Preexisting plasma cell disorders, monoclonal gammopathy of undetermined significance, or smoldering myeloma are present in at least one-third of multiple myeloma patients. However, the proportion of patients with a preexisting plasma cell disorder has never been determined by laboratory testing on prediagnostic sera. We cross-referenced our autologous stem cell transplantation database with the Department of Defense Serum Repository. Serum protein electrophoresis, immunofixation electrophoresis, and serum free light-chain analysis were performed on all sera collected 2 or more years before diagnosis to detect a monoclonal gammopathy (M-Ig). In 30 of 90 patients, 110 prediagnostic samples were available from 2.2 to 15.3 years before diagnosis. An M-Ig was detected initially in 27 of 30 patients (90%, 95% confidence interval, 74%-97%); by serum protein electrophoresis and/or immunofixation electrophoresis in 21 patients (77.8%), and only by serum free light-chain analysis in 6 patients (22.2%). Four patients had only one positive sample within 4 years before diagnosis, with all preceding sera negative. All 4 patients with light-chain/nonsecretory myeloma evolved from a light-chain M-Ig. A preexisting M-Ig is present in most multiple myeloma patients before diagnosis. Some patients progress rapidly through a premalignant phase. Light-chain detected M-Ig is a new entity that requires further study.
Introduction
Multiple myeloma (MM) is a mostly incurable malignant disorder of plasma cells diagnosed in approximately 20 000 patients in the United States annually.1 MM is known to evolve from premalignant plasma cell disorders, such as non Ig-M monoclonal gammopathy of undetermined significance (MGUS) or smoldering multiple myeloma (SMM), in at least one-third of patients.2 The progression to MM occurs at average rates of 1% per year for MGUS2 and 10% per year for SMM.3 The risk of progression from these premalignant conditions to MM is affected by the level of monoclonal immunoglobulin, the presence of non-IgG gammopathy, an abnormal serum free light-chain (sFLC) ratio, the fraction of bone marrow plasma cells bearing an aberrant phenotype, increased bone marrow plasma cells, decreased levels of polyclonal immunoglobulin, and aneuploidy.2,,,,–7 However, the proportion of MM that develops from a preexisting MGUS or SMM is unknown and remains an important unresolved issue in the understanding of the pathogenesis of myeloma.8
These premalignant plasma cell disorders are asymptomatic and usually discovered during investigation for unrelated symptoms or laboratory abnormalities.9 Therefore, it is probable that previous studies have substantially underestimated the true proportion of MM patients with a preexisting plasma cell disorder. Epidemiologic studies support the notion that a preexisting plasma cell disorder is nearly always present.10 Others have suggested that a proportion of MM arises de novo without a premalignant plasma cell disorder.11 It has also been postulated that MM that arises from a preexisting plasma cell disorder has distinct genomic features, a unique pattern of response to therapy, and a more favorable outcome.12,,–15
We sought to determine the proportion of patients with newly diagnosed myeloma who had a preexisting plasma cell disorder (PPCD), as manifested by an M-Ig, using serum collected before their diagnosis. We retrieved samples from the Department of Defense Serum Repository, which contains the unused sera from the mandatory, periodic blood tests performed on active duty US military service members.
Methods
A database of patients who underwent high-dose chemotherapy and autologous stem cell transplantation for MM at Walter Reed Army Medical Center was cross-referenced with the Department of Defense Serum Repository by the Armed Forces Health Surveillance Agency. The Department of Defense Serum Repository prospectively collects the unused sera from periodic mandatory blood tests performed on active duty US military service members for medical surveillance purposes. The repository contains 27 million samples on more than 7 million persons who have served since 1990.16 We intentionally chose a transplantation population because these younger than average myeloma patients would have probably been serving on active duty after 1990 when the repository began. All available sera collected 2 or more years before the diagnosis of MM were retrieved. Serum samples less than 2 years before diagnosis were not tested because these patients probably had undetected MM. Serum protein electrophoresis (SPEP) was performed using agarose gels (Helena Laboratories, Beaumont, TX) and inspected by a technician and one of the investigators (J.A.). Immunofixation electrophoresis (IFE) with antisera to immunoglobulin A (IgA), IgM, IgG, κ, and λ was performed (Helena Laboratories) on all cases, and IgD antisera for selected cases. sFLC levels were determined by automated immunoturbidimetric assays for free κ (normal range, 3.3-9.4 mg/L) and λ (normal range, 5.7-26.3 mg/L) on a Bayer Advia 1650 (Bayer Diagnostics, Tarrytown, NY) using commercial reagents (Freelite, The Binding Site, Birmingham, United Kingdom). The κ/λ ratio (normal, 0.26-1.65) was calculated. Subjects with ratios below the normal range have clonal λ disorders, and subjects with ratios above the normal range have clonal κ disorders. A PPCD was defined as an M-Ig on SPEP, a positive IFE for IgG, IgA, IgD, κ, and/or λ, or an abnormal sFLC ratio. At the time of initial detection of a PPCD and at the immediate prediagnostic sample, the risk of progression to MM was determined by the Mayo Clinic Risk Stratification Model, which uses the following 3 factors to define 4 risk groups: monoclonal immunoglobulin level greater than 1.5 g/dL, abnormal sFLC ratio, and non-IgG gammopathy.
Using SPSS for Windows version 14.0 (SPSS, Chicago, IL) for all data analyses, categorical variables were compared between groups using Fisher exact test (2-tailed), and continuous data were compared using the Wilcoxon rank-sum test. Ninety-five percent confidence intervals for proportions were estimated using the modified Wald method. This retrospective study was approved by the Human Use Committee at Walter Reed Army Medical Center, and informed consent was waived.
Results
Serum samples were available for 30 of 90 (33%) patients. The median number of samples per patient was 3.5 (range, 1-14), ranging from 2.2 to 15.3 years before the diagnosis of MM. The characteristics of the patients are shown in Table 1. Patients with available sera were younger at myeloma diagnosis (48.1 years vs 58.6 years, P < .001) and overwhelmingly male (96% vs 53%, P < .001). White and black patients composed 53% and 47%, respectively, of the serum cohort.
A PPCD was detected initially in 27 of 30 patients (90%; 95% confidence interval, 74%–97%), by SPEP and/or IFE in 21 patients (78%), and sFLC in 6 patients (22%). The PPCD was initially detected by sFLC alone in 6 of 27 (22.2%), IFE alone in 1 of 27 (7.4%), SPEP + IFE in 5 of 27 (18.5%), IFE + sFLC in 1 of 27 (3.7%), and by all 3 assays in 14 of 27 (51.2%). The Mayo Clinic Risk stratification for MGUS was determined for all evaluable patients at their initial (n = 18) and immediate prediagnostic (n = 20) samples. At initial detection of a PPCD, 7 (39%) were low risk, 9 (50%) were low intermediate, and 2 (11%) were high intermediate. Immediately before diagnosis, 4 (20%) were low risk, 10 were low intermediate (50%), and 6 (30%) were high intermediate.
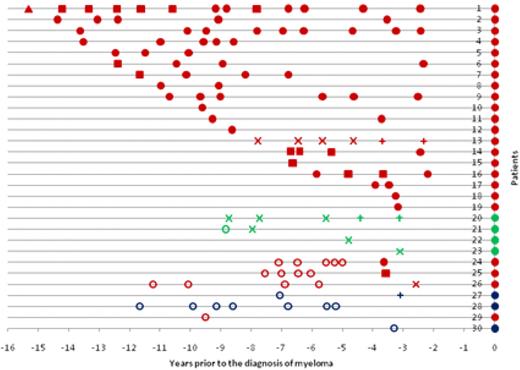
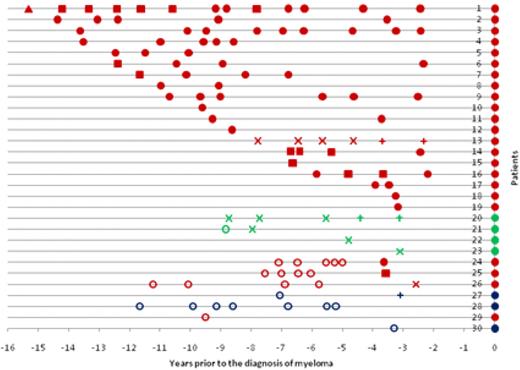
The pattern of serum results before the diagnosis of myeloma is shown in Figure 1. For patients 1 to 23 (except one sample from patient 21), all sera up to 15 years before diagnosis were positive. There were 4 patients (patients 20-23) with light-chain or nonsecretory myeloma. All of these patients had a PPCD initially detected by sFLC alone. Patients 24 to 27 had a PPCD detected initially within 2.6 to 3.7 years before the diagnosis, but with all preceding sera testing negative. Patients 24 and 25 had prediagnostic monoclonal IgG immunoglobulin levels of 0.36 g/dL and 0.40 g/dL, but only patient 24 had an abnormal sFLC ratio (2.05). Patient 26 (IgG myeloma) was detected by an abnormal sFLC ratio (2.71) alone, and patient 27 (IgD myeloma) was detected by sFLC assay and immunofixation. Three patients (patients 28-30) had no evidence of a PPCD. One patient (patient 29) with IgG myeloma had only one prediagnostic sample available 9.5 years before diagnosis. The 2 remaining patients without a PPCD were IgD myeloma patients, with their most recent prediagnostic samples at 5.2 and 3.3 years, respectively.
Serum results before the diagnosis of myeloma. Positive results are shown by ●, ■, ▲, ×, and +. Samples that were negative for all 3 tests (ie, no M-Ig present) are shown by ○. ● represents positive SPEP, IFE, and sFLC assay; ■, positive SPEP and immunofixation; ×, positive sFLC assay only; ▲, positive IFE only; and +, serum-free light assay and immunofixation. The color indicates the myeloma isotype: red represents IgG; green, light-chain/nonsecretory; and blue, IgD. Because the sFLC assay was not available at diagnosis, all patients are depicted with ● at the time of diagnosis for clarity.
Serum results before the diagnosis of myeloma. Positive results are shown by ●, ■, ▲, ×, and +. Samples that were negative for all 3 tests (ie, no M-Ig present) are shown by ○. ● represents positive SPEP, IFE, and sFLC assay; ■, positive SPEP and immunofixation; ×, positive sFLC assay only; ▲, positive IFE only; and +, serum-free light assay and immunofixation. The color indicates the myeloma isotype: red represents IgG; green, light-chain/nonsecretory; and blue, IgD. Because the sFLC assay was not available at diagnosis, all patients are depicted with ● at the time of diagnosis for clarity.
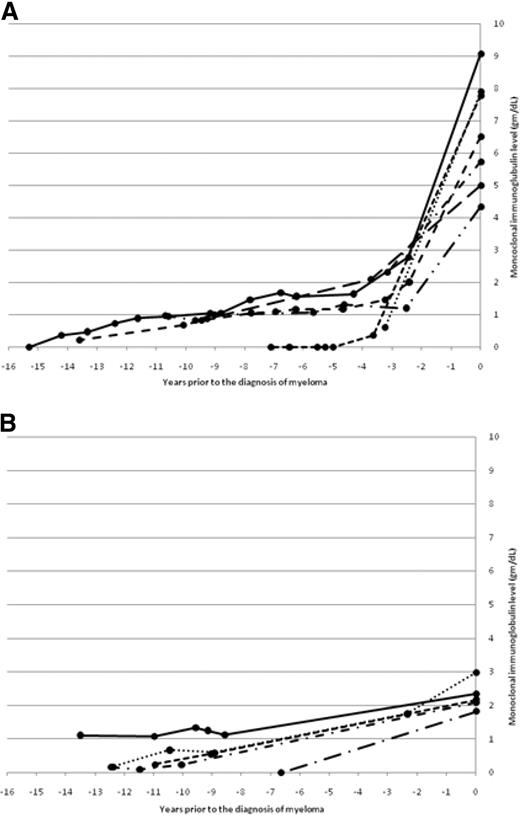
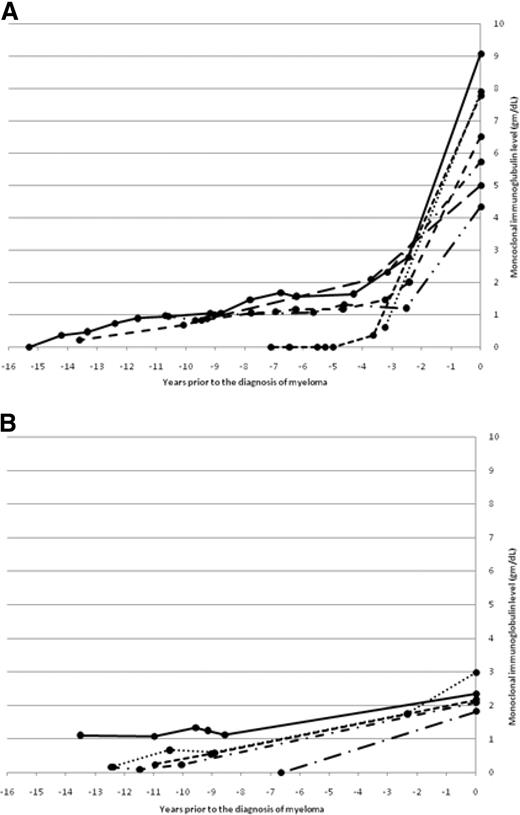
The changes in monoclonal immunoglobulin (M-Ig) levels during progression to MM in patients with available immunoglobulin levels at the time of diagnosis of MM are shown in Figure 2. Two patterns were evident. In Figure 2A, generally stable, low (< 1.5 g/dL) monoclonal immunoglobulin (M-Ig) levels are present for years before diagnosis, with a gradual increase resulting in a subsequent diagnostic M-Ig level of greater than 3 g/dL. In Figure 2B, generally stable, low (< 1.5 g/dL) M-Ig levels again are present for years before diagnosis followed by a gentle rise toward a diagnostic M-Ig level of less than 3 g/dL.
Two patterns of changes in monoclonal immunoglobulin level during progression to myeloma. (A) The change in monoclonal immunoglobulin level before the diagnosis of myeloma in patients with a diagnostic monoclonal immunoglobulin greater than 3 g/dL. (B) The change in monoclonal immunoglobulin level before the diagnosis of myeloma in patients with a diagnostic monoclonal immunoglobulin less than 3 g/dL.
Two patterns of changes in monoclonal immunoglobulin level during progression to myeloma. (A) The change in monoclonal immunoglobulin level before the diagnosis of myeloma in patients with a diagnostic monoclonal immunoglobulin greater than 3 g/dL. (B) The change in monoclonal immunoglobulin level before the diagnosis of myeloma in patients with a diagnostic monoclonal immunoglobulin less than 3 g/dL.
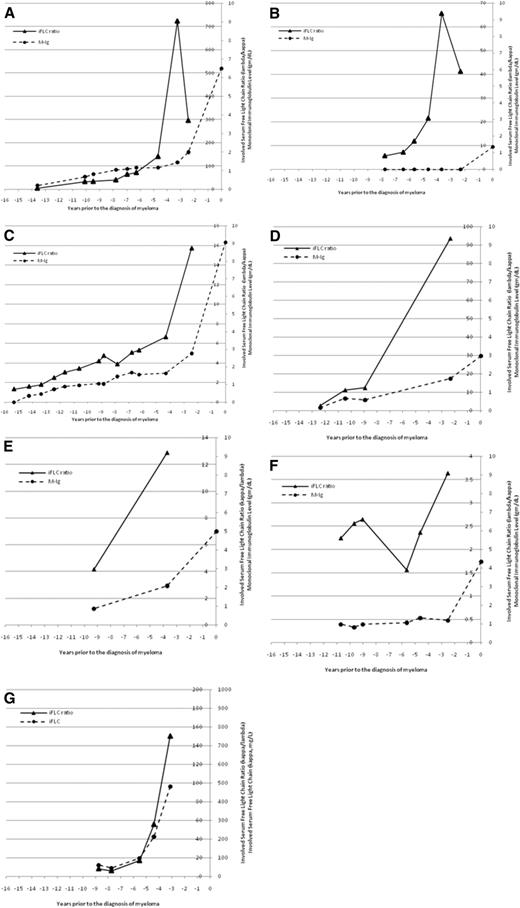
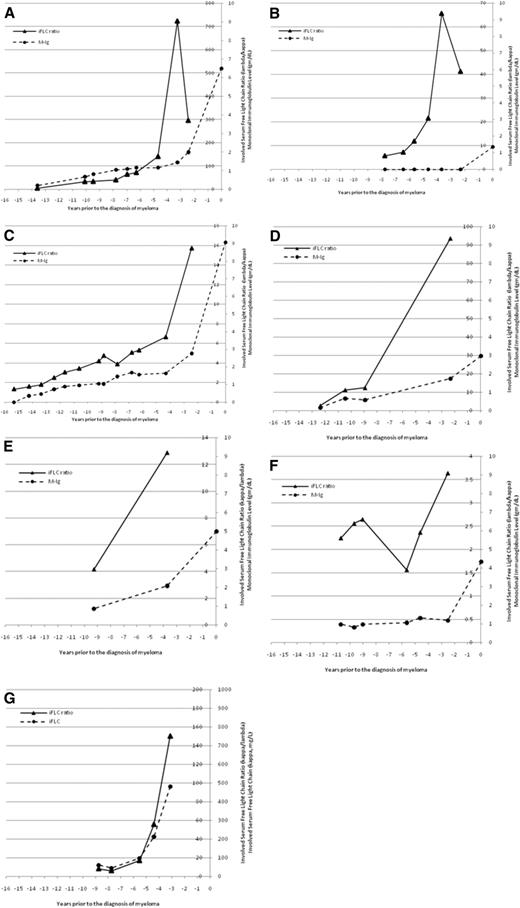
The temporal changes in M-Ig and/or sFLC ratios were fully evaluable in 7 individual patients (Figure 3). For 1 patient (Figure 3A), there was a gradual increase in M-Ig and the sFLC ratio but a sharp increase only in the sFLC ratio 3.5 years before the diagnosis of MM. For a second patient (Figure 3B), there was no M-Ig detectable until the time of diagnosis, but the sFLC ratio was abnormal up to 8 years before the diagnosis of MM and showed a dramatic increase 4 years before the diagnosis of MM. For a third patient (Figure 3C), there was a gradual increase in both M-Ig and the sFLC ratio for several years and then a more substantial increase, particularly for the sFLC ratio, which occurred 2.5 years before the diagnosis of MM. Finally, Figure 3G illustrates a patient with light-chain MM, in whom the sFLC ratio and free κ light chain increased substantially 3 to 4 years before the diagnosis of MM. Altogether, there appeared to be a substantial increase in the sFLC ratio 2 or more years before the diagnosis of MM in 7 evaluable patients (Figure 3).
An increasingly abnormal serum-free light chain ratio may be a harbinger of symptomatic myeloma. The temporal changes in monoclonal immunoglobulin level and involved serum free light-chain ratio (iFLC) are shown for 6 patients with intact immunoglobulin myeloma (A-F) and 1 patient with light-chain myeloma (G). The M-Ig is plotted on the outside axis and the iFLC ratio on the inside axis. The iFLC ratio is expressed as λ/κ for patients with clonal λ MM. For the 1 patient with light-chain myeloma (G), the involved sFLC is plotted on the outside axis and the involved free light-chain ratio is plotted on the inside axis.
An increasingly abnormal serum-free light chain ratio may be a harbinger of symptomatic myeloma. The temporal changes in monoclonal immunoglobulin level and involved serum free light-chain ratio (iFLC) are shown for 6 patients with intact immunoglobulin myeloma (A-F) and 1 patient with light-chain myeloma (G). The M-Ig is plotted on the outside axis and the iFLC ratio on the inside axis. The iFLC ratio is expressed as λ/κ for patients with clonal λ MM. For the 1 patient with light-chain myeloma (G), the involved sFLC is plotted on the outside axis and the involved free light-chain ratio is plotted on the inside axis.
Discussion
In this study using sera available before the diagnosis of MM, we demonstrated that at least 27 of 30 (90%) of patients with MM evolve from a PPCD. We suspect that the proportion of patients with a PPCD may have been higher than 90%, as the characteristics of the negative cases may have reduced our ability to detect a PPCD. One case of IgG myeloma had only one prediagnostic serum sample available at 9.5 years before diagnosis. The remaining 2 negative cases were IgD myeloma with sera available only 3 and 5 years before the diagnosis. This rare isotype is characterized by the secretion of very low levels of monoclonal immunoglobulins.17 An additional case of IgD myeloma in our study was positive by sFLC assay and immunofixation without a quantifiable monoclonal immunoglobulin. Although 3 cases of IgD MGUS have been reported, the laboratory and clinical features, including measurement of sFLCs, are not well known.18,19 Therefore, with the availability of samples closer to the diagnosis and perhaps more sensitive detection methods for patients with IgD myeloma, we suspect that these patients might have had a PPCD.
It is clear from this study that a fraction of patients progress rapidly through a premalignant phase to MM. This is consistent with recent studies stratifying MGUS patients into groups with differing rates of annual progression, from 0.25% to 11.2%, based on several factors: an abnormal sFLC assay, non-IgG gammopathy, the level of bone marrow plasmacytosis, and the immunophenotypic features of bone marrow plasma cells.5,7 However, it is notable that in our study there were no patients who satisfied the criteria for high-risk MGUS by the Mayo Clinic model. Indeed, 2 of the 4 rapidly progressing patients were low or low intermediate risk by the Mayo Clinic risk stratification model. Unfortunately, there are no published data on progression rates for light-chain MGUS.
A new entity called light-chain MGUS, defined as an abnormal sFLC ratio without detectable immunoglobulin heavy chain, has been described by the Mayo Clinic in a population-based prevalence study performed in Olmsted County, Minnesota.20 The investigators reported a prevalence of approximately 2% in those older than 50 years. This is close to the prevalence of intact immunoglobulin MGUS; thus, more information on the natural history of this condition is needed. Our study is the first detailed report of the natural history of this entity. Of 6 patients, 4 patients progressed to light-chain/nonsecretory myeloma, and the remaining to intact immunoglobulin myeloma. For light-chain only or nonsecretory myeloma, it has been unclear to what extent this occurs de novo, is preceded by MGUS expressing an intact immunoglobulin, or is preceded by MGUS that expresses only a light chain. Therefore, it is significant that all 4 cases of light-chain/nonsecretory myeloma in our study were preceded by light-chain MGUS.
This is the first study documenting the serial changes in both the level of monoclonal immunoglobulin and the sFLC assay before the diagnosis of MM. In 7 evaluable patients, there appeared to be a several-fold increase in the involved free light-chain ratio, either with or without a corresponding change in the intact immunoglobulin level, a few years before the onset of MM (Figure 3). This discordance of serum-free light-chain to intact immunoglobulin may be a harbinger of myeloma in MGUS or SMM patients and would be consistent with prior studies showing that an increasingly abnormal serum free light ratio increases the risk of progression.5 This finding needs confirmation in larger studies that include MGUS patients who do not progress to MM.
Our study includes a unique group of patients. As we analyzed a military population from a serum repository that began in 1990, our study population is distinctly different from other studies of MGUS and MM. First, the median age of our patients was approximately 20 years younger than the median age of 70 years in the general MM population, which allowed us to examine the relationship of MM and MGUS in younger patients.21 Second, nearly 50% of our patients were blacks, a population that is underrepresented in most studies on MGUS and MM despite a more than 2-fold increase of both of these conditions in this group.22 Although it is clear that a larger population of patients needs to be analyzed, our present results show no obvious differences based on age or race for either the frequency of MM without a preceding M-Ig or for rapid progression from MGUS to MM.
In conclusion, myeloma is nearly always preceded by a premalignant plasma cell disorder, most commonly MGUS. MGUS affects at least 3% of adults older than 50 years.23 The current approach to MGUS is for annual clinical follow-up with serum and urine protein electrophoresis, complete blood counts, and serum creatinine and calcium concentrations.9 It has been suggested that patients at very low risk for progression based on monoclonal immunoglobulin levels less than 1.5 g/dL, with or without an abnormal sFLC assay, may not warrant serial follow-up. Our findings suggest that current stratification models are insufficient to change the current practice of careful clinical follow-up. Improved biomarkers are needed to determine the significance of monoclonal gammopathies, and future studies should explore the role of serum free light analysis in addition to proteomic and genomic approaches. Lastly, light-chain–detected MGUS is an important new premalignant entity for which further study is needed.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Karen van Hoeven of The Binding Site Inc.; Drs Angelia Eick and Mark Rubertone at the Armed Forces Health Surveillance Agency; and Ms Debra Marks and Ms Sue Vernigor in the Department of Pathology and Area Laboratory Services, Walter Reed Army Medical Center.
This work was supported by the facilities and resources of the Walter Reed Army Medical Center; the Armed Forces Health Surveillance Agency (Silver Spring, MD); the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research; and a gift of laboratory supplies from The Binding Site Inc. These sponsors had no role in the study design, data analysis, data interpretation, manuscript writing, or the decision to submit for publication.
The views expressed in this paper are those of the authors and not official views of the Department of Defense or the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: B.M.W., P.V., and W.M.K. designed the study; J.A. assisted with study implementation and supervised laboratory procedures; P.V. developed the clinical database; R.S.H. performed statistical analysis; and B.M.W., J.A., and W.M.K. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: The Binding Site, Inc., provided reagents for the serum free light-chain assays and is supporting other studies being performed by J.A. and B.M.W.
Correspondence: Brendan M. Weiss, Hematology-Oncology Service, Walter Reed Army Medical Center, 6900 Georgia Avenue NW, Washington, DC 20307; e-mail: brendan.weiss@us.army.mil.