 loss-of-function mutant mice. In this study, we demonstrate for the first time that CD148 plays a global role in platelet activation by all of the major classes of platelet surface receptors, namely ITAM, integrin, and G protein–coupled receptors. Moreover, CD148 is an essential positive regulator of hemostasis and arterial thrombosis in vivo.
Methods
Mice
CD148 loss-of-function transmembrane-knockout (CD148 TM-KO) mice on a C57BL/6 background were generated as previously described.16 FcR γ-chain–deficient (γ-chain−/−) mice on a C57BL/6 background were kindly provided by Dr Takashi Saito (Chiba University Graduate School of Medicine, Japan).22 All procedures were undertaken with United Kingdom Home Office approval in accordance with the Animals (Scientific Procedures) Act of 1986.
Antibodies
Anti–human CD148, anti–Src-pan, anti-SFK activation loop phospho-Tyr, and anti-Src phospho-Tyr-529 antibodies were obtained from Biosource (Camarillo, CA); hamster anti–mouse CD148 antibody (8A-1) was generated in A.W.'s laboratory23 ; fluorescein isothiocyanate (FITC)–conjugated anti–mouse P-selectin, phycoerythrin (PE)-conjugated anti–mouse active αIIbβ3, FITC-conjugated anti–mouse αIIbβ3, FITC-conjugated anti–mouse α2, anti–mouse GPVI, and anti–mouse GPIbα antibodies were from Emfret Analytics (Würzburg, Germany); FITC-conjugated goat anti–rat IgG and anti-actin antibody from Sigma-Aldrich (St. Louis, MO); anti-phosphotyrosine antibody from Millipore (Billerica, MA); anti-PLCγ2 and anti-Syk antibodies were gifts from Dr Joseph Bolen; anti–Lyn-pan and anti-Lyn phospho-Tyr-507 were obtained from Cell Signaling Technology (Danvers, MA); anti–mouse αIIb antibody from BD Pharmingen (San Diego, CA); anti–rat Alexa488 antibody from Invitrogen (Carlsbad, CA); and DyLight 800–conjugated anti–mouse IgG and DyLight 680–conjugated anti–rabbit IgG from Thermo Fisher Scientific (Waltham, MA).
Chemicals
Monoparin heparin was obtained from CP Pharmaceuticals (Wrexham, United Kingdom); D-Phe-Pro-Ala-chloromethylketone (PPACK), from Calbiochem (San Diego, CA); cross-linked collagen-related peptide (CRP) was prepared as previously described24,25 ; fibrillar-type I equine tendon collagen was from Nycomed (Zurich, Switzerland); human plasma fibrinogen from Enzyme Research Laboratories (South Bend, IN); bovine plasma fibronectin from Calbiochem; bovine thrombin, ADP, and FeCl3 from Sigma-Aldrich; FITC-conjugated phalloidin and DiOC6 from Molecular Probes (Eugene, OR); and recombinant murine SDF-1α from PeproTech (Rocky Hill, NJ).
Platelet aggregation
Blood was collected from the heart or descending thoracic aorta of CO2-asphyxiated mice into 1/10 (vol/vol) acid-citrate-dextrose anticoagulant, and washed platelets (2 × 108/mL) were prepared as previously described.26 Platelet aggregation and adenosine triphosphate (ATP) secretion were measured simultaneously using a lumi-aggregometer (Chrono-Log, Havertown, PA).
Flow cytometry
Washed human platelets were prepared as previously described.26 Surface expression of CD148 was quantified using the Platelet Calibrator Kit (Biocytex, Marseille, France), as previously described.27 Resting and activated (10 μg/mL CRP or 0.06 U/mL thrombin) wild-type and CD148 TM-KO mouse platelets were stained for CD148, GPVI, P-selectin, and resting and activated forms of αIIbβ3. All antibodies were fluorescently conjugated except for anti-CD148 and anti-GPVI antibodies, which were detected using fluorescently conjugated secondary antibodies. Samples were analyzed using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, Franklin Lakes, NJ).
Platelet biochemistry
Washed human and mouse platelet whole-cell lysates (WCLs) were prepared and Western blotting performed as previously described.26 For immunoprecipitations, platelets (5 × 108/mL) were pooled from 3 to 6 mice in modified Tyrodes-HEPES buffer containing 10 μM lotrafiban, 10 μM indomethacin, and 2 U/mL apyrase, and stimulated with 10 μg/mL CRP for 90 and 300 seconds with constant stirring at 1200 rpm at 37°C. The remainder of the protocol was as previously described.26 WCLs were prepared from fibrinogen-spread platelets, from which proteins were immunoprecipitated, as previously described.28 WCLs and immunoprecipitates were resolved on 4% to 12% gradient gels (Invitrogen, Paisley, United Kingdom) and immunoblotted with primary antibodies and either horseradish peroxidise- or fluorescently conjugated secondary antibodies. For standard Western blots, proteins were detected by enhanced chemiluminescence (GE Healthcare, Little Chalfont, United Kingdom) and autoradiography. The Odyssey Infrared Imaging System (Li-Cor, Cambridge, United Kingdom) was used to quantify specific band intensities. Phosphospecific bands were normalized to actin, Lyn-pan, or Src-pan on the same membrane and presented as percentage of wild-type basal levels.
Platelet spreading
Washed platelets (2 × 107/mL) from wild-type and CD148 TM-KO mice were placed on fibrinogen-coated coverslips for 45 minutes at 37°C, fixed and imaged, as previously described.29 Platelet spreading was also imaged in real time, as previously described.29 Platelets were treated with 10 μM indomethacin and 2 U/mL apyrase in the absence and presence of either 10 μM PP1 or 1 U/mL thrombin before being plated.
Platelet flow adhesion
Megakaryocyte culture and functional assays
A highly purified population of bone marrow–derived megakaryocytes was cultured from wild-type and CD148 TM-KO mice, as previously described.31 Megakaryocyte chemotaxis toward a SDF-1α gradient on a fibronectin-coated surface was measured in a Dunn chamber, as previously described.31 Megakaryocyte spreading on fibrinogen-, fibronectin-, and collagen-coated surfaces was measured, as previously described.31 Wild-type and CD148 TM-KO megakaryocytes (1 × 106/mL) were lysed with an equal volume of 2 × lysis buffer and Western blotted with an anti-SFK activation loop phospho-Tyr antibody.
Tail-bleeding assay
Experiments were conducted on 20-32 g CD148 TM-KO and litter-matched wild-type mice. Mice were anesthetized with isofluorane, and buprenorphine was used as an analgesic. A 3-mm portion of the tail tip was excised with a razor blade. Mice were allowed to bleed until they lost either 15% blood volume (assuming a blood volume of 70 mL/kg) or for 20 minutes.
Ferric chloride–induced vascular occlusion model
Experiments were conducted on 20-35 g CD148 TM-KO, γ-chain−/−, and litter-matched wild-type mice, as previously described.32 Mice were anesthetized intraperitoneally with ketamine and xylazine. After a laparotamy, a mesenteric arteriole (60-75 μm diameter) was isolated. Alexa488-labeled platelets were injected into mice intravenously via the carotid artery. Filter paper soaked in 5% FeCl3 solution was placed on the dissected arteriole for 1 minute. Real-time intravital brightfield and fluorescent real-time images were captured simultaneously of the developing thrombus.
Laser-induced thrombus formation model
Experiments were conducted on 20- to 35-g CD148 TM-KO and litter-matched wild-type mice, as previously described.32 Mice were anesthetized intraperitoneally with ketamine and xylazine. The cremaster muscle surrounding the testicle was exteriorized on a cover-slip. Alexa 488–labeled platelets were injected into mice via the carotid artery. Laser-induced thrombi were generated at the luminal surface of selected arterioles, as previously described.32 Real-time intravital brightfield and fluorescent real-time images of the developing thrombus were captured simultaneously.
Immune thrombocytopenia
Thrombocytopenia was induced in 2- to 4-month-old wild-type and CD148 TM-KO mice by intraperitoneal injection of anti–mouse GPIbα antibody (2 μg/g of mouse), as previously described.31 Blood samples were collected 7 days preinjection (time = 0) and at 3, 48, 72, 96, 120, 144, and 172 hours after injection by tail bleeding. Platelet counts were measured using an ABX Pentra 60 Hematology Analyzer (Block Scientific, Nutley, NJ).
Statistical analysis
Student t test (one sample or independent samples) and 2-way analysis of variance were used to compare sample means and determine statistical significance. P values of less than .05 were considered significant.
Results
Surface expression of CD148 in human platelets
We recently identified CD148 as the only RPTP expressed in platelets through analysis of the membrane proteome.21 In the present study, we confirmed expression of CD148 in human platelets by Western blotting and flow cytometry. CD148 was detected as a single band of 220 kDa in human platelets by Western blotting (data not shown). Human platelets were found to express 2834 plus or minus 90 (mean ± SE; n = 6) copies of CD148 on their surface when using a flow cytometry–based assay (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). This is comparable with the level of the collagen receptor integrin, α2β1, and approximately 75% of that of GPVI, measured using the same assay, but is substantially lower than integrin αIIbβ3, the major platelet glycoprotein at more than 80 000 copies.27,33 Surface expression of CD148 was not altered in thrombin-stimulated platelets, suggesting the absence of an intracellular pool (Figure 1A). These studies demonstrate that CD148 is expressed on the surface of human platelets and that its expression is tightly regulated.
CD148 is expressed in human and mouse platelets. (A) Human platelets. (Ai) Resting and (Aii) thrombin-activated (1 U/mL) human platelets were stained with a mouse anti–human CD148 primary antibody (CD148, gray line) that recognizes the extracellular region of CD148, or the same amount of an isotype control antibody (IgG, black line), followed by a FITC-conjugated anti–mouse IgG secondary antibody and analyzed by flow cytometry. No detectable change in CD148 surface expression was observed in thrombin-activated platelets. (B) Mouse platelets. (Bi) Resting wild-type (WT) mouse platelets were incubated with either hamster anti–mouse CD148 primary antibody (CD148, gray line) that recognizes the extracellular region of mouse CD148, or an isotype control antibody (IgG, black line). Platelets were subsequently stained with FITC-conjugated anti–hamster secondary antibody and analyzed by flow cytometry. (Bii) Whole-cell lysates (WCLs) prepared of WT and CD148 transmembrane-knockout (KO) mouse platelets were Western blotted for CD148. A 220-kDa band was detected in the WT sample but not in the KO sample.
CD148 is expressed in human and mouse platelets. (A) Human platelets. (Ai) Resting and (Aii) thrombin-activated (1 U/mL) human platelets were stained with a mouse anti–human CD148 primary antibody (CD148, gray line) that recognizes the extracellular region of CD148, or the same amount of an isotype control antibody (IgG, black line), followed by a FITC-conjugated anti–mouse IgG secondary antibody and analyzed by flow cytometry. No detectable change in CD148 surface expression was observed in thrombin-activated platelets. (B) Mouse platelets. (Bi) Resting wild-type (WT) mouse platelets were incubated with either hamster anti–mouse CD148 primary antibody (CD148, gray line) that recognizes the extracellular region of mouse CD148, or an isotype control antibody (IgG, black line). Platelets were subsequently stained with FITC-conjugated anti–hamster secondary antibody and analyzed by flow cytometry. (Bii) Whole-cell lysates (WCLs) prepared of WT and CD148 transmembrane-knockout (KO) mouse platelets were Western blotted for CD148. A 220-kDa band was detected in the WT sample but not in the KO sample.
Platelets from CD148 transmembrane-knockout mice exhibit impaired GPVI-mediated aggregation and secretion
The recent generation of a CD148 loss-of-function TM-KO mouse provided the opportunity to probe the role of this RPTP in platelets.16 Flow cytometry using a specific monoclonal antibody (clone 8A-1) to the extracellular region of mouse CD148 confirmed that the RPTP is expressed on the surface of wild-type mouse platelets (Figure 1Bi).23 Platelets from CD148 TM-KO mice did not express detectable CD148 by Western blotting or flow cytometry (Figure 1Bii and data not shown). Interestingly, surface levels of GPVI were reduced to 42% plus or minus 1.5% (mean ± SE; n = 9) of control levels, whereas integrin α2β1 and αIIbβ3 levels were found to be normal on platelets from CD148-deficient mice (Figure S2).
The ability of CD148-deficient platelets to aggregate and secrete ATP was tested simultaneously using a lumi-aggregometer. Platelets from FcR γ-chain heterozygous-deficient (γ-chain+/−) mice were tested in parallel as they have a concomitant 50% reduction in GPVI levels, which is therefore similar to the reduction in expression in the CD148-deficient platelets.34 CD148-deficient platelets exhibited marked impairment in response to the GPVI-specific agonist CRP (Figure 2Ai). Inhibition was observed at low and intermediate concentrations of CRP (1 and 3 μg/mL). Even at high concentrations of CRP (10 μg/mL), CD148-deficient platelets exhibited reduced aggregation and secretion, demonstrating that CD148 is an essential, positive regulator of GPVI-mediated aggregation and dense granule secretion. The magnitude of this inhibition was much greater than that observed in γ-chain+/− platelets (Figure 2Ai). Aggregation and ATP secretion of CD148-deficient platelets were also inhibited in response to low and intermediate concentrations of the physiologic agonist collagen (1 and 3 μg/mL), which acts through the receptors GPVI and α2β1 (Figure 2Aii). This effect was largely overcome at a higher concentration of collagen (10 μg/mL), which induced almost full aggregation and marked ATP secretion (Figure 2Aii). Moreover, as with CRP, the defect in response to collagen was much greater than that observed in the γ-chain+/− platelets, suggesting that the defect is due to the loss of CD148 and not the reduction in GPVI levels (Figure 2Aii).
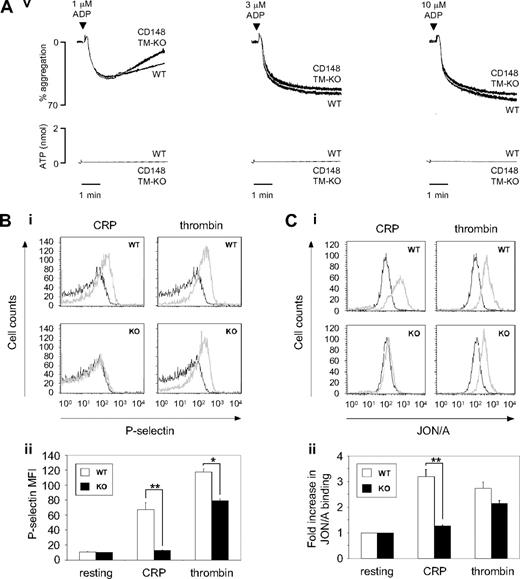
CD148-deficient platelets exhibit impaired GPVI-mediated platelet aggregation, secretion, and integrin activation. (A) Washed platelets (2 × 108/mL) prepared from wild-type (WT), CD148 transmembrane-knockout (CD148 TM-KO), and FcR γ-chain heterozygous-deficient (γ-chain+/−) mice were stimulated with low, intermediate, and high doses of: (Ai) collagen-related peptide (CRP; 1, 3, and 10 μg/mL); (Aii) collagen (1, 3, and 10 μg/mL); and (Aiii) thrombin (0.03, 0.09, and 0.3 U/mL). Platelet-rich plasma prepared from WT and CD148 TM-KO mice was stimulated with low, intermediate, and high doses of (Aiv) thromboxane A2 analog U46619 (1, 3, and 10 μM) and (Av) ADP (1, 3, and 10 μM). Platelet aggregation was measured as a change in light transmission, and ATP secretion was measured as luciferin/luciferase-mediated luminescence, using a lumi-aggregometer. Representative images are shown (n = 3-6 mice per condition). (B) Surface expression of P-selectin and (C) “active” integrin αIIbβ3 was quantified on resting and activated (10 μg/mL CRP or 0.06 U/mL thrombin) platelets from litter-matched WT and CD148 TM-KO (KO) mice by flow cytometry. Resting (black lines) and activated (gray lines) platelets were stained with either (Bi) FITC-conjugated rat anti–mouse P-selectin antibody or (Ci) PE-conjugated JON/A antibody to “active” integrin αIIbβ3. Data presented as (Bii) P-selectin geometric mean fluorescence intensity (MFI) and (Cii) fold increase in JON/A binding relative to total αIIbβ3 staining. Representative histograms are shown (n = 3 mice per condition). Bar height and error bars represent mean ± SEM (*P < .05, **P < .01).
CD148-deficient platelets exhibit impaired GPVI-mediated platelet aggregation, secretion, and integrin activation. (A) Washed platelets (2 × 108/mL) prepared from wild-type (WT), CD148 transmembrane-knockout (CD148 TM-KO), and FcR γ-chain heterozygous-deficient (γ-chain+/−) mice were stimulated with low, intermediate, and high doses of: (Ai) collagen-related peptide (CRP; 1, 3, and 10 μg/mL); (Aii) collagen (1, 3, and 10 μg/mL); and (Aiii) thrombin (0.03, 0.09, and 0.3 U/mL). Platelet-rich plasma prepared from WT and CD148 TM-KO mice was stimulated with low, intermediate, and high doses of (Aiv) thromboxane A2 analog U46619 (1, 3, and 10 μM) and (Av) ADP (1, 3, and 10 μM). Platelet aggregation was measured as a change in light transmission, and ATP secretion was measured as luciferin/luciferase-mediated luminescence, using a lumi-aggregometer. Representative images are shown (n = 3-6 mice per condition). (B) Surface expression of P-selectin and (C) “active” integrin αIIbβ3 was quantified on resting and activated (10 μg/mL CRP or 0.06 U/mL thrombin) platelets from litter-matched WT and CD148 TM-KO (KO) mice by flow cytometry. Resting (black lines) and activated (gray lines) platelets were stained with either (Bi) FITC-conjugated rat anti–mouse P-selectin antibody or (Ci) PE-conjugated JON/A antibody to “active” integrin αIIbβ3. Data presented as (Bii) P-selectin geometric mean fluorescence intensity (MFI) and (Cii) fold increase in JON/A binding relative to total αIIbβ3 staining. Representative histograms are shown (n = 3 mice per condition). Bar height and error bars represent mean ± SEM (*P < .05, **P < .01).
Aggregation and ATP secretion of CD148-deficient platelets were also tested in response to various G protein–coupled receptor agonists, which act through distinct receptors and signaling pathways. Aggregation and ATP secretion were marginally reduced in response to low, intermediate, and high doses of thrombin and U46619 (TxA2 analog), which act through the PAR-4 receptor in mouse platelets and the TP receptor, respectively (Figure 2Aiii,iv). In contrast, CD148-deficient platelets exhibited normal aggregation and ATP secretion responses to ADP, which signals through the P2Y1 and P2Y12 receptors (Figure 2Av). Together, these results demonstrate that CD148 is essential for tyrosine kinase–linked receptor-mediated aggregation and secretion responses, and also plays a minor role in G protein–coupled receptor-mediated responses.
Further studies were carried out to investigate the role of CD148 in α-granule secretion and integrin αIIbβ3 activation. P-selectin expression on the surface of platelets was used as a measure of α-granule secretion (Figure 2B). Inside-out activation of αIIbβ3 integrin was detected using JON/A antibody binding, which only recognizes the high affinity or active conformation of the integrin (Figure 2C). As for the aggregation and ATP secretion findings, CRP-mediated P-selectin expression and integrin αIIbβ3 activation were markedly reduced in the CD148-deficient platelets compared with wild-type platelets, whereas thrombin-mediated responses were marginally reduced (Figure 2B,C). These findings further demonstrate the critical role of CD148 downstream of GPVI but also confirm that it plays a minor role in signaling through the mouse platelet thrombin receptor, PAR-4.
GPVI proximal signaling defect in CD148-deficient platelets
We next investigated the molecular mechanism of the GPVI-mediated functional defects in CD148-deficient platelets by analyzing tyrosine phosphorylation in resting and CRP-stimulated platelets. There was a striking inhibition of the increase in tyrosine phosphorylation of most proteins to CRP in the CD148-deficient platelets throughout a 300-second time course (Figure 3A). This included a marked reduction in tyrosine phosphorylation of the 12-kDa doublet that has been previously identified as FcR γ-chain, thereby suggesting a proximal signaling defect in the GPVI pathway.35,36 Consistent with this observation, there was a reduction in inducible tyrosine phosphorylation of important downstream signaling molecules, such as Syk and PLCγ2 (Figure 3B,C).
GPVI proximal signaling is defective in CD148-deficient platelets. (A) Whole-cell lysates (WCLs) prepared of resting and collagen-related peptide (CRP)–activated platelets from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were Western blotted with an anti-phosphotyrosine antibody (p-Tyr). Platelets were stimulated with 10 μg/mL CRP for 90 and 300 seconds (sec). Bands corresponding to Src family kinases (SFKs) and FcR γ-chain are indicated. (B) Syk and (C) PLCγ2 were immunoprecipitated from equal amounts of WCLs and blotted with a anti-phosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti-Syk and anti-PLCγ2 antibodies. (Di-vi) WCLs prepared of platelets stimulated for 0, 30, and 90 seconds with CRP were Western blotted for (Di) Lyn p-Tyr-507, (Diii) Src p-Tyr-529, and (Dv) SFK activation loop p-Tyr. Blots are representative of 4 to 6 experiments. (Dii, iv, vi) Band intensities were quantified from 4 separate experiments (mean ± SEM; *P < .05, **P < .01).
GPVI proximal signaling is defective in CD148-deficient platelets. (A) Whole-cell lysates (WCLs) prepared of resting and collagen-related peptide (CRP)–activated platelets from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were Western blotted with an anti-phosphotyrosine antibody (p-Tyr). Platelets were stimulated with 10 μg/mL CRP for 90 and 300 seconds (sec). Bands corresponding to Src family kinases (SFKs) and FcR γ-chain are indicated. (B) Syk and (C) PLCγ2 were immunoprecipitated from equal amounts of WCLs and blotted with a anti-phosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti-Syk and anti-PLCγ2 antibodies. (Di-vi) WCLs prepared of platelets stimulated for 0, 30, and 90 seconds with CRP were Western blotted for (Di) Lyn p-Tyr-507, (Diii) Src p-Tyr-529, and (Dv) SFK activation loop p-Tyr. Blots are representative of 4 to 6 experiments. (Dii, iv, vi) Band intensities were quantified from 4 separate experiments (mean ± SEM; *P < .05, **P < .01).
We hypothesized that the signaling defect might be due to defective SFK activation, as these kinases are responsible for FcR γ-chain ITAM phosphorylation, which was markedly reduced in CD148-deficient platelets. We therefore turned our attention to the GPVI-associated SFK, Lyn, which directly phosphorylates the FcR γ-chain.5,37 In the absence of agonist stimulation, Lyn exhibited increased phosphorylation at its C-terminal inhibitory tyrosine residue, Tyr-507, in CD148-deficient platelets, suggesting that a higher proportion of Lyn was in an inactive conformation in resting CD148-deficient platelets (Figure 3Di,ii). Phosphorylation of this site remained approximately 2-fold higher in CD148-deficient platelets compared with controls after CRP stimulation. Src also exhibited increased phosphorylation at inhibitory Tyr-529 in resting CD148-deficient platelets (Figure 3Diii,iv). In line with this, SFKs were markedly hypophosphorylated at their respective activation loop tyrosine residues in CD148-deficient platelets (Figure 3Dv,vi). Because this is a trans-autophosphorylation event, it is indicative of SFK activity and suggests a general reduction in SFK activity in the absence of CD148. The level of phosphorylation at the inhibitory site was not substantially altered by CRP stimulation in wild-type and mutant platelets; however, a significant (approximately 20%; P < .02) increase in phosphorylation of the activation loop tyrosine of SFKs was observed in wild-type platelets (Figure 3Dv,vi). Together, these results demonstrate that SFKs are in a state of reduced activation in both resting and CRP-stimulated CD148-deficient platelets.
Impaired spreading of CD148-deficient platelets on fibrinogen
A series of experiments were undertaken to investigate whether CD148 contributes to signaling by αIIbβ3. We initially investigated the ability of CD148-deficient platelets to adhere to and spread on a fibrinogen-coated surface under static conditions. Although several SFKs interact with the cytoplasmic tail of the β3 subunit, including Src, Fyn, Lyn, Hck, and Yes, only Src has been shown to be essential for αIIbβ3-mediated signaling and spreading on fibrinogen.7,8,38,39 CD148-deficient platelets exhibited a marked reduction in spreading on a fibrinogen-coated surface, comparable with that seen when platelets were pretreated with the general SFK inhibitor PP1 (Figure 4A). The block in fibrinogen spreading could be overcome by preactivating CD148-deficient platelets with the PAR-4 agonist, thrombin (Figure 4A). These findings suggest that CD148 is a positive regulator of αIIbβ3 proximal signaling, as is the case for GPVI.
CD148 is essential for αIIbβ3-mediated platelet spreading. (Ai) Washed platelets (2 × 107/mL) from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were treated with 10 μM indomethacin and 2 U/mL apyrase in the absence (basal) and presence of either 10 μM PP1 or 1 U/mL thrombin before being placed on a fibrinogen-coated surface for 45 minutes. Platelets were fixed and images captured by differential interference contrast (DIC) microscopy (5 μm scale bar). (Aii) Surface areas of platelets per condition shown in (Ai) were measured using ImageJ software (mean ± SEM; n = 250-500 individual platelets per condition). (Bi) Representative DIC images of WT and KO platelets captured in real time at 0, 5, 10, 15, and 20 minutes (min) after being placed on a fibrinogen-coated surface, in the presence of 10 μM indomethacin and 2 U/mL apyrase. (Bii) Surface areas of individual platelets were measured at various time points during spreading on fibrinogen using ImageJ software (mean ± SEM; n = 6-10 platelets per time point). P value was calculated by 2-way analysis of variance. Results are representative of 3 WT and 3 KO mice. See also Figure S4 Videos S1,S2.
CD148 is essential for αIIbβ3-mediated platelet spreading. (Ai) Washed platelets (2 × 107/mL) from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were treated with 10 μM indomethacin and 2 U/mL apyrase in the absence (basal) and presence of either 10 μM PP1 or 1 U/mL thrombin before being placed on a fibrinogen-coated surface for 45 minutes. Platelets were fixed and images captured by differential interference contrast (DIC) microscopy (5 μm scale bar). (Aii) Surface areas of platelets per condition shown in (Ai) were measured using ImageJ software (mean ± SEM; n = 250-500 individual platelets per condition). (Bi) Representative DIC images of WT and KO platelets captured in real time at 0, 5, 10, 15, and 20 minutes (min) after being placed on a fibrinogen-coated surface, in the presence of 10 μM indomethacin and 2 U/mL apyrase. (Bii) Surface areas of individual platelets were measured at various time points during spreading on fibrinogen using ImageJ software (mean ± SEM; n = 6-10 platelets per time point). P value was calculated by 2-way analysis of variance. Results are representative of 3 WT and 3 KO mice. See also Figure S4 Videos S1,S2.
We further investigated the fibrinogen-spreading defect of CD148-deficient platelets by measuring the kinetics of spreading in real time. Results from these experiments were complimentary to the endpoint spreading data (above), providing a more complete picture of the fibrinogen-spreading defect. In this assay, CD148-deficient platelets took longer to form filopodia and generated fewer filopodia, which tended to retract with time (Figure 4B). Further, they rarely formed lamellipodia, compared with wild-type platelets, which underwent lamellipodia formation, as illustrated by the surface area (Figure 4B, Figure S4 Videos S1,S2). Together, these findings demonstrated that CD148 is a positive regulator of αIIbβ3 signaling and is essential for normal platelet spreading on fibrinogen.
αIIbβ3 proximal signaling defect in CD148-deficient platelets
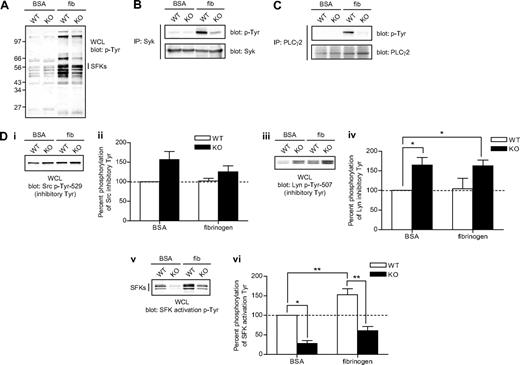
We next investigated the molecular mechanism underlying the fibrinogen-spreading defect exhibited by CD148-deficient platelets. We hypothesized that this is due to a signaling defect as platelets from mutant mice expressed normal levels of integrin αIIbβ3 (Figure S2). Consistent with this hypothesis, whole-cell lysates prepared from fibrinogen-spread platelets and subjected to Western blotting with an anti-phosphotyrosine antibody revealed a reduction in phosphorylation in CD148-deficient platelets, providing evidence of a proximal signaling defect (Figure 5A). Two of these proteins, Syk and PLCγ2, were hypophosphorylated in fibrinogen-spread CD148-deficient platelets (Figure 5B,C). Both Syk and PLCγ2 are essential components of the αIIbβ3 signaling cascade and lie downstream of SFKs. Because Src interacts with the β3 subunit in platelets, is essential for initiating and propagating αIIbβ3 signaling, and has also been shown to be a substrate of CD148,8,40 we investigated the hypothesis that Src was in an inactive conformation in fibrinogen-spread CD148-deficient platelets relative to controls. In line with this hypothesis and the result for GPVI activation, Src was hyperphosphorylated at its C-terminal inhibitory tyrosine residue (Tyr-529), as was Lyn (Tyr-507), in fibrinogen-spread CD148-deficient platelets (Figure 5Di-iv). Concomitantly, SFKs were significantly hypophosphorylated at their activation loop tyrosines in BSA-nonadherent (approximately 30%; P < .05) and fibrinogen-adherent (approximately 46%; P < .02) CD148-deficient platelets compared with controls (Figure 5Dv,vi). These findings suggest that SFKs tend to be in a closed, inactive conformation in CD148-deficient platelets. Together, these results demonstrate that CD148 positively regulates SFKs downstream of αIIbβ3, providing a molecular mechanism for the fibrinogen-spreading defect exhibited by CD148-deficient platelets.
Defective αIIbβ3 signaling in CD148-deficient platelets. Platelets from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were plated on BSA- and fibrinogen-coated surfaces for 45 minutes. Whole cell lysates (WCLs) were prepared of BSA (BSA) nonadherent and fibrinogen (fib) adherent platelets. (A) Equal amounts of total protein were resolved by SDS-PAGE and Western blotted with an anti-phosphotyrosine antibody (p-Tyr). (B) Syk and (C) PLCγ2 were immunoprecipitated from equal amounts of WCLs and blotted with an anti-phosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti-Syk and anti-PLCγ2 antibodies. (Di-vi) WCLs were Western blotted with (Di) an anti-Src p-Tyr-529 antibody, (Diii) an anti-Lyn p-Tyr-507 antibody, and (Dv) an anti-Src family kinase (SFK) activation loop p-Tyr antibody. Blots are representative of 4 to 6 experiments. (Dii, iv, vi) Band intensities were quantified from 4 separate experiments (mean ± SEM; *P < .05, **P < .01).
Defective αIIbβ3 signaling in CD148-deficient platelets. Platelets from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were plated on BSA- and fibrinogen-coated surfaces for 45 minutes. Whole cell lysates (WCLs) were prepared of BSA (BSA) nonadherent and fibrinogen (fib) adherent platelets. (A) Equal amounts of total protein were resolved by SDS-PAGE and Western blotted with an anti-phosphotyrosine antibody (p-Tyr). (B) Syk and (C) PLCγ2 were immunoprecipitated from equal amounts of WCLs and blotted with an anti-phosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti-Syk and anti-PLCγ2 antibodies. (Di-vi) WCLs were Western blotted with (Di) an anti-Src p-Tyr-529 antibody, (Diii) an anti-Lyn p-Tyr-507 antibody, and (Dv) an anti-Src family kinase (SFK) activation loop p-Tyr antibody. Blots are representative of 4 to 6 experiments. (Dii, iv, vi) Band intensities were quantified from 4 separate experiments (mean ± SEM; *P < .05, **P < .01).
CD148-deficient megakaryocytes do not spread or migrate
To determine whether CD148 is important in megakaryocytes, the platelet progenitor, we investigated the ability of CD148-deficient megakaryocytes to spread on various surfaces and migrate on fibronectin. It is well established that integrins and SFKs are essential for cell movement, and in light of the platelet-spreading and αIIbβ3 signaling defects observed in CD148-deficient platelets, we hypothesized that megakaryocytes would exhibit similar functional defects. Indeed, CD148-deficient megakaryocytes exhibited reduced spreading on collagen-, fibrinogen- and fibronectin-coated surfaces (Figure 6A). Spreading on collagen is mediated by GPVI and the integrin α2β1, on fibrinogen by the integrin αIIbβ3, and on fibronectin by α5β1 and αIIbβ3. Further, CD148-deficient megakaryocytes failed to migrate toward a SDF-1α gradient over a fibronectin-coated surface (Figure 6B, Figure S6 Videos S3,S4). These findings raised the possibility of a general integrin functional defect in megakaryocytes and platelets in the absence of CD148. Biochemical data demonstrated reduced SFK activity in nonstimulated CD148-deficient megakaryocytes, supporting a common function of CD148 in regulating basal SFK activity in megakaryocytes and platelets (Figure 6C).
CD148-deficient megakaryocytes do not spread or migrate. (Ai) Cultured bone marrow–derived megakaryocytes from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were plated on fibrinogen-, collagen-, and fibronectin-coated surfaces. Adherent megakaryocytes were fixed, permeablized, and actin fibers stained with rhodamine-phalloidin. Representative images from 3 separate experiments (20 μm scale bar). (Aii) The surface area of spread megakaryocytes was quantified and presented as mean ± SEM. (Bi) WT and KO megakaryocytes were placed on a fibronectin-coated surface and allowed to migrate toward a SDF-1α gradient in a Dunn chamber. Images were captured at the indicated times by differential interference contrast microscopy (20 μm scale bar). (Bii) The direction and distance traveled by individual megakaryocytes was tracked in real time and plotted as shown. Each trace was generated by a single megakaryocyte over a 4-hour period. Representative images from 3 separate experiments. See also Figure S6 Videos S3,S4. (C) Whole-cell lysates (WCLs) prepared of nonadherent WT, and KO megakaryocytes were Western blotted with an anti-Src family kinase (SFK) activation p-Tyr antibody. Membranes were stripped and reblotted with an anti–Src-pan antibody (Src). Representative images from 2 experiments. (D) Delayed thrombopoiesis in CD48 TM-KO mice after complement-mediated immune thrombocytopenia. Thrombocytopenia was induced in WT and KO mice by an intraperitoneal injection of anti–mouse GPIbα antibody (2 μg/g of mouse). Peripheral platelet counts were measured 7 days before injection (time = 0) and at 3, 48, 72, 96, 120, 144, and 172 hours after injection (n = 3-4 mice per time point). Mean platelet count (± SEM) was plotted as a function of time (**P < .01).
CD148-deficient megakaryocytes do not spread or migrate. (Ai) Cultured bone marrow–derived megakaryocytes from wild-type (WT) and CD148 transmembrane-knockout (KO) mice were plated on fibrinogen-, collagen-, and fibronectin-coated surfaces. Adherent megakaryocytes were fixed, permeablized, and actin fibers stained with rhodamine-phalloidin. Representative images from 3 separate experiments (20 μm scale bar). (Aii) The surface area of spread megakaryocytes was quantified and presented as mean ± SEM. (Bi) WT and KO megakaryocytes were placed on a fibronectin-coated surface and allowed to migrate toward a SDF-1α gradient in a Dunn chamber. Images were captured at the indicated times by differential interference contrast microscopy (20 μm scale bar). (Bii) The direction and distance traveled by individual megakaryocytes was tracked in real time and plotted as shown. Each trace was generated by a single megakaryocyte over a 4-hour period. Representative images from 3 separate experiments. See also Figure S6 Videos S3,S4. (C) Whole-cell lysates (WCLs) prepared of nonadherent WT, and KO megakaryocytes were Western blotted with an anti-Src family kinase (SFK) activation p-Tyr antibody. Membranes were stripped and reblotted with an anti–Src-pan antibody (Src). Representative images from 2 experiments. (D) Delayed thrombopoiesis in CD48 TM-KO mice after complement-mediated immune thrombocytopenia. Thrombocytopenia was induced in WT and KO mice by an intraperitoneal injection of anti–mouse GPIbα antibody (2 μg/g of mouse). Peripheral platelet counts were measured 7 days before injection (time = 0) and at 3, 48, 72, 96, 120, 144, and 172 hours after injection (n = 3-4 mice per time point). Mean platelet count (± SEM) was plotted as a function of time (**P < .01).
Because SDF-1α–mediated megakaryocyte migration from the endosteal niche to the vascular niche is essential for platelet release into the circulation,41 we also measured platelet recovery in wild-type and CD148 TM-KO mice made thrombocytopenic through intraperitoneal injection of anti-GPIbα antibody. Platelet counts were normal in unchallenged CD148 TM-KO mice but showed a delayed recovery postinjection of anti-GPIbα antibody compared with control mice (Figure 6D). Platelet counts eventually returned to normal in CD148 TM-KO mice 172 hours postinjection. These defects were similar to those previously reported in PECAM-1–deficient megakaryocytes.31
Reduced aggregate formation of CD148-deficient platelets on collagen under flow
Further studies were performed to investigate platelet activation on collagen at arteriolar shear, as a more physiologically relevant model of platelet function. Platelet adhesion and aggregate formation on collagen was measured by flowing anticoagulated blood from wild-type and CD148 TM-KO mice through collagen-coated capillary tubes under arterial flow conditions (1000 s−1). Under these conditions, CD148-deficient platelets exhibited impaired adhesion and failed to support formation of large aggregates compared with wild-type platelets, leaving primarily a monolayer of single platelets with occasional small aggregates (Figure 7A, Figure S7 Videos S5,S6). Moreover, at higher magnification, a large proportion of collagen adherent CD148-deficient platelets exhibited filopodia formation, but lacked the characteristic lamellipodia of collagen-activated platelets, confirming a defect in activation (Figure 7A). The severe defect in aggregate formation on collagen under flow in the absence of CD148 is consistent with the marked impairment in collagen-induced aggregation and secretion described above. Interestingly, however, the defect is not as severe as the almost total abolition of adhesion of GPVI-FcR γ-chain complex–deficient (γ-chain−/−) platelets, as previously described.27,42 This difference can be partially explained by the fact that collagen signaling is markedly reduced, but not completely abolished in the absence of CD148.
CD148 positively regulates platelet aggregate formation on collagen under flow and thrombus formation in vivo. (Ai) Anticoagulated blood from wild-type (WT) and CD148 transmembrane-knockout (KO) mice was flowed through collagen-coated capillary tubes at 1000 s−1. Platelets were fluorescently labeled with DiOC6 before being flowed. Representative images were taken in real time by fluorescence microscopy (10 μm scale bar). (Aii) Differential interference contrast (DIC) images of fixed platelets on collagen fibrils after being flowed through collagen-coated capillary tubes at 1000 s−1 for 4 minutes (10 μm scale bar). (Aiii) High magnification DIC images of adherent platelets from panel (Aii) (5 μm scale bar). Results are representative of 3 WT and 3 KO mice. See also Figure S7 Videos S5,S6. (B-D) The functional role of CD148 in thrombosis and hemostasis was investigated using 3 different in vivo assays: (B) the tail bleeding assay, (C) the ferric chloride injury model, and (D) the laser injury model. (B) CD148 transmembrane-knockout (CD148 TM-KO) mice exhibited prolonged bleeding compared with litter-matched WT mice, after excision of a small portion of the tail tip. Symbols represent individual mice. Horizontal lines intersecting datasets represent the mean. (Ci) FcR γ-chain–deficient (γ-chain−/−) and (Cii) CD148 TM-KO mice exhibited delayed vascular occlusion after ferric chloride–induced injury of mesenteric arterioles compared with litter-matched WT mice. (Di) Platelets from WT and CD148 TM-KO mice were fluorescently labeled ex vivo with rat anti–mouse αIIb primary antibody and Alex488-conjugated secondary antibody before being reintroduced into recipient mice. Arterioles in cremaster muscles of recipients were subsequently injured by laser, and the accumulation of platelets (green) into the thrombi was assessed. Representative images from 5 WT and 5 CD148 TM-KO mice are shown. (Dii) Each curve represents the median integrated thrombus fluorescence intensity in arbitrary units (a.u.) for 25 thrombi induced in 5 mice of each genotype. See also Figure S7 Videos S7,S8.
CD148 positively regulates platelet aggregate formation on collagen under flow and thrombus formation in vivo. (Ai) Anticoagulated blood from wild-type (WT) and CD148 transmembrane-knockout (KO) mice was flowed through collagen-coated capillary tubes at 1000 s−1. Platelets were fluorescently labeled with DiOC6 before being flowed. Representative images were taken in real time by fluorescence microscopy (10 μm scale bar). (Aii) Differential interference contrast (DIC) images of fixed platelets on collagen fibrils after being flowed through collagen-coated capillary tubes at 1000 s−1 for 4 minutes (10 μm scale bar). (Aiii) High magnification DIC images of adherent platelets from panel (Aii) (5 μm scale bar). Results are representative of 3 WT and 3 KO mice. See also Figure S7 Videos S5,S6. (B-D) The functional role of CD148 in thrombosis and hemostasis was investigated using 3 different in vivo assays: (B) the tail bleeding assay, (C) the ferric chloride injury model, and (D) the laser injury model. (B) CD148 transmembrane-knockout (CD148 TM-KO) mice exhibited prolonged bleeding compared with litter-matched WT mice, after excision of a small portion of the tail tip. Symbols represent individual mice. Horizontal lines intersecting datasets represent the mean. (Ci) FcR γ-chain–deficient (γ-chain−/−) and (Cii) CD148 TM-KO mice exhibited delayed vascular occlusion after ferric chloride–induced injury of mesenteric arterioles compared with litter-matched WT mice. (Di) Platelets from WT and CD148 TM-KO mice were fluorescently labeled ex vivo with rat anti–mouse αIIb primary antibody and Alex488-conjugated secondary antibody before being reintroduced into recipient mice. Arterioles in cremaster muscles of recipients were subsequently injured by laser, and the accumulation of platelets (green) into the thrombi was assessed. Representative images from 5 WT and 5 CD148 TM-KO mice are shown. (Dii) Each curve represents the median integrated thrombus fluorescence intensity in arbitrary units (a.u.) for 25 thrombi induced in 5 mice of each genotype. See also Figure S7 Videos S7,S8.
Delayed thrombus formation in CD148 TM-KO mice
The physiologic role of CD148 in thrombosis and hemostasis was investigated using 3 complimentary in vivo assays: (1) the tail bleeding assay, which measures blood loss after excision of a small portion of the tail tip; (2) the ferric chloride–induced vascular occlusion model, which measures the time until a thrombus occludes a chemically induced injury to an arteriole; and (3) the laser-induced thrombus formation model, which measures thrombus formation as a function of time. In the tail-bleeding assay, blood loss was measured as a percentage of the permitted maximum. In this assay, CD148-deficient mice bled significantly more than litter-matched wild-type mice (wild-type: 26.2 ± 10.3% permitted blood loss in 10 minutes [mean ± SEM] vs CD148 TM-KO: 55.5 ± 10.2%, P < .03; Figure 7B). Further, CD148 TM-KO mice exhibited a bimodal distribution of bleeding, with some mice bleeding excessively while others bled to the same extent as wild-type mice (Figure 7B). This distribution was similar to the tail bleeding phenotype exhibited by GPVI-deficient mice and suggests the possible contribution of a modifier locus.43
In the ferric chloride–induced vascular occlusion model, thrombus formation was visualized in real time by fluorescent intravital microscopy. We initially tested γ-chain−/− mice, which also do not express GPVI, in this assay to confirm a role for platelets in contributing to thrombus formation after treatment with ferric chloride and also to aid comparison to the result obtained in the absence of CD148.22,27,36 As expected, γ-chain−/− mice exhibited a significantly longer time to vessel occlusion compared with litter-matched wild-type mice (wild-type, 28.3 ± 10 minutes [mean ± SEM] vs γ-chain−/−, 55.4 ± 7.4 minutes, P < .001; Figure 7Ci). The time to vessel occlusion was also significantly increased in CD148 TM-KO mice compared with litter-matched wild-type mice (wild-type, 20.2 ± 2.9 minutes vs CD148 TM-KO, 36.5 ± 4.2 minutes, P < .01; Figure 7Cii).
Laser-induced vessel injury performed in this study generates less tissue damage than ferric chloride, normally ablating a relatively small number of endothelial cells lining the lumen of the vessel. The Furie group have shown this model to be heavily dependent on thrombin generation and PAR-4,44,45 but we and others have found the model to be dependent on GPVI and its downstream signaling proteins,32,46,47 possibly due to different severities of injury between different groups. In our model, we found that thrombus formation was severely delayed, peak thrombus size was significantly reduced, and thrombi receded more rapidly in CD148 TM-KO mice (Figure 7D, Figure S7 Videos S7,S8). This suggests that thrombus formation and stability were compromised in mutant mice. Together with the bleeding and ferric chloride–induced vascular occlusion data, these results demonstrate that CD148 is an essential positive regulator of hemostasis and thrombosis.
Discussion
This is the first study of the functional role of CD148, the only RPTP expressed in platelets, in hemostasis and thrombosis in vivo.21 The present study shows the following: (1) CD148 is a global regulator of SFKs in platelets. (2) CD148 plays a critical role in signaling through GPVI and the major platelet integrin, αIIbβ3. (3) CD148 contributes to signaling by the G protein-coupled receptors for thrombin and TxA2, PAR-4, and TP, respectively. (4) CD148 is required for megakaryocyte spreading and migration on a variety of surfaces. (5) CD148 plays a vital role in mediating platelet aggregation under flow and in hemostasis and thrombus formation in vivo, with the latter being shown using 3 distinct models. These findings, in addition to the absence of pathologic hemorrhaging in mice lacking cell surface expression of CD148, make this RPTP an attractive novel antithrombotic drug target.
Platelet functional responses are regulated by a diverse repertoire of tyrosine kinase–linked and G protein–coupled receptors. SFKs are essential for initiating and propagating signaling from several major platelet tyrosine kinase-linked receptors. They also contribute to signaling downstream of several stimulatory G protein–coupled receptors.48,49 SFK activity is tightly regulated by tyrosine phosphorylation and intramolecular interactions that constrain the kinase domain.10,11 Phosphorylation of the C-terminal inhibitory tyrosine maintains the SFK in an inactive conformation, whereas dephosphorylation of this site in parallel with trans-autophosphorylation of the activation loop tyrosine renders it maximally active.15,50,51 One of the main findings of this study was that global SFK activity, as detected using phosphospecific antibodies, was significantly lower in both resting and activated CD148-deficient platelets compared with wild-type platelets, demonstrating for the first time that CD148 is required for maintaining a pool of active SFKs in resting platelets and increasing SFK activity upon platelet activation. We hypothesize that the pool of active SFKs in resting platelets is to maintain essential constitutive biologic processes and to initiate a rapid response to a stimulatory signal.
Until now, the earliest characterized signaling event downstream of GPVI is the activation of constitutively associated SFKs.5,6,37 It has yet to be elucidated, however, how SFKs become activated after GPVI-collagen engagement.6 Our biochemical data demonstrated a GPVI proximal signaling defect in the absence of CD148 as tyrosine phosphorylation of the FcR γ-chain, Syk, and PLCγ2 were all substantially reduced in response to CRP. Consistent with a major signaling defect, functional and biochemical defects exhibited by CD148-deficient platelets were substantially more severe than those observed in platelets from FcR γ-chain heterozygous-deficient mice, which express a similar reduction in the GPVI-FcR γ-chain complex.27,34 Additionally, SFKs exhibited significantly reduced phosphorylation of their activation loop tyrosines in parallel with increased phosphorylation of their C-terminal inhibitory tyrosines in the absence of CD148. These findings demonstrate that CD148 plays a critical role in activating SFKs downstream of GPVI. CD148 would therefore appear to play a similar role to that of the structurally distinct RPTP CD45 in B- and T-cell receptor signaling.16,20
The reduction in the signaling response to engagement of integrin αIIbβ3 in the absence of CD148 is similarly explained by the altered tyrosine phosphorylation of the SFKs. Interestingly, the group of Shattil has also demonstrated a role for the nontransmembrane tyrosine phosphatase, PTP-1B, in signaling by the major platelet integrin, although it does not play a role in platelet activation by the snake venom toxin, convulxin.9 Thus, CD148 and PTP-1B appear to work together to regulate αIIbβ3 integrin signaling.
Results from this study demonstrate a critical role of CD148 in regulating global SFK activity in platelets and suggest that SFKs may be direct physiologic substrates of CD148. In support of this hypothesis, CD148 has previously been shown to interact directly with Src and to dephosphorylate both of its regulatory phosphorylation sites in vitro.40 The interaction was also observed in transfected cells; however, CD148 only dephosphorylated the inhibitory site and not the activation site in transfected cells.40 Other potential physiologic substrates of CD148 in platelets and other cells include: the tyrosine kinase-linked receptors Met and PDGFβ, the adapter proteins LAT and Gab1, the adherens junction protein p120catenin, PLCγ1, and more recently the p85 subunit of PI 3-kinase, although the latter is not tyrosine phosphorylated in platelets.52–57 Interestingly, LAT, PLCγ1 and PI 3-kinase all lie downstream of SFKs in the GPVI signaling cascade; therefore, CD148 may be regulating multiple points of the GPVI signaling pathway.58–61
Residual GPVI signaling in CD148-deficient platelets raises the possibility that one or more other PTPs partially compensate for the absence of CD148. Because we were unable to identify another RPTP in platelets using a proteomic approach, we hypothesize that a nontransmembrane PTP may fulfill the role as has been shown to be the case for signaling by αIIbβ3.7–9,21 Possible candidates include the SH2 domain-containing PTPs, Shp1 and Shp2, as they have been previously shown to regulate ITAM receptor signaling in immune cells. Shp1 has also been shown to interact with Src in platelets and to positively regulate Src activation by preferentially dephosphorylating inhibitory Tyr-529.62 In support of this model, Shp1 was demonstrated to play a positive regulatory role in GPVI-mediated platelet activation.63 This was shown through the use of naturally occurring motheaten viable mice, which have reduced Shp1 activity.63
A question that arises from our proposed mechanism is how approximately 2800 copies of CD148 molecules can regulate the pools of SFKs associated with approximately 4000 copies of GPVI and more than 80 000 copies of αIIbβ3.33,64 We hypothesize the explanation lies in the high catalytic activity of PTPs, which have kcat values up to 3 orders of magnitude greater than those of protein tyrosine kinases.65 Regulation of CD148 activity and localization in the platelet plasma membrane are now critical to understanding how CD148 regulates both GPVI and αIIbβ3 signaling. This might be mediated by interaction with a ligand or through compartmentalization into membrane microdomains. The ligand for CD148 is presently not known. One report, however, suggests that it may be an extracellular matrix protein.66 Moreover, work done in Jurkat T cells has shown that CD148 can be regulated by membrane compartmentalization, as it is excluded from the immunologic synapse by mechanical forces.18,19
Both our ex vivo flow adhesion studies and in vivo analysis of hemostasis and thrombosis indicate that CD148 plays a novel physiologic role in preventing blood loss from sites of vascular injury. The lack of evidence for a severe bleeding diathesis in CD148 mutant mice makes it a potentially exciting antithrombotic drug target. Structural and functional features of CD148 also lend it to drug targeting, including its large extracellular domain that could be targeted by small molecule inhibitors without the need to cross the plasma membrane. These features, together with its unique function in platelets, make it an ideal target for development of a new class of antiplatelet agents.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all members of the Birmingham Biomedical Sciences Unit for the exceptionally high standard at which mouse colonies are maintained. We also would like to thank Professor Shaun Coughlin from the Cardiovascular Research Institute, University of California San Francisco, for allowing us to perform initial aggregation studies on the CD148 TM-KO mice in his laboratory; Dr Daniel Palmer from the Coughlin laboratory for assisting with the initial aggregation studies; and Mr Majd Protty from the University of Birmingham for excellent technical assistance.
Y.A.S. is a British Heart Foundation (BHF) Intermediate Research Fellow (FS/08/034/25 085); M.G.T. is a BHF Senior Research Fellow (FS/08/062/25 797); S.P.W. holds a BHF Chair. This work was supported by the BHF (PG/07/034/22 775, FS/05/085/19 460, CH/03/003); Wellcome Trust (073107); National Institutes of Health (AI066120); MRC New Investigator Award to MGT (10 717); and MRC Cooperative Grant (65 282). Finally, we thank the Wellcome Trust and British Society for Hemostasis and Thrombosis for providing travel grants to Y.A.S. and M.G.T. to visit A.W.'s laboratory and initiate studies on CD148 TM-KO mice.
National Institutes of Health
Wellcome Trust
Authorship
Contribution: Y.A.S. and M.G.T. designed research, performed research, collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; S.E., A.M., J.L., and Y.Z. performed research, collected data, analyzed and interpreted data, and performed statistical analysis; K.N.K. performed research, collected data, and analyzed and interpreted data; J.M.A. and S.G.T. performed research, collected data, analyzed and interpreted data, and performed statistical analysis; T.D. and N.K. contributed analytical tools; J.W.Z. and A.W. contributed vital new reagents; and S.P.W. contributed analytical tools and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yotis A. Senis, Centre for Cardiovascular Sciences, Institute of Biomedical Research, School of Clinical and Experimental Medicine, College of Medical and Dental Sciences, University of Birmingham, Wolfson Dr, Edgbaston, Birmingham, B15 2TT, United Kingdom; e-mail: y.senis@bham.ac.uk.
References
Author notes
*Y.A.S. and M.G.T. contributed equally to this work.