Spectrin and ankyrin participate in membrane organization, stability, signal transduction, and protein targeting; their interaction is critical for erythrocyte stability. Repeats 14 and 15 of βI-spectrin are crucial for ankyrin recognition, yet the way spectrin binds ankyrin while preserving its repeat structure is unknown. We have solved the crystal structure of the βI-spectrin 14,15 di-repeat unit to 2.1 Å resolution and found 14 residues critical for ankyrin binding that map to the end of the helix C of repeat 14, the linker region, and the B-C loop of repeat 15. The tilt (64°) across the 14,15 linker is greater than in any published di-repeat structure, suggesting that the relative positioning of the two repeats is important for ankyrin binding. We propose that a lack of structural constraints on linker and inter-helix loops allows proteins containing spectrin-like di-repeats to evolve diverse but specific ligand-recognition sites without compromising the structure of the repeat unit. The linker regions between repeats are thus critical determinants of both spectrin's flexibility and polyfunctionality. The putative coupling of flexibility and ligand binding suggests a mechanism by which spectrin might participate in mechanosensory regulation.
Introduction
First discovered in the human erythrocyte and closely associated with a variety of familial hemolytic anemias, the spectrin-ankyrin cytoskeleton has emerged as the classical paradigm of a polyfunctional organizing membrane scaffold. Ubiquitous in higher eukaryotes, the spectrin-ankyrin skeleton contributes to membrane stability, the organization of membrane proteins and lipids, the recruitment to membranes of cytosolic proteins and signaling complexes, the tethering of organized protein mosaics to filamentous actin or to the motors effecting microtubule-directed transport, and the facilitated transport of membrane proteins through the secretory and endocytic pathways.1 Reflecting these diverse but fundamental roles, hereditary or experimental disruption of spectrin or ankyrin leads to many pathologies, including hemolytic disease,2 embryonic lethality, cancer and developmental defects,3 pump and channel failures and endoplasmic reticulum (ER) retention disorders,4 neuromuscular syndromes and sudden cardiac death.5,6
The participation of spectrin and ankyrin in so many cellular processes reflects their ability to organize multiple membrane and cytosolic proteins and lipids into membrane microdomains, linking them to the filamentous skeleton. Polyfunctionality is a critical attribute of both proteins. Ankyrins derive this capacity largely by the juxtaposition of multiple 33-residue repeat units, each composed of 2 helices linked by a β-turn.7 Selectivity is achieved by minor sequence variation within the β-turn of each ankyrin-repeat and by the juxtaposition of repeats with differing sequence. Less is known about how spectrin binds its ligands with high affinity and specificity. In humans, there are 7 spectrin genes encoding 5 β-spectrins and 2 α-spectrins. These usually exist as antiparallel αβ heterodimers that undergo self-association to form tetramers and higher oligomers. Each spectrin is composed of multiple triple helical units of ∼106 residues. Other members of the spectrin gene superfamily include α-actinin, utrophin, and dystrophin; spectrin-like repeats also are found in unrelated proteins, including kalirin, plectin, MACF1, AKAP6, Syne-1, and Syne-2 (Nesprin-1 and 2).8,–10 Only 5 canonical protein-protein or lipid interaction motifs are found within the spectrins: (1) 2 calponin homology (CH) domains near the N terminus of β spectrin responsible for actin and dynactin binding11,12 ; (2) EF-hand domains near the C terminus of the α-spectrins that bind Ca2+13 ; (3) a SH3 domain inserted near the middle of α-spectrin14 ; (4) the calmodulin-binding domain also near the middle of αII-spectrin15 ; and (5) the PH domain near the C terminus of several β-spectrins.16 All other ligands, including ankyrin, the Lutheran (Lu) blood group antigen,17 N-CAM,18 EAAT4,6 NMDA-R2,19 and lipids,20 bind to regions of spectrin composed only of triple helical spectrin repeats (summarized in De Matteis and Morrow1 ). In other proteins also, spectrin-like repeats are responsible for binding specific ligands such as a nuclear receptor21 or possibly histone deacetylase22 in the proteins mAKAP or BPAG1, respectively. Yet, few insights have emerged to explain how specificity of ligand binding is derived from regions with such a conserved and repetitive structure.
This problem has been most thoroughly explored for the interaction between erythroid βI-spectrin and ankyrin. Kennedy et al23 and Ipsaro et al24 established that the ankyrin-binding site in βI-spectrin lay between codons 1768-1898, a sequence bridging the terminal third of the 14th repeat (βI-14) and most of the 15th repeat (βI-15). This region is well conserved in all classical β-spectrins (βI-βIV); its most unusual feature is an absence of highly conserved tryptophan and histidine residues in βI-15. Otherwise, the sequence of this region reveals no features that distinguish it from other spectrin di-repeat units.
In the present work, we explore the mechanistic basis of how ankyrin binds to spectrin and address the general question of how ligand-binding specificity can be achieved within the spectrin-repeat structure. We used alanine-scanning mutagenesis to identify residues critical for ankyrin-binding within the βI-14,15 di-repeat peptide and solved its crystal structure to 2.1 Å resolution. We found 14 residues critical for ankyrin binding; they map to helix C of the 14th repeat (h14C), a helical linker between the repeat units (14-15 linker), and to the loop linking helices B and C of the 15th repeat unit (15B-C loop). These residues are either closely involved with stabilizing h14C or form a hydrophilic binding surface extending along h14C and the 14-15 linker. The residues in the loop 15B-C contribute to the binding site by stabilizing an unusual tilt between the two repeats. Restoration of the conserved Trp and His residues missing in repeat 15 does not affect ankyrin binding, suggesting that their unusual absence in βI-15 relates to other functions. We postulate that sequence variations in the linker between repeats and in helix C of the upstream repeat, together with variations in the juxtaposed loop between helices B and C of the downstream repeat, provide an attractive mechanism for modulating spectrin's flexibility and ligand-binding specificity. The implications of this mechanism for allosteric control within spectrin25 and the participation of spectrin in mechanosensing26,27 are discussed.
Methods
Cloning
The human spectrin peptides βI-14,15, residues 1680 to 1898 (accession NM_000347), and αI-14,15, residues 1489-1705 (accession NM_003126), were cloned and expressed in pGEX-4T (Invitrogen, Carlsbad, CA) as glutathione S-transferase (GST) fusion proteins. Residue substitutions (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) used the Quick-Change Site-Directed Mutagenesis Kit (Stratagene, Cedar Creek, TX). Full-length HIS-tagged human ankyrin R (hisAnkR) was cloned and expressed in pTRC-his-B (Invitrogen). The preparation of full-length recombinant ankyrin required special precautions. All constructs were transformed into BL21-Gold competent cells from Stratagene. Bacteria were induced with 0.5 mmol/L IPTG after reaching an O.D. 595 = 0.5 and allowed to induce over night at 18°C. This procedure yielded full-length soluble ankyrin, although at yields that were approximately 1/10 (M/M) of that realized for the smaller spectrin peptides.
Ankyrin pull-down binding assay
Bacteria expressing hisAnkR were cosedimented with bacteria expressing GST-spectrin peptide and resuspended in 1 mL binding buffer (50 mmol/L Tris, pH 8.0; 250 mmol/L NaCl; 1 mol/L ethylene diamine tetraacetic acid (EDTA); 2 mmol/L 2-mercaptoethanol (βME); 1% (vol/vol) glycerol; 0.1% (vol/vol) Triton X-100). After lysis by 3 freeze-thaw cycles and sedimentation at 21 000g for 20 minutes, the supernatant was incubated with glutathione Sepharose 4B (GE Healthcare, Waukesha, WI) at 4°C. The resin was washed 3 times with binding buffer and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) or Western blot.
Circular dichroism measurements and thermal melt analysis
GST-spectrin peptides were bound to glutathione-sepharose and cleaved with thrombin to liberate the spectrin peptide. After dilution to 10 μmol/L in phosphate-buffered saline (PBS), circular dichroism measurements and thermal melt analysis were conducted with the use of an Aviv model 215 CD scanning spectrophotometer. Secondary structure was estimated by K2d software.28
Purification and crystallization of βI-14,15
The bacterial cell paste was resuspended in lysis buffer (50 mmol/L Tris-HCl, pH 8.0; 50 mmol/L NaCl; 1 mmol/L EDTA; 2 mmol/L βME; 10% glycerol; 0.1% Triton X-100) and sonicated; after centrifugation, the supernatant was incubated with glutathione–sepharose at 4°C. The matrix was rinsed with the following buffers. (W1): 50 mmol/L Tris-HCl, pH 8.00; 300 mmol/L NaCl; 1 mmol/L EDTA; 2 mmol/L βME; 10% glycerol; 0.1% Triton X-100. (W2): 50 mmol/L Tris-HCl, pH 8.00; 50 mmol/L NaCl; 1 mmol/L EDTA; 2 mmol/L βME. (W3): 20 mmol/L Tris-HCl, pH 8.00; 50 mmol/L NaCl. After overnight thrombin treatment at 21°C, the GST-free βI peptide was eluted with 4 mL W3 buffer, concentrated to 3 mg/mL, and stored at 4°C. Crystals were obtained at 16°C by mixing equal volumes of the protein solution (3 mg/mL) and the crystallization buffer (0.1 mol/L bis-tris-propane, pH 6.2; 0.2 mol KSCN; 20% PEG 3350; 10 mmol/L spermine-tetrachloride). Se-Met–labeled protein crystals were obtained at 16°C by mixing equal volumes of the protein solution (2.5 mg/mL) with 0.1 mol/L bis-tris-propane, pH 5.8; 0.3 mol/L KSCN; 22% PEG 3350; 5 mmol/L spermine-tetrachloride/10 mmol/L NaBr. Crystals were incubated for 5 minutes in the crystallization buffer containing 20% (vol/vol) ethylene glycol before freezing in liquid propane.
Data collection, structure determination, and model refinement
Datasets were collected at beam line ID24E of the Advanced Photon Source (APS, Argonne, IL). Data were indexed, scaled, and merged with the program HKL2000.29 The initial phase was calculated with the SHELX software package.30 Structure refinement was performed with Phenix,31 whereas model rebuilding used Coot.32 Procheck and MolProbity verified the final model geometry. The complete structure factors of spectrin βI-14,15 were deposited in the Protein Data Bank (PDBID: 3edu; http://www.rcsb.org/pdb/search/structidSearch.do?structureId=3EDU).
Structure analysis and figures
Structure superpositioning was performed both in Lsqman33 and Coot.32 A rotation matrix describing structural relationship between the βI repeats was calculated by superimposing βI-15 over βI-14. The matrix was then decomposed in either Lsqman33 or CNS, version 1.2.34 The same procedure was used to evaluate the tilt angle in other spectrin structures. All figures were composed in PyMOL (PyMOL Molecular Graphics System; DeLano Scientific, San Carlos, CA).
Results
Alanine-scanning mutagenesis identifies residues critical for ankyrin binding
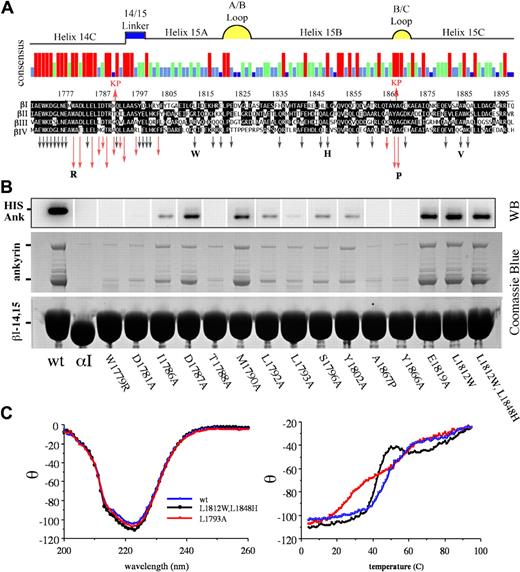
Studies that used serial truncation of βI-14,15,23 interpreted with the use of the repeat phasing suggested by structural studies of other spectrin repeats35,36 defined the boundaries of the ankyrin-binding domain of spectrin (residues 1768-1898) as portions of the h14C and all but the most distal parts of repeat 15 (Figure 1A). To determine which residues were most critical for ankyrin binding, a series of GST-βI-14,15 spectrin di-repeat peptides were evaluated in which residues were systematically mutated by site-directed mutagenesis to alanine. The selection of mutated codons was biased toward residues conserved in the 14-15 repeats of βI to βIV spectrin but not conserved across other spectrin repeats, on the assumption that residues conserved in the nonankyrin-binding repeat units were likely to be fundamental to maintaining the structural stability of the triple helical unit. The substitutions examined and their impact on ankyrin-binding are listed in Table S2 and depicted graphically by the arrows in Figure 1A; representative pull-down assay data for selected peptides is presented in Figure 1B. The native βI-14,15 peptide reliably bound both full-length ankyrin as well as the smaller ankyrin present in the recombinant lysate (Figure 1B lane 1). The genesis of the smaller ankyrin band, whether it was an incomplete transcript or a proteolytic fragment, was not explored, but its identity as hisAnkR was verified by Western blotting (Figure 1B). There was no difference in the binding of either the full-length or the smaller ankyrin. An αI-spectrin peptide (αI-14,15) served as a negative control (Figure 1B lane 2). A total of 42 mutant peptides, each with a single residue substitution, were evaluated. All substitutions were alanine unless otherwise specified. Those with diminished ankyrin-binding activity are depicted with red arrows in Figure 1A. The length of the arrow designates the relative potency of the mutation in suppressing ankyrin-binding activity as determined by densitometric analysis of 2 to 6 independent experiments (Table S2). Residues critical for ankyrin-binding activity were clustered near the C-terminus of the 14th repeat and in the 14-15 linker. Three additional residues in or adjacent to the B-C loop were also critical for ankyrin recognition.
Alanine-scanning mutagenesis identifies the critical residues within the ankyrin-binding domain of erythroid βI-spectrin. (A) Sequence alignment of the ankyrin-binding region of βI spectrin23 (codons 1768-1898) with βII, βIII, and βIV spectrin. The red arrows above the alignment labeled KP (Kennedy peptide) represent the limits of ankyrin-binding activity previously identified, ie, deletion of sequences outside this region did not perturb ankyrin-binding activity.23 The histogram summarizes the strength of homology among these spectrins; red, conserved in all 4 spectrins; green conserved in 3 of 4; light blue, conserved in 2 of 4; and dark blue, not conserved. The putative structural feature of each region of sequence also is depicted. Arrows below the alignment indicate the position of each of the 42 mutants examined in this study. Red arrows highlight amino acids that abrogated ankyrin binding; the length of the arrow approximates the inhibitory potency of the mutation. Black arrows indicate amino acid changes that had no effect. All amino acids mutations were to alanine unless otherwise indicated by the bold letters. The W1779R mutant explored the effect of deletion of the highly conserved trp at that locus; L1812W replaced the “invariant” trp that characterizes most spectrin repreats; L1848H restored the H that is conserved in other spectrin repeats; A1867P was designed to mimic the pro at this position in βV spectrin (sequence not shown); and A1884V explored the impact of the Saõ Paolo mutation. (B) Western blot using anti-HIS antibody to detect his-ankR (top) and Coomassie blue stained SDS-PAGE (bottom) show the results of a single pull-down assay of 17 representative peptides. The first lane is the wt βI-14,15 peptide; lane 2 is αI-14,15 spectrin peptide that does not bind ankyrin. The next 12 lanes represent mutations that diminished ankyrin binding; their position is as labeled. Lane 15 is representative of an alanine substitution that has no effect on ankyrin binding; the last 2 lanes are the 2 peptides incorporating tryptophan at position 1812 and histidine at position 1848. (C) CD and thermal-melt analysis of selected peptides. The panel on the left shows the CD analysis of 3 peptides. The wt peptide in blue is nearly super-imposable with L1793A (red), a mutant peptide that abrogates ankyrin binding, and the Trp/His double mutant (black) that binds ankyrin normally. Each of these peptides had a calculated α-helix content of ≈70%. Thermal melt analysis (right panel) of the same peptides reveals a loss of cooperative unfolding of the L1793A mutant. Other peptides selected for CD and thermal melt analysis were similar to the wt peptide.
Alanine-scanning mutagenesis identifies the critical residues within the ankyrin-binding domain of erythroid βI-spectrin. (A) Sequence alignment of the ankyrin-binding region of βI spectrin23 (codons 1768-1898) with βII, βIII, and βIV spectrin. The red arrows above the alignment labeled KP (Kennedy peptide) represent the limits of ankyrin-binding activity previously identified, ie, deletion of sequences outside this region did not perturb ankyrin-binding activity.23 The histogram summarizes the strength of homology among these spectrins; red, conserved in all 4 spectrins; green conserved in 3 of 4; light blue, conserved in 2 of 4; and dark blue, not conserved. The putative structural feature of each region of sequence also is depicted. Arrows below the alignment indicate the position of each of the 42 mutants examined in this study. Red arrows highlight amino acids that abrogated ankyrin binding; the length of the arrow approximates the inhibitory potency of the mutation. Black arrows indicate amino acid changes that had no effect. All amino acids mutations were to alanine unless otherwise indicated by the bold letters. The W1779R mutant explored the effect of deletion of the highly conserved trp at that locus; L1812W replaced the “invariant” trp that characterizes most spectrin repreats; L1848H restored the H that is conserved in other spectrin repeats; A1867P was designed to mimic the pro at this position in βV spectrin (sequence not shown); and A1884V explored the impact of the Saõ Paolo mutation. (B) Western blot using anti-HIS antibody to detect his-ankR (top) and Coomassie blue stained SDS-PAGE (bottom) show the results of a single pull-down assay of 17 representative peptides. The first lane is the wt βI-14,15 peptide; lane 2 is αI-14,15 spectrin peptide that does not bind ankyrin. The next 12 lanes represent mutations that diminished ankyrin binding; their position is as labeled. Lane 15 is representative of an alanine substitution that has no effect on ankyrin binding; the last 2 lanes are the 2 peptides incorporating tryptophan at position 1812 and histidine at position 1848. (C) CD and thermal-melt analysis of selected peptides. The panel on the left shows the CD analysis of 3 peptides. The wt peptide in blue is nearly super-imposable with L1793A (red), a mutant peptide that abrogates ankyrin binding, and the Trp/His double mutant (black) that binds ankyrin normally. Each of these peptides had a calculated α-helix content of ≈70%. Thermal melt analysis (right panel) of the same peptides reveals a loss of cooperative unfolding of the L1793A mutant. Other peptides selected for CD and thermal melt analysis were similar to the wt peptide.
The L1793A mutation abrogates ankyrin binding and uncouples the di-repeat unfolding, whereas the Saõ Paolo mutation has no effect on ankyrin binding
For several of the nonbinding peptides, preservation of overall secondary and tertiary structure was confirmed by circular dichroism and thermal denaturation. The wild-type peptide and all of the mutants examined preserved their α-helical content (68%-75%) (Figure 1C); however, the peptide containing the L1793A mutation uniquely displayed a biphasic melting curve indicating that the cooperative thermal denaturation of the two repeats was now decoupled. This mutation also strongly abrogated ankyrin-binding, corroborating a contribution from both repeats 14 and 15 to the binding site.
Also examined was the impact of the A1884V mutation in β-spectrin São Paolo that is associated with a familial spherocytosis.37 Typically, spherocytosis is characterized by a loss of spectrin from the membrane as the result of deletions or missense mutations in β-spectrin or to defects in its ankyrin-mediated linkage to the membrane.2 However, the single point mutation in spectrin São Paolo, a conservative replacement of alanine with valine in helix 15C, did not impair ankyrin binding in our in vitro assay (Figure 1).
The structure of the ankyrin-binding domain of βI-spectrin
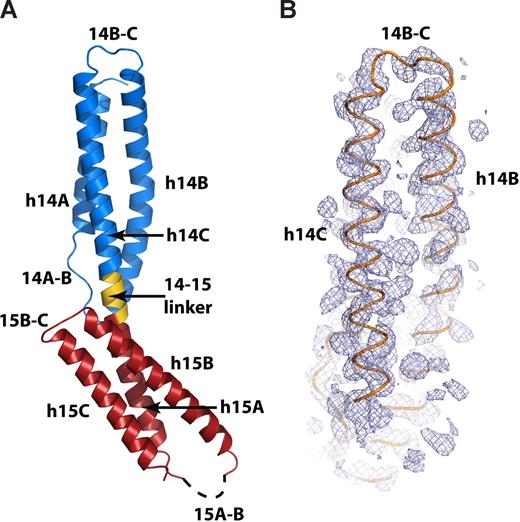
To more precisely interpret the implications of the mutagenesis data, the structure of the spectrin βI-14,15 peptide was solved to 2.1 Å by SAD phasing (Table S3). Crystals of the seleno-methionine–labeled protein diffracted to 2.6 Å, and all 3 selenium sites were ordered and fully occupied. The experimental electron-density map was readily interpretable, allowing the placement of all α helices (Figure 2B); the selenium sites resolved the correct sequence register.
The crystal structure of the ankyrin-binding domain of erythroid spectrin. (A) Ribbon diagram of the βI-14,15 di-repeat. The 14th repeat (βI-14) is shown in blue, the 14,15 linker is shown in gold, and the 15th repeat (βI-15) is red. The dashed line designates the disordered 15A-B loop. Other secondary structure elements are labeled as in the text. (B) Experimental SAD-phased electron-density map covering the 14th repeat of βI spectrin (blue mesh) calculated to 2.5 Å and contoured at 1.5σ. The Cα trace of the final model of βI-14,15 (orange) is superimposed.
The crystal structure of the ankyrin-binding domain of erythroid spectrin. (A) Ribbon diagram of the βI-14,15 di-repeat. The 14th repeat (βI-14) is shown in blue, the 14,15 linker is shown in gold, and the 15th repeat (βI-15) is red. The dashed line designates the disordered 15A-B loop. Other secondary structure elements are labeled as in the text. (B) Experimental SAD-phased electron-density map covering the 14th repeat of βI spectrin (blue mesh) calculated to 2.5 Å and contoured at 1.5σ. The Cα trace of the final model of βI-14,15 (orange) is superimposed.
The model was refined against the isomorphous 2.1 Å native dataset to the final Rcryst = 21.6% and Rfree = 25.3% (Table S3). The asymmetric unit included one molecule; the model contained 190 amino acids and 128 water molecules. Model geometry was excellent, with 98.33% and 1.67% of residues falling, respectively in preferred and allowed region of the Ramachandran plot. The βI-14,15 peptide was asymmetric and elongated (∼93 × ∼30 Å) and displayed a typical spectrin di-repeat fold (Figure 2A). The two repeats overlapped for approximately half the length of the molecule. Each repeat had 3 helices, labeled as h14A, h14B, h14C, h15A, h15B, and h15C, and 2 loops labeled 14A-B, 14B-C, 15A-B, and 15B-C (Figure 2A). The two repeats were joined by a 5 amino-acid α-helical link (14-15 linker). The 14-15 linker connected helices 14C and 15A into a single long continuous α-helix similar to that observed in previous spectrin-like di-repeat structures.35,36,38,39 Fourteen C-terminal residues (residues C1892-R1907) and 13 residues in the 15A-B loop (residues L1820-H1835) were disordered and could not be modeled.
Hydrophobic residues critical for ankyrin-binding stabilize the di-repeat structure, whereas the solvent-exposed residues from h14C form the ankyrin-recognition surface
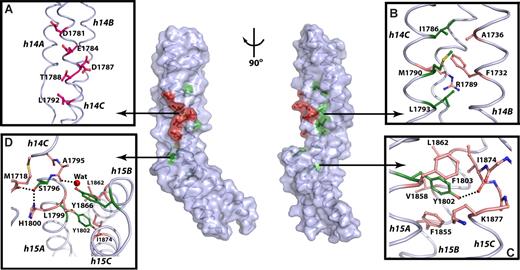
All residues implicated in ankyrin binding (W1779, D1781, E1784, I1786, D1787, T1788, M1790, L1792, L1793, S1796, Y1802, T1864, Y1866, and A1867) are spatially located within or near the 14-15 linker (colored residues, Figure 3; center; also see Figure S3). The mutagenesis data indicate that ankyrin binding requires the hydrophobic residues from the helices 14C and 15A to stabilize the structure of each repeat, whereas the solvent-exposed residues from h14C form the putative ankyrin-recognition surface.
Residues critical for ankyrin binding are located around and within the 14,15 linker. In the center panel, the molecular surface and the coil representation of the βI spectrin di-repeat structure are shown. Solvent-exposed residues critical for ankyrin recognition are red; buried residues are green; the remaining residues are colored light blue. The view in the left panel is the same as in Figure 2A, whereas the right panel view is rotated ∼90° clockwise around the vertical axis. (A) Detailed view of the putative ankyrin-recognition surface with the critical residues represented as red ball-and-stick. (B) Interactions in the hydrophobic core of βI-14 with the important hydrophobic residues in green and other interacting residues shown as beige ball-and-stick. (C) The role of Y1802 in stabilizing the structure of βI-15. The side-chain of Y1802 (green) forms interactions with residues from all 3 helices of the 15th repeat (beige). (D) Interactions in the 14,15 linker region. The side-chains of S1796 and Y1866 (green) interact with residues from both repeats (beige) and appear to be critical for determining the relative orientation of the two repeats. The water molecule is shown as red sphere, while the dashed lines indicate hydrogen bonds. The major secondary structure elements are labeled in italic.
Residues critical for ankyrin binding are located around and within the 14,15 linker. In the center panel, the molecular surface and the coil representation of the βI spectrin di-repeat structure are shown. Solvent-exposed residues critical for ankyrin recognition are red; buried residues are green; the remaining residues are colored light blue. The view in the left panel is the same as in Figure 2A, whereas the right panel view is rotated ∼90° clockwise around the vertical axis. (A) Detailed view of the putative ankyrin-recognition surface with the critical residues represented as red ball-and-stick. (B) Interactions in the hydrophobic core of βI-14 with the important hydrophobic residues in green and other interacting residues shown as beige ball-and-stick. (C) The role of Y1802 in stabilizing the structure of βI-15. The side-chain of Y1802 (green) forms interactions with residues from all 3 helices of the 15th repeat (beige). (D) Interactions in the 14,15 linker region. The side-chains of S1796 and Y1866 (green) interact with residues from both repeats (beige) and appear to be critical for determining the relative orientation of the two repeats. The water molecule is shown as red sphere, while the dashed lines indicate hydrogen bonds. The major secondary structure elements are labeled in italic.
The side-chains of D1781, E1784, D1787, T1788, and L1792 from helix 14C are critical for ankyrin binding. These side-chains are solvent exposed and form a common face along the molecular surface (Figure 3A). It is therefore unlikely that they play a role in stabilizing the structure of the di-repeat. This surface is positioned near the linker region and is supported by the hydrophobic core of βI-14. The molecular surface formed by D1781, E1784, D1787, T1788, and L1792 thus constitutes the putative ankyrin-recognition surface. Most of these residues are negatively charged. Although there are additional basic residues located in helices 14A and 14B, these do not appear to play a role because their deletion does not affect the ankyrin-binding ability of the spectrin di-repeat (Figure 1).23
The side-chain of W1779 from h14C flanks the N-terminal end of the 14-15 linker and makes Van der Walls contacts with V1747 from h14B, and with L1782 and L1783 from h14C. The Nϵ1 atom of W1779 also forms a hydrogen bond with the Oϵ1 atom of Q1744 from h14B (not shown in Figure 3). The hydrophobic core of the 14th repeat (Figure 3B) is further composed of I1786, M1790, and L1793 (Figure 3B). These residues form several hydrophobic interactions with side-chains from h14B, further strengthening the entire structure of the 14th repeat. The side-chain of I1786 interacts with A1736 and F1732, whereas M1790 forms Van der Walls contacts with the aliphatic chain of R1733 (not shown in Figure 3). Further, L1793 interacts with M1718 from the 14A-B loop. Finally, the side-chains of L1793 and F1732 sandwich the aliphatic chain of R1789. Thus, W1779, I1786, M1790 and L1793 stabilize the 14th repeat structure and anchor the N-terminal end of the ankyrin-binding region.
The C-terminal end of the 14-15 linker (Figure 3C) is stabilized by Y1802 from helix 15A. The Y1802 side-chain is in the hydrophobic pocket composed of F1855, V1858, and L1862 all from helix 15B (Figure 3C). Its hydroxyl group forms a strong hydrogen bond with the carbonyl oxygen of I1874 and a weak one with the backbone amide of E1878 from helix 15C. This structure thus reveals that Y1802 plays a critical role in stabilizing the 15th repeat and anchors the conformation of the C-terminal end of the ankyrin-binding region.
The linker residues S1796 and Y1866 from the 15B-C loop influence interrepeat orientation
Ankyrin recognition is abrogated when either S1796 from the 14-15 linker or Y1866 from the 15B-C loop are mutated to alanine. The structure (Figure 3D) suggests that these side-chains stabilize the relative orientation of the 2 repeats and interact with residues from both repeats and from the linker region. For instance, the Oγ atom of S1796 forms a tight hydrogen bond with the carbonyl oxygen of M1718 in the 14A-B loop and with the Nδ1 atom of H1800 from the helix 15A. Similarly, the side chain of Y1866 interacts with both helix 14C and the linker. This side-chain sits atop the hydrophobic core of the 15th repeat and forms Van der Walls interactions with L1799, L1862, and I1874 from helices 15A, 15B, and 15C, respectively (Figure 3D). Its hydroxyl group coordinates a water molecule hydrogen-bonded to the carbonyl oxygen of A1795 in the 14-15 linker (Figure 3D). Thus, the side-chains of S1796 and Y1866 provide a critical link between the two repeats. We propose that interactions of these residues with other structural elements determine the relative orientation of the two repeats.
Structural flexibility and orientation between βI-14,15 may be important for function
The individual repeats of βI-14,15 are oriented with a larger tilt angle between repeats than in all previously determined spectrin di-repeat structures. This conformational feature of the ankyrin-binding domain may be of significance for its physiologic function, and implies a potentially high level of possible flexibility between the two repeats.
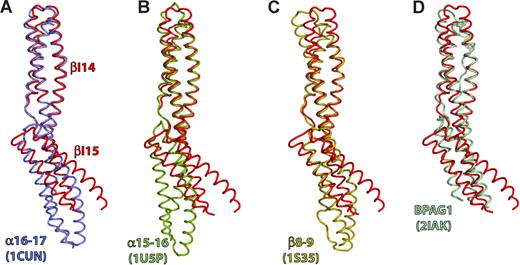
The structure of βI-14,15 was compared with the structures of βI-8,9, αI-15,16, αI-16,17, and BP antigen 1 (BPAG1).36,37,39,–41 Least squares super-positioning of the entire βI-14,15 structure yielded root mean square deviation (rmsd) values from 2.2 to 3 Å (Table 1). However, when either of the βI repeats was used as a reference the rmsd values decreased to 1.0 to 2.2 Å, suggesting that the individual repeats are very similar to one another, whereas their relative orientations vary greatly (Figure 4). When βI-14 was used as a reference for superpositioning, the C-terminal repeats of different di-repeats assumed distinct orientations (Figure 4). The tilt between βI-14 and βI-15 is 64°, whereas for βI-8,9, αI-16,17 and αI-15,16 the angles were 11°, 37° and 49°, respectively. The BPAG1 repeats were very similar to that of βI-14,15 (tilt 63°). Consequently, βI-14,15 and BPAG1 are contracted by ∼10 Å compared with other di-repeats, and their C termini and the A-B loop of the C-terminal repeat is disordered. It is plausible that a larger rotation around the linker region induces structural rearrangements in the downstream repeat, a possible mechanisms of long-range signal transduction along the spectrin filament such as observed in the linkage of ankyrin binding to spectrin tetramer formation.25
The tilt angle between repeats is large in βI-14,15. Comparison with previously determined spectrin di-repeat structures reveals that the tilt angle between repeats βI-14 and βI-15 is the largest observed so far in any spectrin, and is similar to that observed in some plakin repeats. Depicted is least-squares superpositioning of βI-14,15 (red) with: (A) Repeats 16 and 17 of the chicken α-spectrin (blue, PDBID: 1CUN); (B) Repeats 15 and 16 of chicken α-spectrin (green; PDBID: 1U5P); (C) Erythroid spectrin repeats 8 and 9 (gold; PDBID: 1S35); (D) The BPAG1 di-repeat structure (light blue; PDBID: 2IAK). The βI-14 repeat was used as a reference molecule for superpositioning.
The tilt angle between repeats is large in βI-14,15. Comparison with previously determined spectrin di-repeat structures reveals that the tilt angle between repeats βI-14 and βI-15 is the largest observed so far in any spectrin, and is similar to that observed in some plakin repeats. Depicted is least-squares superpositioning of βI-14,15 (red) with: (A) Repeats 16 and 17 of the chicken α-spectrin (blue, PDBID: 1CUN); (B) Repeats 15 and 16 of chicken α-spectrin (green; PDBID: 1U5P); (C) Erythroid spectrin repeats 8 and 9 (gold; PDBID: 1S35); (D) The BPAG1 di-repeat structure (light blue; PDBID: 2IAK). The βI-14 repeat was used as a reference molecule for superpositioning.
The unusual absence of both Trp and Trp-His couple in repeat 15 does not affect the hydrophobic core of repeat 15 nor ankyrin-binding activity
We analyzed the consequences of the absence of the nearly invariant Trp at position 14 in helix A and the semi-invariant His in helix B of the 15th repeat. A tryptophan at the end of the second heptad of helix A is spectrin's most highly conserved feature, present in 14 of 16 βI repeats. A second well-conserved Trp residue is located in the first position of the third heptad of helix C, and a third well-conserved residue is His in the middle of helix B. Previously determined spectrin di-repeat structures36,38 indicate that the side-chains of these 3 residues form a tightly packed hydrophobic core that has been proposed to contribute to peptide folding and stability and possibly to its physiologic role.23,26,41,42 However, we find that the absence of Trp and the Trp-His couple in βI-15 is not critical for stability of this di-repeat, nor does it modify ankyrin recognition (Figure 1). Furthermore, we find in our structure that the hydrophobic core of βI-15 is tightly packed, similar to that of other spectrin repeats, regardless of its lack of the invariant Trp residue in helix A.
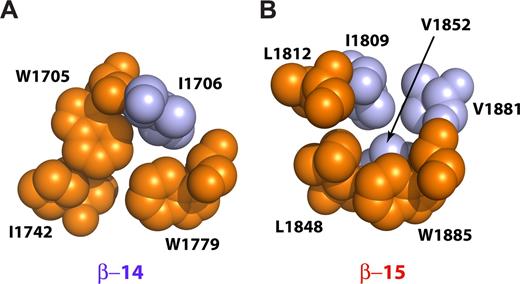
Two peptides were prepared with an L1812W mutation or with L1812W, L1848H double mutation. These mutations return both the critical Trp in h15A as well as its interacting His side-chain in h15B, presumably restoring the conventional packing of the 3 helices.38 Both of these peptides retained full ankyrin-binding activity (Figure 1B lanes 16,17). The thermal stability of the double mutant was slightly reduced but remained highly cooperative with a transition temperature near 42°C (Figure 1C). Thus, the loss of the invariant tryptophan and the absence of the Trp-His pair in βI-15 is not a requirement for ankyrin binding. Further, in βI-14,15 the invariant Trp residue in h15A is replaced by L1812, whereas the semi-invariant His in h15B is replaced with L1848. The second semi-invariant Trp (W1885) in hC is preserved. In the published structures that preserve the invariant tryptophan, a Trp-His couple from hA and hB forms a critical interaction,38 whereas the semiconserved Trp from hC further stabilizes the couple. In our structure, L1848 from h15B interacts with the semi-invariant W1885 from h15C, whereas the L1812 side-chain from h15A interacts with I1809 from h15A. I1809 along with V1852 from h15B and V1881 from h15C close the hydrophobic core of the repeat (Figure 5B).39 Thus, although both the invariant Trp from h15A and the semi-invariant His from h15B are absent, the core is compact because of contributions from other smaller hydrophobic side-chains resident in helices 15B and 15C. The core of the 14th repeat, on the other hand, is reminiscent of the typical spectrin-repeat hydrophobic core where W1705 (h14A) interacts with I1742 (h14B) in a fashion similar to the Trp-His couple in other di-repeats (Figure 5A).
Interactions near the conserved tryptophan residues in the βI-14 and βI-15 repeats. The diagrams show space-filling models with the conserved hydrophobic triplets depicted in orange and other interacting residues colored light blue. (A) The 14th repeat contains 2 conserved tryptophan residues, W1705 and W1779. W1705 is in the highly conserved g position of the second heptad of h14A and interacts with the d position residue of the third heptad of h14B (I1742). The semiconserved W1779 is in the a position of the second heptad of h14C and interacts with both W1705 and I1742 as well as with I1706. (B) The 15th repeat lacks both the “invariant” tryptophan in h15A and the semiconserved histidine in h15B. Instead, this repeat contains L1812 and L1848 and the semi-invariant W1885. L1848 interacts with W1885, whereas L1812 interacts with I1809. The core is further stabilized by V1852 and V1881 that interact with I1809 and W1885, respectively. The cores of both βI-14 and βI-15 are thus tightly packed and hydrophobic, without included water. This is unlike the structure reported for βI-9 that also lacks the invariant tryptophan.39
Interactions near the conserved tryptophan residues in the βI-14 and βI-15 repeats. The diagrams show space-filling models with the conserved hydrophobic triplets depicted in orange and other interacting residues colored light blue. (A) The 14th repeat contains 2 conserved tryptophan residues, W1705 and W1779. W1705 is in the highly conserved g position of the second heptad of h14A and interacts with the d position residue of the third heptad of h14B (I1742). The semiconserved W1779 is in the a position of the second heptad of h14C and interacts with both W1705 and I1742 as well as with I1706. (B) The 15th repeat lacks both the “invariant” tryptophan in h15A and the semiconserved histidine in h15B. Instead, this repeat contains L1812 and L1848 and the semi-invariant W1885. L1848 interacts with W1885, whereas L1812 interacts with I1809. The core is further stabilized by V1852 and V1881 that interact with I1809 and W1885, respectively. The cores of both βI-14 and βI-15 are thus tightly packed and hydrophobic, without included water. This is unlike the structure reported for βI-9 that also lacks the invariant tryptophan.39
Discussion
Together, the scanning mutagenesis findings and 3-dimensional structure of the βI-14,15 di-repeat allows a comprehensible view of the ankyrin-binding domain in βI-spectrin. 3 characteristics define this domain: (1) the presence of 8 critical residues in the C-terminal end of h14C and in the 14-15 linker that form a tight hydrophobic core stabilizing the di-repeat, (2) a highly charged hydrophilic surface of h14C and the linker region that forms the putative ankyrin-recognition surface, and (3) a larger tilt angle between the 14th and 15th repeat units stabilized by the residues S1796 and Y1866 that interact with residues from both repeats. We also find, contrary to our earlier speculation,23 that the absence of the nearly invariant tryptophan at position 14 within the 15th repeat (L1812) has no effect on the structure of the hydrophobic core of βI-15, nor does it affect ankyrin binding. In this respect our structure differs significantly from the structure of βI-9 that also lacks the conserved tryptophan but displays a more open and presumably less stable central core.38 Thus, the changes that bestow ankyrin-binding specificity are subtle and involve primarily sequence changes in the terminal portions of h14C, the 14-15 linker, and in the 15B-C loop.
Ankyrin and other ligands such as the Lutheran blood group antigens,17 the neural cell adhesion molecule NCAM,18 the delta glutamate receptor,43 and excitatory amino acid transporters6 bind with high specificity and affinity to the spectrin repeat (summarized in De Matteis and Morrow1 ). Other proteins with spectrin-like repeats such as BPAG122 and mAKAP21 also use their repeat regions for ligand binding. We speculate that the evolutionary adaptability provided by restricting changes to the helix C-linker region, with contributions from the spatially adjacent B-C loop of the down-stream repeat, provides a general model of how ligand specificity can be achieved in spectrin di-repeat units without disrupting the folding or stability of each triple-helical unit. The primary structure of the different spectrins supports this hypothesis in that there is high repeat-to-repeat and linker conservation across the different spectrins (Figure S1B), but much less conservation within any given spectrin (Figure S1A; also see Baines10 ). Interestingly, divergence is most apparent in the terminal portions of the helix C, the linkers, and the interhelix loops. There is almost a total absence of linker homology within a given spectrin, but very high linker conservation at similar positions between the different β-spectrins (Figure S1B). Correspondingly, the predominant sites of sequence insertion, either by evolution or alternative transcription, concentrate in the helix C-linker and B-C loop regions of every spectrin (Figure S2), as do many mutations that lead to hemolytic disease.27 Thus, the helix C, linker, and interhelix loops (particularly the B-C loop), are sites that readily accommodate modification without disruption of the structural repeat. Although the linker provides a crucial bridge coupling the folding and unfolding of paired repeats to each other,42 we propose that the linker has additional roles. Specifically, we propose that the spectrin di-repeat unit represents a novel and here-to-fore unappreciated general protein-protein interaction motif. As with the ankyrin-repeat unit, evolution has used the spectrin di-repeat in a modular fashion in a variety of proteins to create specific protein-protein interaction sites ordered along an extended scaffold. A model of the putative spectrin di-repeat protein-protein interaction motif is depicted in cartoon form in Figure 6.
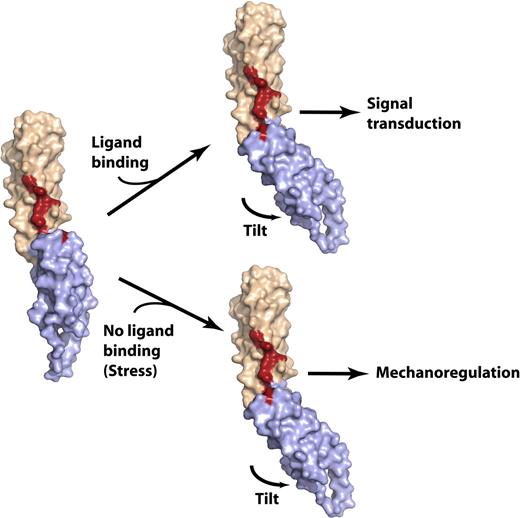
Model of the spectrin di-repeat as a generalized mechanosensitive protein–protein interaction motif. The N-terminal repeat is colored beige, the C-terminal repeat is light blue, and the residues critical for ankyrin binding are colored red. (Left) The βI-14,15 di-repeat is shown in a hypothetical extended conformation similar to that observed in most of the previously determined spectrin di-repeat structures. The conformation of βI-15 and the 15A-B loop are modeled in this structure using the βI-8,9 structure (PDBID: 1S35) as a reference. In this conformation, the continuity of the ankyrin-binding surface is disrupted; mutagenesis data suggest that loss of residues stabilizing the tilt angle abrogates ankyrin binding. Such a conformation might conceivably be generated by stretch across the di-repeat44 or perhaps by other factors such as point mutations or by lipid binding.20 (right) Ligand binding would alter the conformation of the linear di-repeat, inducing down-stream events (signal transduction), or the same conformational change may occur in the absence of ligand binding and under mechanical stress, inducing ligand binding. In either case, the delicate conformation of the ligand-binding pocket generated by spectrin di-repeats makes this structure an attractive candidate as a mechanosensory switch. This switch is dynamically depicted in Figure S3.
Model of the spectrin di-repeat as a generalized mechanosensitive protein–protein interaction motif. The N-terminal repeat is colored beige, the C-terminal repeat is light blue, and the residues critical for ankyrin binding are colored red. (Left) The βI-14,15 di-repeat is shown in a hypothetical extended conformation similar to that observed in most of the previously determined spectrin di-repeat structures. The conformation of βI-15 and the 15A-B loop are modeled in this structure using the βI-8,9 structure (PDBID: 1S35) as a reference. In this conformation, the continuity of the ankyrin-binding surface is disrupted; mutagenesis data suggest that loss of residues stabilizing the tilt angle abrogates ankyrin binding. Such a conformation might conceivably be generated by stretch across the di-repeat44 or perhaps by other factors such as point mutations or by lipid binding.20 (right) Ligand binding would alter the conformation of the linear di-repeat, inducing down-stream events (signal transduction), or the same conformational change may occur in the absence of ligand binding and under mechanical stress, inducing ligand binding. In either case, the delicate conformation of the ligand-binding pocket generated by spectrin di-repeats makes this structure an attractive candidate as a mechanosensory switch. This switch is dynamically depicted in Figure S3.
Beyond providing a binding site, we also propose that the sensitivity of spectrin repeats and their helical linker to thermal unfolding and stretch-deformation26,45 provides a compelling mechanism whereby mechanical deformation or stress may be transduced into alterations in ligand binding, or conversely provides a mechanism whereby allosteric coupling between functional sites in different repeats maybe be effected (Figure 6 and Figure S4). For example, in the βI-14,15 structure, both an acute angle and cooperative folding between the two repeats are required for high-affinity ankyrin binding. Mutations that interrupt the bonds necessary to maintain the di-repeat angle (S1796 and Y1866) or that disable the cooperative unfolding of the two repeat units (L1793A) degrade ankyrin affinity. In earlier work, we demonstrated that ankyrin-binding to this locus promotes the self-association of spectrin heterodimers to form tetramers and larger oligomers25 via an interaction mediated by the partial 17th repeat of βI spectrin and the N-terminal partial repeat of αI-spectrin. The ability of the linkers to convey conformational information down the spectrin molecule, or distortions generated by altering the tilt between repeats such as may be induced by ankyrin-binding, provides an attractive model that would explain how ankyrin binding and self-association are coupled.
An even more interesting possibility that follows from this model is that the spectrin di-repeat unit provides a modular mechanical switch used in spectrin and other spectrin-repeat containing proteins as a mechanism for coupling mechanical deformation or stretch to ligand binding and signal transduction pathways. There is already considerable evidence that the flexibility of spectrin, especially as defined by the nature of its linker sequences, is critical for its function, and that even under physiologic deformation in the red cell localized unfolding of spectrin occurs.44 We hypothesize that spectrin proteins containing spectrin di-repeat structures may provide not only a controlled flexibility to membrane-associated scaffolds, but also an intrinsic mechanosensing switch designed to control the disposition of ligands and signal-transducing molecules in response to cellular deformation or stretch. In future work, it will be important to evaluate the predictions of this model, that is, that di-repeat spectrin units can form deformation sensitive protein-protein interaction domains.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the Northeastern Collaborative Access Team (NE-CAT; ID-24E) beam line and C. Axel Innis for help during data collection.
This study was supported by National Institutes of Health (Bethesda, MD) grants R01-HL28560 and R01-DK43812 to J.S.M.
National Institutes of Health
Authorship
Contribution: P.R.S. designed and performed research, collected and analyzed data, and wrote portions of manuscript; I.S. performed research, contributed vital new reagents, and collected and analyzed data; M.A.R. performed research and collected and analyzed data; M.S.A. performed research and collected and analyzed data; T.A.S. contributed vital analytical tools, analyzed, and interpreted data; M.S. designed and performed research, collected and analyzed data, interpreted data, and wrote portions of manuscript; and J.S.M. designed research, analyzed and interpreted data, and wrote portions of manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for Ms Ivana Simonović and Dr Miljan Simonović is Department of Biochemistry and Molecular Genetics, University of Illinois at Chicago, Chicago, IL 60607.
Correspondence: Jon S. Morrow, MD, PhD, Yale University, New Haven, CT 06520; e-mail: jon.morrow@yale.edu; or Miljan Simonović, PhD, Department of Biochemistry and Molecular Genetics, University of Illinois at Chicago, Chicago, IL 60607; e-mail: msimon5@uic.edu.