Current approaches for treatment of late-stage breast cancer rarely result in a long-term cure. In part this is due to tumor stroma that prevents access of systemically or intratumorally applied therapeutics. We propose a stem cell gene therapy approach for controlled tumor stroma degradation that uses the pathophysiologic process of recruitment of inflammatory cells into the tumor. This approach involves genetic modification of hematopoietic stem cells (HSCs) and their subsequent transplantation into tumor-bearing mice. We show that inducible, intratumoral expression of relaxin (Rlx) either by transplanting tumor cells that contained the Rlx gene or by transplantation of mouse HSCs transduced with an Rlx-expressing lentivirus vector delays tumor growth in a mouse model of breast cancer. The antitumor effect of Rlx was mediated through degradation of tumor stroma, which provided increased access of infiltrating antitumor immune cells to their target tumor cells. Furthermore, we have shown in a human/mouse chimeric model that genetically modified HSCs expressing a transgene can access the tumor site. Our findings are relevant for cancer gene therapy and immunotherapy.
Introduction
The histology of late-stage breast cancer is often characterized by tumor nests surrounded by stroma.1 Access of antitumor therapeutics (such as antitumor immune cells, monoclonal antibodies, immunotoxins, and oncolytic viruses) and their intratumoral diffusion is limited by tumor stroma.2,–4 Tumor stroma is composed of stroma cells and a complex matrix containing collagen, laminin, and proteoglycans. Stroma cells include inflammatory cells, predominantly derived from myeloid lineage progenitor cells located in the bone marrow. Most of these tumor-infiltrating hematopoietic cells are macrophages (tumor-associated macrophages or TAMs).5 Tumor cells, among other cytokines, produce monocyte chemo-attractant protein-1 (MCP-1) and colony-stimulating factor-1, which participate in mobilization of TAM progenitors from the bone marrow and homing to tumor stroma. Homing of TAMs to tumors is also supported by the specific architecture of tumor blood vessels that promote efficient trafficking of blood cells. There is convincing evidence that the extent of MCP-1 expression in human cancers, including breast cancer, correlates with both TAM infiltration and tumor malignancy, whereby the correlation of the number of TAMs and malignancy is particularly well documented for patients with breast cancer.6,–8 TAMs produce immunosuppressive cytokines, including IL-10 and TGF-β1, that contribute to immune evasion as well as factors that promote tumor growth and invasion, including HGF, FGF, PDGF, and estrogens.
We propose a stem cell gene therapy approach for treatment of breast cancer that uses the pathophysiologic process of recruitment of hematopoietic cells into the tumor. Because long-term presence of genetically modified stem cells is a key component of our strategy to enable control of cancer and to prevent the relapse of tumor growth, our target cells for genetic modification will be hematopoietic stem cells (HSCs). HSCs are able to provide multilineage reconstitution of blood cells and a source for TAMs. Long engraftment of transplanted HSCs can be achieved after nonmyeloablative cytoreduction by standard cancer chemotherapy.9,10 Ultimately, we plan to transduce ex vivo, autologous HSCs with optimized lentivirus vectors containing transgenes under the control of TAM-specific expression cassettes, transplant these genetically modified cells into patients with cancer after chemotherapy, where they engraft in the bone marrow and provide a constant source of genetically modified cells that home to tumors. Candidate therapeutic genes to be expressed by this approach include (1) membrane-localized enzymes that are able to activate a prodrug resulting in the killing of TAMs and neighboring tumor cells, (2) immunostimulatory molecules, and (3) proteins that are able to permeabilize the tumor stroma to provide access to antitumor therapeutics, specifically antitumor immune cells. In this study we focus on the expression of a stroma-degrading protein to facilitate immune responses in a breast cancer model. T cells specific for tumor-associated antigens (TAAs), such as Her2/neu, are frequently found in patients with untreated breast cancer, even at late stages of disease, and transfer of anti–TAA-specific cells into mice has been shown to reject xenotransplanted autologous tumors.11,12 However in patients, this naturally induced immunity is not sufficient to block tumor growth. Among several mechanisms, tumor stroma contributes in at least 2 critical ways to immune evasion: (1) by creating a physical barrier formed by stroma proteins that prevents direct contact between tumor-infiltrating immune effector cells (eg, CTLs or natural killer [NK] cells) and malignant cells and (2) by production of immunosuppressive cytokines that directly block activation of immune cells or attract/activate immunosuppressive cells such as regulatory T cells (Tregs).
Several approaches have been tested to degrade stroma proteins by application or expression of proteases. Intratumoral injection of collagenase has been shown to remove diffusive hindrance to the penetration of therapeutic molecules within tumors.13 In addition, direct administration of collagenase/dispase or trypsin into glioma xenografts has been shown to enhance the extent of infection of a nonreplicating adenoviral vector expressing a reporter gene.14 Two recent studies have shown that virus-mediated intratumoral expression of MMP1 or MMP8 or both can degrade collagen and improve the spread of oncolytic adenovirus (Ad)15 or herpes simplex virus.16 No evidence of metastatic spread after Ad-mediated MMP8 expression was observed. Another protein that was used to degrade tumor stroma proteins is relaxin (Rlx), a peptide hormone that, during pregnancy, is involved in softening the uterine cervix, vagina, and interpubic ligaments in preparation for parturition.17 This is achieved by remodeling or degrading collagen and altering the connective tissue matrix composition, specifically by up-regulation of matrix metalloproteinases, thus triggering degradation of stroma proteins and blocking TGF-β1–stimulated collagen production. Rlx is the ligand for 2 leucine-rich repeat-containing G-protein coupled receptors (LGRs), LGR7 and LGR8, now classified as Rlx family peptide receptors 1 and 2 (RXFP1 and RXFP2), respectively.18 These receptors have been found on Rlx target tissues, particularly on endometrial stromal cells and tumor stroma fibroblasts.18 In preclinical studies, Ad-mediated mouse Rlx expression from liver reversed cardiac fibrosis without adversely affecting normal collagen levels on other organs.19 In tumor models, chronic application of Rlx by osmotic pumps resulted in collagen degradation and increased intratumoral diffusion of macromolecules, whereby the new tumor matrix, that is created after Rlx is removed, is more porous and hence has a weaker diffusion hindrance.13 In cancer virotherapy studies, Rlx expression enhanced the spread of oncolytic Ad.20,21
In this study, we have tested the effect of regulated, intratumoral Rlx expression on tumor growth in a mouse model of breast cancer involving neu-transgenic mice (neu-tg) and syngeneic mammary carcinoma cells (MMCs). Intratumoral Rlx expression was achieved either by transplanting MMCs that contained the Rlx gene or by transplantation of mouse HSCs transduced with an Rlx-expressing lentivirus vector. In both systems, Rlx expression was inducible by doxycycline (Dox).
Methods
Cells
To obtain mouse HSCs, donor mice were injected with 5-FU (150 mg/kg) intravenously 2 days before bone marrow isolation. A lineage cell depletion kit (Miltenyi Biotec, Auburn, CA) was used to obtain Lin− cells. Lin− cells were analyzed by flow cytometry using antimouse CD3-FITC antibodies (BD PharMingen, San Diego, CA) and antimouse CD117-PE antibodies (BD PharMingen). Bone marrow cells were cultured for 3 days, and nonadherent cells were collected for transplantation or lentivirus infection. Isolation and transduction of human CD34+ cells is described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Animal studies
All experimental procedures involving animals were conducted in accordance with the institutional guideline set forth by the University of Washington and were approved by the university's Institutional Review Board and Institutional Animal Care and Use Committee.
MMC model.
For subcutaneous tumors, a total of 5 × 105 MMCs was subcutaneously injected into neu-tg mice or immunodeficient CB17 severe combined immunodeficient (SCID)/beige mice. For mammary fat pad tumors, 5 × 105 MMCs in 50 μL complete medium were mixed with 50 μL Matrigel (BD Biosciences, San Jose, CA) or PBS and injected into mammary fat pads.
HSC/MMC model.
A total of 106 whole bone marrow cells or lineage cell–depleted bone marrow cells from 5-FU–treated mice were transplanted into lethally irradiated (1050 cGy) female neu-tg mice. Six weeks after bone marrow transplantation, mice received 5 × 105 MMCs by subcutaneous injection.
Human/mouse chimera.
Transplant recipients were 6- to 10-week-old, female CB17 SCID/beige mice, sublethally irradiated with 375 cGy immediately before tail vein injection with 3 × 105 lentivirus vector–transduced CD34+ umbilical cord blood (UCB) cells. After engraftment of human cells in the recipients' bone marrow was confirmed, a total of 2 × 106 MDA-MB435 (HTB-129; ATCC, Manassas, VA) human breast cancer cells were injected into the portal vein through a permanently placed catheter as described elsewhere.22
Immunohistochemistry and cytochemistry
At 3 weeks after tumor cell transplantation, mice were killed, and organs were harvested and processed as follows. Samples were (1) placed in OCT compound (Sakura Finetech, Torrance, CA) without fixation; (2) fixed in 4% paraformaldehyde, dehydrated in 5%, 10%, and 20% sucrose in PBS, and then embedded in OCT; or (3) fixed in 10% formalin and then embedded in paraffin. All antibodies used are listed in Table S1. Analysis of paraffin sections from human breast cancer tissues was described previously.23
Analysis of tumor-infiltrating lymphocytes in tumor-draining lymph nodes and MMC tumors
Isolated MMC tumors or lymph nodes were minced and filtered through a 70-μm cell strainer. Tumor-infiltrating lymphocytes (TILs) were then isolated from tumor cells/erythrocytes by centrifugation in a Ficoll gradient. TILs as well as splenocytes from tumor-bearing mice were used for analysis of Neu-specific T cells with the use of a Neu-tetramer assay. The PE-labeled H-2Dq/RNEU420-429 (H-2D(q)PDSLRDLSVF) tetramer was obtained from the National Institute of Allergy and Infectious Diseases MHC Tetramer Core Facility (Atlanta, GA). Intracellular IFNγ staining was performed according to the manufacturer's intracellular staining protocol (BD Biosciences). All samples were treated with Fc-block (anti-CD16/CD32; BD Biosciences). Corresponding isotope controls yielded no significant staining.
Blood cell counts
Mouse blood was analyzed with the use of a HemaVet 950FS machine (Drew Scientific, Dallas, TX).
Microphotographs
All microphotographs were taken on a Leica DMLB microscope (Wetzlar, Germany) using the following objectives without oil: 40×/0.65 numeric aperture (NA), 20×/0.4 NA, and 10×/0.25 NA. For immunofluorescence analysis, sections were embedded in Vector Shield (Vector, Burlingame, CA). Photographs were taken with a Leica DFC 300FX camera. Leica Application Suite (version 2.4.1 R1) was used for image acquisition. Each image shows a scale bar representing 100 μm.
Results
Analysis of tumor-infiltrating leukocytes in human breast cancer samples and in mouse model for breast cancer
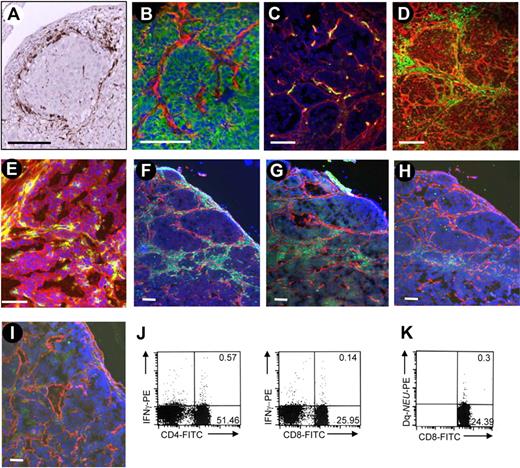
We stained tumor section from patients with breast cancer for CD68, a marker that is expressed on TAMs in a variety of cancers.7,24 We found CD68+ cells at high density in the tumor stroma in all biopsies; a characteristic section is shown in Figure 1A. To reproduce this histopathology in an animal model, we used FVB/N-TgN 220 (MMTV/neu) mice that overexpress rat neu in breast tissue (neu-tg mice). Neu-tg mice develop active immune tolerance toward Neu, which is dependent on Tregs and is similar to what is observed in patients with breast cancer.25 We transplanted MMCs, a cell line derived from a spontaneous mammary tumor from neu-tg mice,25 subcutaneously into neu-tg mice and analyzed tumor sections 3 weeks later (Figure 1B-I). MMCs formed nests of epithelial (E-cadherin–positive) tumors surrounded by laminin (Figure 1B). Blood vessels rarely penetrate the layer of stroma proteins (Figure 1C). Tumor stroma was massively infiltrated with CD45+ leukocytes (Figure 1D). Tumor-infiltrating leukocytes were separated from MMCs by tumor stroma proteins. Tumor-infiltrating leukocytes expressed the mouse ortholog for the human Rlx receptor LGR8 (Figure 1E). Most tumor infiltrating leukocytes were F4/80+ macrophages (Figure 1F). Infiltrating cells also contained CD4, CD8, and NK cells (Figure 1G-I). Detectable amounts of Neu-specific CD8 cells (measured by a Neu-tetramer assay26 ) and IFNγ-producing CD4 and CD8 cells were found in the tumor-draining lymph nodes (Figure 1J,K) and in tumor-infiltrating lymphocytes (S.T., Y.L., Z.Y.L., et al, Vaccine, accepted March, 2009). In summary, tumor nests and lymphocyte compartments appear to be physically separated in patient tumors and MMC tumors by tumor stroma. Despite the presence of tumor-specific CD8 cells, tumor progression cannot be controlled. We hypothesize that physical barriers formed by tumor stroma contribute to inhibition of tumor-reactive immune cells.
Analysis of tumor-infiltrating cells in human and mouse tumors. (A) Tumor sections from patients with breast cancer were stained for the macrophage marker CD68 (brown) and counterstained with hematoxylin. (B-I) Sections of subcutaneous MMC tumors in neu-tg mice. Tumors were analyzed 3 weeks after MMC transplantation. (B) Adherens junction marker E-cadherin (green) and stroma marker laminin (red); (C) endothelial marker CD31 (green) and laminin (red); (D) panleukocyte marker CD45 (green) and laminin (red); (E) CD45 (green) and relaxin receptor (red); (F) macrophage marker F4/80 (green) and laminin (red); (G) CD4 T-cell marker (green) and laminin (red); (H) CD8 T-cell marker (green) and laminin (red); (I) NK cell marker NK1.1 (green) and laminin (red). Nuclei are stained with DAPI (blue). The scale bar represents 100 μm. (J) Quantification of immune cells in tumor-draining lymph nodes. Intracellular cytokine staining for IFNγ and CD4, using anti–IFNγ-PE antibodies and anti-CD8, anti–CD4-FITC antibodies. (K) Quantification of immune cells in tumor-draining lymph nodes. Frequency of Neu-specific CD8+ cells in splenocytes was determined by Tetramer assay.26 Characteristic samples are shown. The numbers in the quadrants represent the precentage of positive cells.
Analysis of tumor-infiltrating cells in human and mouse tumors. (A) Tumor sections from patients with breast cancer were stained for the macrophage marker CD68 (brown) and counterstained with hematoxylin. (B-I) Sections of subcutaneous MMC tumors in neu-tg mice. Tumors were analyzed 3 weeks after MMC transplantation. (B) Adherens junction marker E-cadherin (green) and stroma marker laminin (red); (C) endothelial marker CD31 (green) and laminin (red); (D) panleukocyte marker CD45 (green) and laminin (red); (E) CD45 (green) and relaxin receptor (red); (F) macrophage marker F4/80 (green) and laminin (red); (G) CD4 T-cell marker (green) and laminin (red); (H) CD8 T-cell marker (green) and laminin (red); (I) NK cell marker NK1.1 (green) and laminin (red). Nuclei are stained with DAPI (blue). The scale bar represents 100 μm. (J) Quantification of immune cells in tumor-draining lymph nodes. Intracellular cytokine staining for IFNγ and CD4, using anti–IFNγ-PE antibodies and anti-CD8, anti–CD4-FITC antibodies. (K) Quantification of immune cells in tumor-draining lymph nodes. Frequency of Neu-specific CD8+ cells in splenocytes was determined by Tetramer assay.26 Characteristic samples are shown. The numbers in the quadrants represent the precentage of positive cells.
Relaxin-mediated tumor stroma degradation facilitates antitumor immune response
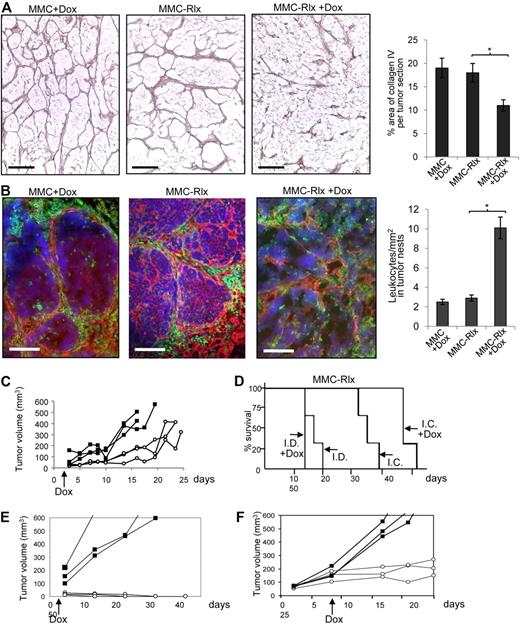
Our final goal is to use tumor-infiltrating inflammatory cells to deliver a gene to the tumor site that is able to transiently degrade tumor stroma and thereby facilitate access of existing antitumor immune cells to malignant cells. We first tested whether this can be achieved by regulated Rlx expression at the tumor site. We transduced, ex vivo, MMCs with a VSV-G–pseudotyped lentivirus vector expressing mouse Rlx under control of a modified Tet-on system (LV-Rlx). Transduced MMCs (MMC-Rlx cells) were expanded and induction of Rlx expression was measured in cells 24 hours after the addition of Dox to culture medium (1 μg/mL). Dox increased Rlx mRNA expression approximately 50-fold (Figure S1). However, there was also detectable Rlx mRNA expression without Dox induction. The biologic activity of Rlx in the supernatant of transduced MMCs was measured based on its ability to induce cAMP production. Using the THP-1 cAMP bioassay, we found 155-fold (± 23-fold) higher cAMP levels in MMC-Rlx cells treated with Dox, compared with MMCs. Next, we subcutaneously injected MMCs and MMC-Rlx cells into neu-tg mice. Two days later, half of the mice received 0.2 mg/mL Dox in drinking water, and mice were killed 27 days after initiation of Dox treatment. Tumor sections were analyzed for stroma matrix proteins (Figure 2A) in relation to infiltrating leukocytes (Figure 2B). Induction of Rlx production by Dox led to a marked fragmentation in the tumor stoma. Furthermore, quantitative morphometry showed significantly less collagen IV in MMC-Rlx + Dox samples compared with MMC + Dox tumors or MMC-Rlx (w/o Dox) tumors (Figure 2A right). Induction of Rlx expression and matrix degradation enabled more direct access of tumor-infiltrating leukocytes to malignant cells (Figure 2B left; Figure S2), which is reflected by more leukocytes detected inside tumor nests (Figure 2B right). Growth of MMC-Rlx tumors was significantly delayed in mice treated with Dox (Figure 2C). To assess whether this antitumor effect was immune mediated, the experiment was repeated in (immunocompetent) neu-tg mice and immunodeficient SCID/beige CB17 mice. Tumor growth was monitored in a Kaplan-Meyer survival study (Figure 2D). Induction of Rlx expression by Dox had a significant antitumor effect in immunocompetent but not in immunodeficient mice. Notably, Dox treatment had no effect on growth of MMC tumors that did not express Rlx in SCID/beige CB17and neu-tg mice (Figure S3).
Rlx-mediated stroma degradation in subcutaneous tumors mediates antitumor responses. (A,B) MMCs transduced with lentivirus vectors expressing mouse Rlx under Dox control (MMC-Rlx) or unmodified MMCs were used to establish tumors in neu-tg mice. Two days after transplantation, Dox was added to drinking water for selected groups of animals. Tumors were harvested 3 weeks after cell implantation, and tumor sections were analyzed for collagen IV (A) and laminin/CD45 (B). The scale bar represents 100 μm. Morphometry for collagen IV and quantification of CD45+ cells in tumor nests are shown on the right sides. Error bar represents SD. (C) Tumor size of MMC-Rlx tumors in neu-tg mice with Dox (○) and without Dox (■) treatment was measured over a period of 45 days. Each line represents one mice. (D) Kaplan-Meyer survival study in neu-tg mice (I.C. indicates immunocompetent) and CB17 SCID/beige (I.D. indicates immunodeficient) mice bearing MMC-Rlx tumors with and without Dox treatment (started at day 2 after tumor cell implantation.) Tumor size was measured daily. End point was the day when tumors reached a size of 1000 mm3 (n = 7 per group). (E) Tumor growth after injection of MMC-Rlx29 without Dox (■) and with Dox (○) treatment that was started at day 2 after tumor cell implantation. (F) Tumor growth after injection of MMC-Rlx29 without Dox (■) and with Dox (○) treatment that was started when tumors reached a size of approximately 70 mm.3
Rlx-mediated stroma degradation in subcutaneous tumors mediates antitumor responses. (A,B) MMCs transduced with lentivirus vectors expressing mouse Rlx under Dox control (MMC-Rlx) or unmodified MMCs were used to establish tumors in neu-tg mice. Two days after transplantation, Dox was added to drinking water for selected groups of animals. Tumors were harvested 3 weeks after cell implantation, and tumor sections were analyzed for collagen IV (A) and laminin/CD45 (B). The scale bar represents 100 μm. Morphometry for collagen IV and quantification of CD45+ cells in tumor nests are shown on the right sides. Error bar represents SD. (C) Tumor size of MMC-Rlx tumors in neu-tg mice with Dox (○) and without Dox (■) treatment was measured over a period of 45 days. Each line represents one mice. (D) Kaplan-Meyer survival study in neu-tg mice (I.C. indicates immunocompetent) and CB17 SCID/beige (I.D. indicates immunodeficient) mice bearing MMC-Rlx tumors with and without Dox treatment (started at day 2 after tumor cell implantation.) Tumor size was measured daily. End point was the day when tumors reached a size of 1000 mm3 (n = 7 per group). (E) Tumor growth after injection of MMC-Rlx29 without Dox (■) and with Dox (○) treatment that was started at day 2 after tumor cell implantation. (F) Tumor growth after injection of MMC-Rlx29 without Dox (■) and with Dox (○) treatment that was started when tumors reached a size of approximately 70 mm.3
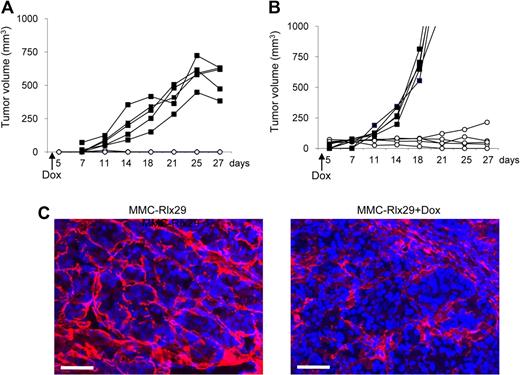
So far, we worked with a population of MMCs transduced with LV-Rlx. However, Rlx expression within this population was very heterogeneous. This is reflected in Rlx mRNA expression studies on clonal cultures derived from MMC-Rlx cells (Figure S4). Expression levels without Dox and induction factors on the addition of Dox varied within several orders of magnitude, which is probably a result of position effects on lentivirus integration and of variegation/silencing of gene expression.27 This is a problem that we are planning to address in the future using insulated self-inactivating lentivirus vectors.28 Here we used a clone of MMC-Rlx cells (MMC-Rlx29) with minimal background expression of Rlx mRNA that could be increased approximately 180-fold by Dox (Figure S4). Transplantation of MMC-Rlx29 into mice resulted in aggressive tumor growth without Dox and a markedly delayed tumor growth when Dox was added to the drinking water 2 days after tumor cell transplantation (Figure 2E). Importantly, in the latter group, most tumors disappeared by day 25 and did not reappear for the time of the experiment. In another experiment, Dox treatment was started only when MMC-Rlx29 tumors reached a size of approximately 70 mm3 (Figure 2F). In this model, induction of Rlx expression also led to an attenuation of tumor growth.
Next, we tested the effect of Rlx expression on MMC tumors growing orthotopically, in mammary fat pads of neu-tg mice. Figure 3A shows that Dox-induction of Rlx in transplanted MMC-Rlx29 tumors inhibited tumor growth. To further model the extracellular environment found in breast cancer, we injected MMC-Rlx29 cells mixed with Matrigel, a gelatinous protein mixture secreted by mouse tumor cells, into the mammary fat pad of neu-tg mice (Figure 3B). In mice without Dox treatment, MMC-Rlx29/Matrigel tumors grew faster than did MMC-Rlx29 tumors. However, even in this more challenging model, Dox-induced Rlx expression attenuated tumor growth. As in subcutaneous tumors, this effect can be attributed to Rlx-mediated stroma degradation as indicated by laminin immunofluorescence analysis on sections of mammary fat pad MMC-Rlx29/Matrigel tumors in mice with and without Dox treatment (Figure 3C).
Therapeutic effect of Rlx expression in orthotopic tumors. (A,B) MMC-Rlx29 cells were injected without (A) or with (B) Matrigel into the mammary fat pad of neu-tg mice. Tumor growth in mice without Dox treatment (■) and with Dox treatment (○) that was started at day 2 after tumor cell implantation is shown. Each line represents one animal. Growth of mammary fat pad MMC tumors with and without Dox did not differ from that of MMC-Rlx29 tumors without Dox (data not shown). (C) Immunofluorescence analysis for laminin (red) on sections of MMC-Rlx29 tumors (without and with Dox) harvested at day 27 after tumor implantation. The scale bar represents 100 μm.
Therapeutic effect of Rlx expression in orthotopic tumors. (A,B) MMC-Rlx29 cells were injected without (A) or with (B) Matrigel into the mammary fat pad of neu-tg mice. Tumor growth in mice without Dox treatment (■) and with Dox treatment (○) that was started at day 2 after tumor cell implantation is shown. Each line represents one animal. Growth of mammary fat pad MMC tumors with and without Dox did not differ from that of MMC-Rlx29 tumors without Dox (data not shown). (C) Immunofluorescence analysis for laminin (red) on sections of MMC-Rlx29 tumors (without and with Dox) harvested at day 27 after tumor implantation. The scale bar represents 100 μm.
In summary, induction of Rlx expression from MMCs resulted in degradation of tumor stroma, increased access of infiltrating leukocytes to tumor cells, and exerted an antitumor effect that appeared to be immune mediated.
Role of immune effector cells in relaxin-mediated stimulation of antitumor responses
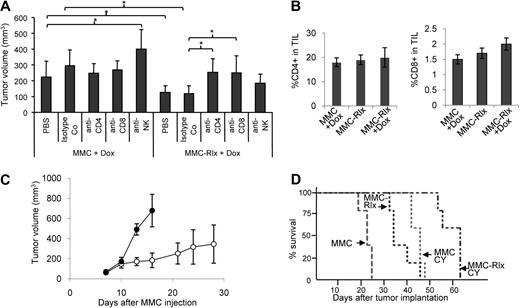
To investigate the role of different immune effector arms in Rlx-mediated antitumor activity, we depleted NK, CD4, and CD8 in neu-tg mice that received MMCs or MMC-Rlx cells. Depletion efficiencies were 61% (± 6%), 96.9% (± 3%), and 98.8% (± 0.5%) for NK, CD4, and CD8 cells, respectively (Figure S5). There was no significant difference in depletion efficiency between MMC- and MMC-Rlx tumor–carrying mice. As expected, in Dox-treated mice, MMC-Rlx tumors were significantly smaller than tumors derived from MMCs that did not express Rlx (Figure 4A). MMC tumor growth appeared to be controlled by NK cells because depletion of NK cells resulted in larger tumors. In MMC-Rlx–bearing mice, CD8 depletion nearly abolished the antitumor effect of Rlx expression. This is also apparent in survival studies; Dox-treated mice bearing MMC-Rlx tumors, which were depleted for CD4 or CD8 cells, lived significantly shorter than mice treated with a corresponding isotype control antibody (P = .005; Figure S6). This indicates that the antitumor effect of Rlx is mediated through CD8 cells. We therefore studied the frequency of anti-Neu CD8 T cells in tumor-infiltrating leukocytes by tetramer assay. In both Dox-treated MMC- and MMC-Rlx tumor–bearing mice, the percentage of CD8 cells in TILs and the percentage of Neu-specific T cells in CD8 cells was comparable (Figure 4B). This suggests that Rlx acted through providing better access of Neu-specific CD8 cells to tumor cells, rather than stimulating expansion of tumor-specific T cells. To test whether intratumoral Rlx expression also enhances systemic and memory antitumor responses, we performed rechallenging experiments (Figure 4C). We established MMC-Rlx29 tumors at the right inguinal area of mice, which were then treated with Dox. Two weeks after vaccination with MMC-Rlx29 cells, mice received a subcutaneous injection of 5 × 105 MMCs at the left inguinal region. Secondary, MMC tumors grew out significantly slower in mice that were vaccinated with Rlx-expressing tumors compared with nonvaccinated mice.
Tumor growth in mice depleted for CD4, CD8, or NK cells. Neu-tg mice received an intraperitoneal injection of anti-CD4, anti-CD8, and anti-GM1 three days before transplantation of MMC-Rlx cells (pool) and then every fourth day. Control mice were injected with isotope control antibody. All mice received Dox. (A) Tumor size at day 14 after injection (n = 5 per group). Significant differences of P < .001 are indicated by an asterisk. (B) Quantification of immune cells in MMC and MMC-Rlx tumors 3 weeks after tumor cell transplantation. All mice received Dox, starting at day 2 after transplantation. Tumor-infiltrating lymphocytes were extracted and analyzed by flow cytometry for the frequency of CD4+ and CD8+ cells in TILs. Differences between groups were not significant. (C) Rechallenge study. Mice received a subcutaneous injection of 105 MMC-Rlx29 cells into the left inguinal region (○) or a PBS injection (■). Both groups were treated with Dox as described in Figure 2F. Two weeks after injection of PBS or MMC-Rlx29 cells, mice received a subcutaneous injection of 5 × 105 MMCs into the right inguinal region. Shown is the volume of challenge tumors at different days after MMC cell implantation. n = 5 per group. Error bars represent SD. (D) Combination of Rlx expression and Treg depletion by low-dose cyclophosphamide. Mice with tumors derived from MMCs or MMCs expressing Rlx (MMC-Rlx) received drinking water with or without Dox, starting day 2 after implantation. At day 12 after tumor transplantation, mice were injected intraperitoneally with 2 mg cyclophosphamide (CY) or PBS. Kaplan-Meyer survival data are shown. End point was the day when tumors reached a size of 500 mm3 (n = 7 per group).
Tumor growth in mice depleted for CD4, CD8, or NK cells. Neu-tg mice received an intraperitoneal injection of anti-CD4, anti-CD8, and anti-GM1 three days before transplantation of MMC-Rlx cells (pool) and then every fourth day. Control mice were injected with isotope control antibody. All mice received Dox. (A) Tumor size at day 14 after injection (n = 5 per group). Significant differences of P < .001 are indicated by an asterisk. (B) Quantification of immune cells in MMC and MMC-Rlx tumors 3 weeks after tumor cell transplantation. All mice received Dox, starting at day 2 after transplantation. Tumor-infiltrating lymphocytes were extracted and analyzed by flow cytometry for the frequency of CD4+ and CD8+ cells in TILs. Differences between groups were not significant. (C) Rechallenge study. Mice received a subcutaneous injection of 105 MMC-Rlx29 cells into the left inguinal region (○) or a PBS injection (■). Both groups were treated with Dox as described in Figure 2F. Two weeks after injection of PBS or MMC-Rlx29 cells, mice received a subcutaneous injection of 5 × 105 MMCs into the right inguinal region. Shown is the volume of challenge tumors at different days after MMC cell implantation. n = 5 per group. Error bars represent SD. (D) Combination of Rlx expression and Treg depletion by low-dose cyclophosphamide. Mice with tumors derived from MMCs or MMCs expressing Rlx (MMC-Rlx) received drinking water with or without Dox, starting day 2 after implantation. At day 12 after tumor transplantation, mice were injected intraperitoneally with 2 mg cyclophosphamide (CY) or PBS. Kaplan-Meyer survival data are shown. End point was the day when tumors reached a size of 500 mm3 (n = 7 per group).
In summary, intratumoral Rlx expression facilitated immune responses that delayed MMC tumor growth and were dependent on CD4 and CD8 T cells. We conclude that this is because of better access of T cells to tumor cells.
Additive effect of Rlx and Treg depletion
As outlined in the Introduction, antitumor immune responses in patients with breast cancer as well as in our mouse model are inhibited by Tregs. Therefore, we studied whether blocking/eliminating Tregs and Rlx expression would have an additive antitumor effect (Figure 4D). We have previously shown that one intraperitoneal injection of low-dose (2 mg) cyclophosphamide per mouse efficiently decreases the number of intratumoral and peripheral Tregs without significantly affecting the levels of CD8 cells.23 In this study, application of low-dose cyclophosphamide to MMC tumor–bearing neu-tg mice alone caused a delay of MMC tumor growth. Importantly, mice with Rlx-expressing tumors that received low-dose cyclophosphamide lived significantly longer than mice treated with a single agent (either MMC-Rlx alone or cyclophosphamide alone). This antitumor effect of low-dose cyclophosphamide alone or in combination with intratumoral Rlx expression was immune mediated and did not occur in immunodeficient mice (Figure S7). Our data give a rationale for future attempts to combine intratumoral Rlx expression with immunostimulatory approaches.
Biodistribution of transplanted mouse bone marrow cells
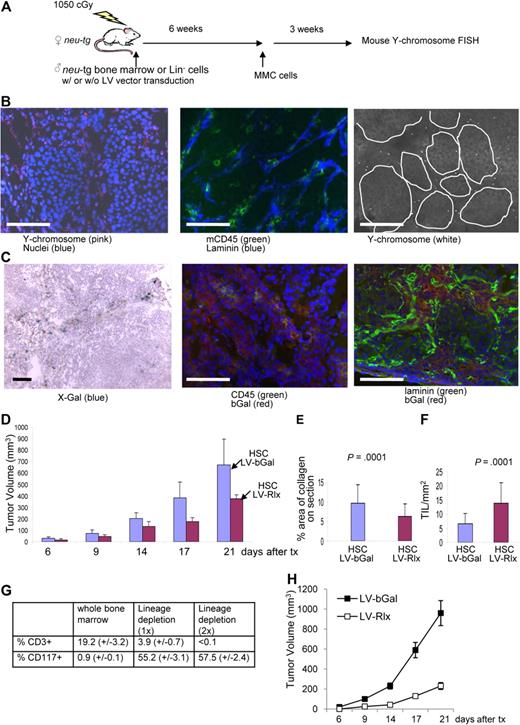
As a proof of principle that stroma degradation has an antitumor effect, so far, we used MMCs that provide intratumoral production of Rlx. Our ultimate goal is to use tumor-infiltrating cells, specifically TAMs, to deliver a regulatable Rlx gene into the tumor. Toward this goal, we first studied whether transplanted mouse HSCs home to MMC tumors in neu-tg mice. In a first series of experiments, as a source of HSCs, we used bone marrow cells from mice that were injected intravenously with 5-FU (150 mg/kg) 2 days before collection of bone marrow. This method allows for enrichment of HSCs and primitive cells.29 Bone marrow cells were cultured for 3 days, and nonadherent cells (enriched for HSCs and primitive progenitors) were collected for transplantation or lentivirus infection. We used 2 methods to trace transplanted HSCs. The first method involved transplantation of HSCs from male neu-tg mice into lethally irradiated female neu-tg mice. Six weeks later, mice received MMCs, and 3 weeks later biodistribution of donor cells was analyzed by fluorescence in situ hybridization (FISH) for mouse Y chromosome DNA (Figure 5A). Most bone marrow cells in female recipients were Y chromosome positive, indicating efficient engraftment of donor cells (Figure S8A). On tumor sections, Y chromosome–positive cells were predominantly found in the tumor stroma and stained CD45+ on consecutive sections (Figures 5B, S9). The second method to confirm homing of genetically modified HSCs to tumors involved ex vivo infection HSCs with an Escherichia coli β-galactosidase–expressing VSVG-pseudotyped lentivirus vector (LV-bGal). Transplantation of marked cells was performed as described in the male-to-female transplantation study. MMC tumors were analyzed 3 weeks later for bGal expression (Figures 5C, S9). Most CD45+ cells, present in MMC tumors, were found to express the transgene bGal, whereby bGal-positive cells were localized to the tumor stroma. These studies indicate that HSCs expressing a transgene can be used as a vehicle to access the tumor. Clearly, donor cells not only homed to tumors but also to normal organs, as shown by Y chromosome FISH and bGal staining (Figure S8B,C).
Studies with transplanted mouse bone marrow stem cells. (A) Scheme of experiment. Whole bone marrow cells (B-G) or Lineage-depleted (Lin−) cells (G,H) of male neu-tg mice were transplanted into lethally irradiated female neu-tg mice by tail injection. Six weeks after HSC engraftment, MMC tumors were established by injection of 5 × 105 MMCs. (B) Three weeks after tumor transplantation, tumor sections were analyzed for mouse Y chromosome (left), mouse CD45, and laminin (middle). (B right) Y chromosome signals are shown in a black and white exposure to better visualize their relation to tumor nests. (C) Tumor localization of transplanted mouse bone marrow cells that were transduced with a VSV-G pseudotyped b-Gal–expressing lentivirus vector (LV-bGal). Tumor sections were analyzed for bGal by X-Gal staining (left) and immunofluorescence with anti-bGal and anti-CD45 antibodies (middle) or anti-bGal and antilaminin antibodies (right). The scale bar represents 100 μm. (D) Mouse bone marrow cells were transduced ex vivo with the relaxin-expressing lentivirus vectors (LV-Rlx) or LV-bGal and transplanted into irradiated neu-tg mice. Six weeks later, MMCs were injected subcutaneously. Two days after MMC transplantation mice received Dox, and tumor size was measured (n = 7 per group). (E,F) Three weeks after tumor cell transplantation, morphometric analysis of collagen IV was performed on tumor sections (E), and the number of CD45+ TILs inside tumor nests in direct contact with tumor cells was counted on 10 sections stained for CD45 and laminin (n = 7 per group; F). (G) Flow cytometric analysis for the T-cell marker CD3 and the HSC marker CD117 (cKit) in whole bone marrow and Lin− cells obtained after 1 (1x) or 2 rounds (2x) of magnetic cell sorting using a Lineage-cell depletion kit. Bone marrow was harvested from femurs of neu-tg mice 2 days after 5-FU treatment (n = 3 per group). (H) LV-bGal- or LV-Rlx-transduced Lin− cells were transplanted into lethally irradiated neu-tg mice. Six weeks later, MMC tumors were established by mammary fat pad injection of 5 × 105 MMCs in Matrigel. Both experimental groups received Dox treatment. Shown is the tumor volume (n = 5 per group). Error bars represent SD.
Studies with transplanted mouse bone marrow stem cells. (A) Scheme of experiment. Whole bone marrow cells (B-G) or Lineage-depleted (Lin−) cells (G,H) of male neu-tg mice were transplanted into lethally irradiated female neu-tg mice by tail injection. Six weeks after HSC engraftment, MMC tumors were established by injection of 5 × 105 MMCs. (B) Three weeks after tumor transplantation, tumor sections were analyzed for mouse Y chromosome (left), mouse CD45, and laminin (middle). (B right) Y chromosome signals are shown in a black and white exposure to better visualize their relation to tumor nests. (C) Tumor localization of transplanted mouse bone marrow cells that were transduced with a VSV-G pseudotyped b-Gal–expressing lentivirus vector (LV-bGal). Tumor sections were analyzed for bGal by X-Gal staining (left) and immunofluorescence with anti-bGal and anti-CD45 antibodies (middle) or anti-bGal and antilaminin antibodies (right). The scale bar represents 100 μm. (D) Mouse bone marrow cells were transduced ex vivo with the relaxin-expressing lentivirus vectors (LV-Rlx) or LV-bGal and transplanted into irradiated neu-tg mice. Six weeks later, MMCs were injected subcutaneously. Two days after MMC transplantation mice received Dox, and tumor size was measured (n = 7 per group). (E,F) Three weeks after tumor cell transplantation, morphometric analysis of collagen IV was performed on tumor sections (E), and the number of CD45+ TILs inside tumor nests in direct contact with tumor cells was counted on 10 sections stained for CD45 and laminin (n = 7 per group; F). (G) Flow cytometric analysis for the T-cell marker CD3 and the HSC marker CD117 (cKit) in whole bone marrow and Lin− cells obtained after 1 (1x) or 2 rounds (2x) of magnetic cell sorting using a Lineage-cell depletion kit. Bone marrow was harvested from femurs of neu-tg mice 2 days after 5-FU treatment (n = 3 per group). (H) LV-bGal- or LV-Rlx-transduced Lin− cells were transplanted into lethally irradiated neu-tg mice. Six weeks later, MMC tumors were established by mammary fat pad injection of 5 × 105 MMCs in Matrigel. Both experimental groups received Dox treatment. Shown is the tumor volume (n = 5 per group). Error bars represent SD.
Effect of Rlx expression from tumor-homing mouse bone marrow cells on tumor growth
Next, we transduced HSCs with LV-Rlx, the lentivirus vector that carried the Rlx gene under Dox control. LV-Rlx–transduced cells were transplanted into myeloablated neu-tg mice. Mice that received transplants with LV-bGal HSCs were used as controls. Six weeks later, MMCs were subcutaneously transplanted into both groups of mice, and Dox was added to the drinking water at day 2 after transplantation. Mice that received LV-Rlx–transduced HSCs had significantly smaller tumors compared with the control animals (Figure 5D). Analysis of tumor histology showed less collagen IV (Figure 5E) and more TILs in contact with tumor cells (Figure 5F) in mice that received a transplant with LV-RLx/HSCs. The antitumor effect in this study is remarkable, considering that the transduction efficiency of HSCs with the LV-Rlx vector was only approximately 5%. In this experiment, we also analyzed differential blood cell counts (Figure S10). No significant differences between Dox-treated mice transplanted with unmodified HSCs or HSCs transduced with LV-bGal or LV-Rlx were found for whole blood cell counts, numbers of neutrophils, lymphocytes, monocytes, erythrocytes, platelets, levels of hemoglobin, or hematocrits. However, numbers of eosinophils and basophils were significantly greater in mice that received a transplant with LV-Rlx/HSCs compared with the other 2 groups.
To show that LV-Rlx–transduced HSCs or primitive cells present in transplanted bone marrow from 5-FU–treated mice mediate the antitumor effect rather than transplanted T cells, we repeated the therapy studies with lineage-depleted bone marrow cells. Lineage cell depletion was achieved using magnetic cell sorting with antibodies against CD5, CD45R, CD11b, Gr-1, 7-4, and Ter-119. Lin− HSCs were analyzed by flow cytometry for the T-cell marker CD3 and the HSC marker cKit/CD117. Although whole (nondepleted) bone marrow contained approximately 19.2% (± 3.2%) CD3+ cells and 0.9% (± 0.1%) cKit/CD117+, one round of depletion reduced the percentage of T cells to 3.9% (± 0.7%), whereas cKit/CD117+ cells were enriched to 55.2% (± 3.1%; Figure 5G; Figure S11). A second round of lineage cell depletion resulted in cell preparations with less than 0.1% CD3+ cells and 57.4% (± 2.4%) cKit/CD117+ cells. Lin− cells were then transduced with LV-bGal or LV-Rlx vectors in the presence of low cytokine concentrations (to prevent differentiation of HSCs) and transplanted into lethally irradiated neu-tg mice. Six weeks later, MMC tumors were established via mammary fat pad injection of 5 × 105 MMCs mixed with Matrigel. We found a significant delay of tumor growth in mice that received transplants with LV-Rlx–transduced Lin− cells compared with mice that received LV-bGal–infected Lin− cells.
In summary, our data provide a support for a HSC-based Rlx gene therapy approach to facilitate antitumor immune responses through degradation of tumor stroma.
Tumor homing of human HSCs in a liver metastasis model for breast cancer
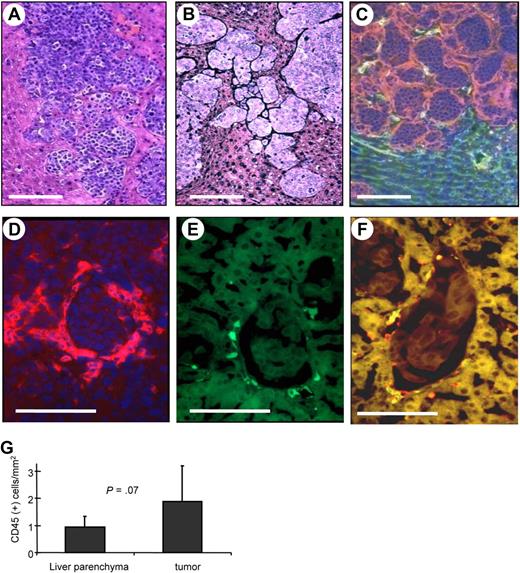
In an attempt to study whether human HSCs would home to tumors and could be used to deliver a gene to the tumor site, we established a mouse model with liver metastases derived from human breast cancer cells. As a tumor model, we used human MDA-MB435 breast cancer cells. Intraportal injection of these cells into immunodeficient mice resulted in clearly distinguishable liver metastases 3 weeks later (Figure 6A). Dense tumor stroma was found surrounding most tumor nests, particularly small tumors, and blood vessels rarely penetrate into the tumor nodule (Figure 6B,C). This morphology is very similar to what is seen in human breast cancer metastases.3 As a source for human HSCs we used umbilical cord blood–derived CD34+ cells. CD34+ cells were transduced with GFP-expressing VSV-G–pseudotyped lentivirus and then transplanted into sublethally irradiated CB17 SCID/beige mice. Bone marrow engraftment of human donor cells was confirmed 6 weeks after transplantation in bone marrow aspirates by flow cytometry for human CD45 and GFP expression. At this time point, mice were injected intraportally with MDA-MB435 cells. Three weeks later, mice were killed, and the distribution of human cells on organ sections was analyzed by immunofluorescence of GFP and the panleukocyte marker CD45. In most tumor nodules, human CD45+ cells were located in the stroma surrounding tumors (Figure 6D). A large number of tumor-associated hCD45+ cells expressed GFP (Figure 6E,F). Quantitative analyses showed significantly higher numbers of human CD45+ cells in and around liver metastases than in liver parenchyma (Figure 6G). As expected, human CD45+ cells were also found in the spleen and, at lower numbers, in the lung and kidney (Figure S12).
Homing of human HSC progeny to liver metastases derived from human breast cancer cells. (A-C) Histology of liver metastases. Two weeks after intraportal MDA-MB435 cell transplantation, liver sections were stained with H&E (A), stained for stroma (black; B), or for laminin (red) and mouse CD31 (green; C). (B-F) Human CD34+ cells were transduced with a GFP-expressing lentivirus vectors and transplanted into sublethally irradiated CB17 SCID/beige mice. Six weeks later, mice received MDA-MB435 cells. Liver sections were analyzed 3 weeks later for laminin (red; D), GFP (green; E), and laminin and GFP (F). Notably, there were no specific fluorescence signals on liver/tumor sections of mice that did not receive LV-GFP–transduced bone marrow cells. The scale bar represents 100 μm. (G) Quantification of human donor cells in liver parenchyma and tumor stroma (N = 15; 5 mice, 3 sections per mouse). Error bars represent SD.
Homing of human HSC progeny to liver metastases derived from human breast cancer cells. (A-C) Histology of liver metastases. Two weeks after intraportal MDA-MB435 cell transplantation, liver sections were stained with H&E (A), stained for stroma (black; B), or for laminin (red) and mouse CD31 (green; C). (B-F) Human CD34+ cells were transduced with a GFP-expressing lentivirus vectors and transplanted into sublethally irradiated CB17 SCID/beige mice. Six weeks later, mice received MDA-MB435 cells. Liver sections were analyzed 3 weeks later for laminin (red; D), GFP (green; E), and laminin and GFP (F). Notably, there were no specific fluorescence signals on liver/tumor sections of mice that did not receive LV-GFP–transduced bone marrow cells. The scale bar represents 100 μm. (G) Quantification of human donor cells in liver parenchyma and tumor stroma (N = 15; 5 mice, 3 sections per mouse). Error bars represent SD.
The expression of several surface markers for human cells was analyzed on liver sections from mice with human donor cells that homed to tumors and on bone marrow smears (Table S2). Positive staining was observed for the human leukocyte markers HLA-DR-DP-DQ and CD45, the human endothelial marker CD31, and the human macrophage markers CD14, CD68, and Tie-2. Infiltrated human cells did not label with markers specific for T or B lymphocytes, NK cells, fibroblasts, myoblasts, or endothelial cells. Our results indicate that tumor-infiltrating human leukocytes are mostly of monocyte lineage, supporting the finding in MMC tumors that most tumor-infiltrating cells are TAMs. In summary, our human/mouse chimeric animal model supports the idea to use genetically modified HSCs as a vehicle to achieve transgene expression at the tumor site.
Discussion
In this study, we tested a stem cell–based approach to deliver the Rlx gene into breast cancer tumors. This approach has several advantages over existing classical or experimental therapies. (1) For long-term control of cancer, most therapies rely on repeated administration of antitumor agents, which can be problematic because of neutralizing immune responses or high financial burden. With our approach, a single stem cell infusion should enable a constant source of genetically modified cells that home to tumors. (2) Genetic targeting/killing of tumor cells is problematic because of their genetic instability and phenotypic heterogeneity, which facilitates the emergence of escape variants. In contrast, stroma cells are genetically stable, and ex vivo transduction of their progenitors is a safe and well-established procedure. (3) The efficacy of cancer gene therapy with virus-based vectors is limited by antiviral immune responses. In contrast, using stem cells as a gene delivery vehicle should not be hampered by immune responses.
Previous stem cell–based approaches focused on genetic modification of bone marrow–derived mesenchymal stem cells (MSCs), and it has been shown that genetically modified MSCs home to tumors after intravenous injection into mouse models.30,31 Our strategy is based on the modification of HSCs. An advantage of HSCs is that, after transplantation into irradiated recipients, they engraft and provide a life-long source of genetically modified cells, specifically of TAMs, which represent the predominant cell type in inflammatory infiltrates of breast cancer. In a previous pivotal study, De Palma et al32 transplanted lentivirus vector–transduced mouse bone marrow cells expressing transgenes under the control of the Tie-2 promoter into myeloablated immunocompetent mice bearing syngeneic tumors and showed that transgene expression was restricted to donor bone marrow–derived macrophages that homed to tumors. Compared with work of De Palma et al,32 our study is novel with regard to the use of genetically modified HSCs to degrade tumor stroma and enable existing immune responses to become tumor destructive. In addition, the therapeutic effect of our approach can be directly controlled through Dox application. Furthermore, we have shown in a chimeric human/mouse model that HSCs can be used for gene delivery to metastatic tumors.
A potential concern is the use of retroviruses for gene transfer. Transduction of human CD34+ cells with mouse leukemia virus (MLV)–based retrovirus vectors has been used in clinical trials for genetic blood diseases such as SCID adenosine deaminase deficiency, X-linked SCID, and sickle cell disease.33 A critical problem with the use of MLV-based retroviruses became apparent in X-linked SCID trials, in which several patients developed leukemia because of insertional mutagenesis and activation of the oncogene(s). However, several recent studies in tumor-prone mouse models have shown that tumorigenesis does not occur with self-inactivating lentivirus vectors, and the use of insulated vectors would decrease the potential risk even more.33 In our own studies, we have not seen any insertional mutagenesis in dogs that received a transplant with lentivirus-transduced HSCs, with a follow-up of more than 5 years.34 In this context, it is noteworthy that we are developing other vector systems that allow for targeted integration in HSCs.35,36
Long-term engraftment of genetically modified HSCs is usually achieved after myeloablative chemotherapy. Although autologous bone marrow transplantation after myeloablative chemotherapy is a procedure that is also performed in patients with nonhematologic cancers,10 it causes great risks and side effects to patients. Notably, engraftment of gene-modified cells has also been successful after (nonmyeloablative) chemotherapy conditioning,10,37 and giving the gene-modified HSCs after a chemotherapy treatment for breast cancer is clinically relevant because the patient will benefit from the chemotherapy and from our new approach.
There are concerns that tumor stroma degradation by Rlx increases metastasis. Studies by Silvertown et al38 reported that permanent Rlx overexpression increased in vivo prostate xenograft tumor growth and angiogenesis. These results were recently revised by the same group.39 The general consensus is now that Rlx expression alone is not sufficient to induce metastasis, a process that involves epithelial to mesenchymal transition, dissociation of cells from the primary tumor, enhanced cell motility, and the ability of cells to invade blood vessels.20,40,41 Notably, in studies involving Ad-mediated Rlx expression, no metastases were found.20,21 In fact, Ad-mediated Rlx expression reversed the spread of tumor cells that normally would metastasize.20 We followed mice that received Rlx-expressing MMC-Rlx29 cells and initially developed tumors for 3 months and at necropsy did not observed metastases macroscopically and on tissue sections.
Rlx expression in our studies did not result in obvious toxicity; however, it caused elevations in basophil and eosinophil counts. On the basis of recent evidence that TAMs have a unique gene expression signature that distinguishes it from other tissue macrophages,42 we are currently working on TAM-specific expression systems in the context of insulated SIN lentivirus vectors to increase the safety of our approach.
Removing physical barriers formed by tumor stroma using intratumoral Rlx expression not only facilitates antitumor immune responses but might also sensitize tumors to other therapies based on oncolytic viruses, cytostatic drugs, tumor-specific antibodies, T cells, viruses, liposomes, or nanoparticles. In this context, we showed that Rlx expression from MMCs improves tumor transduction with an Ad vector (Figure S10). Finally, recruitment of hematopoietic cells appears to be a general phenomenon of cancer, implying that our stem therapy approach might also be relevant for malignancies other than breast cancer. We found infiltrating CD68+ cells on sections of all tumor types analyzed, including osteosarcoma, melanoma, liposarcoma, ovarian cancer, and kidney cancer (Figure S14). Furthermore, flow cytometric analysis of glioma biopsies showed significant amounts of CD45+ cells as well as CD34+, CD33+, or KDR/VEGFR2+ hematopoietic cells in tumors (Figure S15).
In summary, this study describes 2 important findings. HSCs expressing a transgene can be used to access the tumor site, and intratumoral Rlx expression facilitates antitumor immune responses. Both findings are relevant for tumor gene and tumor immunotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Hua Cao and Jonas Persson for help with lineage depletion of bone marrow cells. We also thank Roma Yumul for editing the manuscript.
This work was supported by the National Institutes of Health (grants R01 CA080192, A.L.; R01 HLA078836, A.L.; DK56465, H.K.); by the Pacific Ovarian Cancer Research Consortium (Seattle, WA) Specialized Program of Research Excellence in Ovarian Cancer (grant P50 CA83636); and by the Fred Hutchinson Cancer Research Center (grant P30 DK056465).
National Institutes of Health
Authorship
Contribution: Z.Y.L., Y.L., S.T., Y.X., X.F., L.M., and A.L performed the research; Q.F., N.K., H.-P.K., and M.L.D. contributed vital new reagents; and A.L. wrote paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: André Lieber, University of Washington, Division of Medical Genetics, Box 357720, Seattle, WA 98195; e-mail: lieber00@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal