Abstract
The canonical mode of transcriptional activation by both the Epstein-Barr viral protein, Epstein-Barr virus–encoded nuclear antigen 2 (EBNA2), and an activated Notch receptor (Notch-IC) requires their recruitment to RBPJ, suggesting that EBNA2 uses the Notch pathway to achieve B-cell immortalization. To gain further insight into the biologic equivalence between Notch-IC and EBNA2, we performed a genome-wide expression analysis, revealing that Notch-IC and EBNA2 exhibit profound differences in the regulation of target genes. Whereas Notch-IC is more potent in regulating genes associated with differentiation and development, EBNA2 is more potent in inducing viral and cellular genes involved in proliferation, survival, and chemotaxis. Because both EBNA2 and Notch-IC induced the expression of cell cycle–associated genes, we analyzed whether Notch1-IC or Notch2-IC can replace EBNA2 in B-cell immortalization. Although Notch-IC could drive quiescent B cells into the cell cycle, B-cell immortalization was not maintained, partially due to an increased apoptosis rate in Notch-IC–expressing cells. Expression analysis revealed that both EBNA2 and Notch-IC induced the expression of proapoptotic genes, but only in EBNA2-expressing cells were antiapoptotic genes strongly up-regulated. These findings suggest that Notch signaling in B cells and B-cell lymphomas is only compatible with proliferation if pathways leading to antiapototic signals are active.
Introduction
The Notch signaling network is an evolutionarily conserved intercellular signaling pathway that regulates interactions between physically adjacent cells. In mammals, 4 different Notch receptors are known, referred to as Notch1 through Notch4. Notch is located in the plasma membrane as a hetero-oligomer, consisting of a large extracellular portion (NEC) that associates in a noncovalent interaction with a smaller piece (N™) of the Notch protein composed of a short extracellular region, a single transmembrane pass, and an intracellular region.1
Signal transduction through the classical Notch pathway is initiated by interaction of the Notch extracellular domain with a member of 2 conserved families of ligands (Delta-like and Jagged), and involves a regulated set of proteolytic cleavage events, leading to the translocation of the Notch intracellular fragment (Notch-IC) to the nucleus, where it interacts with the DNA-binding protein RBPJ to activate transcription (for review, see Zimber-Strobl and Strobl2 ). In the lymphoid compartment, Notch1 signaling is indispensable for the generation of T cells,3 whereas Notch2 signaling is essential for the generation of marginal zone B cells.4 The human Notch1 gene was first described in the context of the t(7;9) chromosomal translocation in T-cell acute lymphoblastic leukemia (T-ALL), leading to the overexpression of a constitutively active form of Notch1.5 Since then, deregulated Notch expression and signaling have been described in a wide range of malignancies. The Epstein-Barr viral (EBV) protein, Epstein-Barr virus–encoded nuclear antigen 2 (EBNA2), is regarded to be a functional homolog of an active Notch receptor because it is also tethered to promoter regions by interaction with RBPJ.6–8 However, in contrast to Notch, EBNA2 acts ligand independently and is therefore constitutively active. EBV infects human B cells and epithelial cells and is associated with several human malignancies of lymphoid and epithelial origin, including Burkitt lymphoma, Hodgkin lymphoma, and nasopharygeal carcinoma. EBV is able to immortalize B cells after in vitro infection, leading to the establishment of permanently growing lymphoblastoid cell lines (LCLs). EBNA2 plays an essential role in this immortalization process. Together with EBNA-LP, EBNA2 is the first gene expressed after infection of B cells and activates almost all viral genes expressed in immortalized cell lines by interacting with RBPJ. EBNA2 strongly up-regulates the latent membrane proteins 2A (LMP2A) and 1 (LMP1),9,10 which are mimicking constitutively active forms of the B-cell receptor (BCR) and the CD40 receptor, respectively.11,12,13 In addition, EBNA2 regulates several cellular genes, including CD21, CD23, MYC, and CCR7.14–20 Inactivation of EBNA2 in immortalized cell lines leads to cell-cycle arrest and to cell death, which illustrates its importance in immortalized B cells.14
The oncogenic potential of deregulated Notch1 signaling in T cells and other tumors suggests that EBNA2 contributes to B-cell immortalization by constitutively activating the Notch signaling pathway. However, in the EBV context, constitutive Notch1 signaling could not replace EBNA2 in its function to maintain B-cell proliferation. Partial rescue of proliferation could only be achieved by coexpressing LMP1 independently of EBNA2 or by expressing Notch1-IC at extremely high levels.21,22 This biologic difference between EBNA2 and Notch1-IC might be due either to (1) the circumstance that Notch1 is the inappropriate Notch family member for studies in B cells, because mainly Notch2 is expressed in mature B cells, or to (2) differences of Notch1-IC and EBNA2 in the quantitative and/or qualitative regulation of target genes.
To gain further insight into the functional equivalence among Notch1-IC, Notch2-IC, and EBNA2, we introduced doxycycline-regulatable expression vectors for these proteins into the conditionally immortalized LCL EREB2-5. In this cell line, the viral EBNA2 gene is fused to the estrogen receptor hormone–binding domain, rendering its function estrogen-dependent.14 Because EBNA2 is essential for the proliferation of EBV-infected cells, EREB2-5 cells grow only in the presence of estrogen. In this study, we show that neither Notch1-IC nor Notch2-IC can substitute for EBNA2 in the absence of estrogen, which is due to profound differences in the regulation of target genes involved in proliferation, survival, and differentiation.
Methods
Cell lines and culture conditions
EREB2-5 cells were cultured as described previously.14 Cells were deprived of estrogen by washing them 3 times in RPMI medium containing 10% FCS. EREB2-5 cells were stably transfected with expression plasmids (pRTS-1)23 coding for Notch1-IC, Notch2-IC, or chloramphenicol acetyltransferase (CAT). Expression of Notch1-IC or Notch2-IC (Notch1/2-IC) and CAT was induced by the addition of doxycycline to a final concentration of 100 ng/mL medium.
Plasmids
The full-length intracellular parts of Notch1-IC from position 5278 to 7668 of NM_0176173 and Notch2-IC from position 5362 to 7672 of NM_024408.2 were cloned into the pRTS-1 vector23 under the control of a doxycycline-inducible bidirectional promoter.
Immunoblot analysis
Cells were lysed in Nonidet P-40 buffer (150 mM NaCl, 50 mM Tris/HCL, pH 8, and 1% Igepal), and immunoblotting was performed according to standard procedures.12,13 The following antibodies were used: α-Notch1 (c-20, sc-6014), α-MYC (9E10, sc-40; both purchased from Santa Cruz Biotechnology, Santa Cruz, CA), α-Notch2 (C651.6DbHN; developed by S. Artavanis-Tsakonas, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), α-phosphatidylinositol 3-kinase (PI3K) p85 (06-195; purchased from Upstate Biotechnology, Lake Placid, NY), α-LMP1 (cs1-4; purchased from Dianova, Hamburg, Germany), and α-LMP2A (14B6)24 (courtesy of E. Kremmer, Helmholtz Center Munich, Munich, Germany).
Flow cytometry
Cells were stained with α-CD21, α-IgM, and α-nerve growth factor receptor (NGFR) antibodies (all BD Biosciences, San Jose, CA). Data were analyzed with a FACSCalibur (BD Biosciences) using CellQuest software.
The 5-bromo-2′-deoxyuridine incorporation and apoptosis assays
Cells were analyzed 3 days after addition of doxycycline and withdrawal of estrogen. The 5-bromo-2′-deoxyuridine (BrdU) incorporation in pulse-chased ΔNGFR+ cells was measured by fluorescence-activated cell sorter (FACS; fluorescein isothiocyanate [FITC] BrdU flow kit; BD Biosciences). Apoptotic cells were analyzed by annexin V–FITC/7-aminoactinomycin D (7-AAD) staining. Apoptotic cells were determined as annexinV+7-AAD− (annexin V: FITC apoptosis detection kit; BD Biosciences).
Magnetic-activated cell sorting
Before RNA or protein isolation, ΔNGFR+ cells were magnetic-activated cell sorting (MACS) purified after staining with α-NGFR antibody (derived from the ATCC-HB 8737 cell line) and incubation with goat α-mouse magnetic beads (Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions.
Microarray analysis
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's specifications. Double strand (ds) cDNA was synthesized with the Microarray cDNA synthesis kit (Roche Diagnostics, Mannheim, Germany) and purified with the Microarray target purification kit (Roche Diagnostics). Using the Microarray RNA target synthesis kit (Roche Diagnostics), ds-cDNA was converted to biotinylated cRNA, which was hybridized to the Affymetrix HGU 133 PLUS2.0 arrays. For each time point, samples from 3 independent experiments were analyzed, in total on 42 chips. Expression values for all genes were generated by Affymetrix (Santa Clara, CA) Suite 5.0 software (MAS5.0). CEL files were analyzed using ChipInspector (Genomatix, Munich, Germany), comparing each CEL file of a given time point of the kinetics with the respective 0-hour file in an exhaustive way. Significantly changed probes were then defined within ChipInspector by significance analysis of microarrays (SAM) analysis25 at a false discovery rate of 1%. Only significantly changed transcripts with a minimum probe coverage of 3 were accepted for further analysis. Venn diagrams were compiled with the online software available at http://mcbc.usm.edu/genevenn/genevenn.htm. To compare the regulation of selected target genes, MAS5.0-generated expression values were used. Unsupervised hierarchical clustering was performed using GeneCluster (Eisen laboratory, University of California, Berkeley, CA, http://rana.lbl.gov/eisen/?page_id = 42) and displayed with TreeView (Eisen laboratory, University of California, Berkeley, CA, http://rana.lbl.gov/eisen/?page_id = 42). The allocation of genes to functional groups was performed by dCHIP (Department of Biostatistics, Harvard School of Public Health, Boston, MA, http://biosun1.harvard.edu/complab/dchip) using GeneOntology annotation.
Quantitative real-time–polymerase chain reaction
All primer pairs for quantitative real-time–polymerase chain reaction (qRT-PCR) were designed using Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and were chosen spanning 2 exons (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). For qRT-PCR, total RNA was isolated using TRIzol reagent (Invitrogen), according to manufacturer's specifications. cDNA was reversely transcribed from total RNA using first strand cDNA synthesis kit for RT-PCR (Roche Diagnostics) with oligo-p(dT)15 primers, according to the manufacturer's instructions. qRT-PCR was performed using the LightCycler system (Roche Diagnostics), whereas data were collected based on the threshold cycle method in the LightCycler software 3.3. Transcripts were amplified from cDNA using LightCycler FastStart kit (Roche Diagnostics), according to the manufacturer's protocol. Relative expression ratios were calculated using the ΔΔCP method, as described previously.26 Expression was standardized by using a primer pair amplifying the RNAs of the ribosomal proteins RPL23a, similar to ribosomal protein L23A, ribosomal protein L23a-like, and similar to RPL23AP7.
Statistics
The Student t test was applied to determine the significance of qRT-PCR, BrdU, and annexin V values.
Microarray data accession numbers
Normalized expression values were deposited in the Gene Expression Omnibus repository as series number GSE12355 (www.ncbi.nlm.nih.gov/geo/).
Results
Generation of EBV-transformed B-cell lines expressing doxycycline-regulatable human Notch1-IC or Notch2-IC
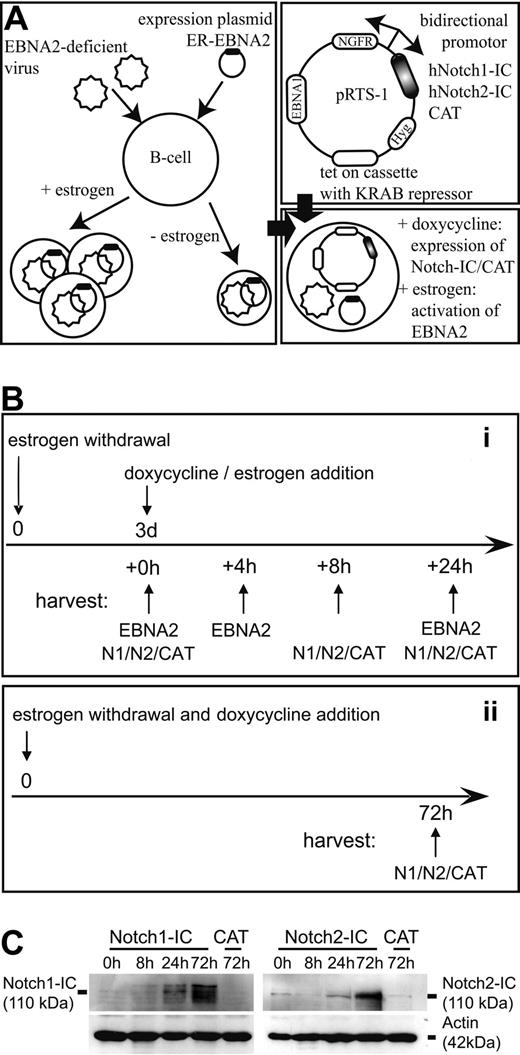
To test the functional equivalence among Notch1-IC, Notch2-IC, and EBNA2 in B cells, we established a cell system that allows separate activation of EBNA2 and Notch1-IC or Notch2-IC by addition of estrogen and doxycyline, respectively (Figure 1A). As parental cell line, we chose EREB2-5 cells, a conditional LCL, in which EBNA2 is fused to the estrogen receptor hormone binding domain.14 Estrogen withdrawal leads to the inactivation of EBNA2, resulting in cell cycle arrest. cDNAs coding for the full-length intracellular parts of human Notch1 (Notch1-IC) and human Notch2 (Notch2-IC) were cloned downstream of a bidirectional doxycycline-dependent promoter (TetO7) on a pRTS-1 vector.23 As negative control, the bacterial CAT gene was introduced instead of Notch1-IC or Notch2-IC (Notch1/2-IC). In the opposite transcriptional orientation, a cDNA coding for a truncated human ΔNGFR was cloned to allow identification and sorting of doxycycline-induced cells. These expression constructs were stably introduced into EREB2-5 cells to establish the cell lines Notch1-IC/EREB, Notch2-IC/EREB, and CAT/EREB, in which the EBNA2 function is dependent on the presence of estrogen and the expression of Notch1-IC or Notch2-IC on doxycycline.
Establishment of Notch1-IC-, Notch2-IC-, and EBNA2-expressing EREB2-5 cells. (A) Expression vectors for Notch1-IC, Notch2-IC, and CAT (negative control) were stably transfected in EREB2-5 cells, a conditionally immortalized lymphoblastoid cell line, in which EBNA2 function depends on the presence of estrogen. Notch1-IC, Notch2-IC, and CAT were cloned downstream of a bidirectional doxycycline-dependent (Tet-O7) promoter. In the opposite orientation, truncated ΔNGFR was cloned to detect or sort Notch1/2-IC– and CAT-expressing cells. The tet-on cassette is coding for the reverse transactivator and the KRAB repressor. The hygromycin expression cassette (Hyg) allows selection of stably transfected cell clones, and the EBNA1 gene ensures episomal maintenance of the expression plasmids. Transfection of the expression plasmids into EREB cells resulted in the following cell lines: Notch1-IC/EREB, Notch2-IC/EREB, and CAT/EREB. Addition of estrogen results in the activation of EBNA2, and addition of doxycycline leads to the expression of Notch1-IC, Notch2-IC, and CAT as well as ΔNGFR. (B) Experimental design that was valid for all kinetic experiments: (i) before induction, cells were deprived of estrogen for 3 days. Cells were harvested directly after estrogen withdrawal (0 hours), 8 hours and 24 hours after doxycycline addition (Notch1-IC, Notch2-IC, CAT) and 4 hours and 24 hours after estrogen addition to CAT/EREB cells (EBNA2). (ii) To examine long-term effects of Notch1/2-IC expression, doxycycline was added immediately after estrogen depletion and cells were harvested 3 days later. The control cell line CAT/EREB was treated in the same way as Notch1-IC/EREB and Notch2-IC/EREB cells. Before the preparation of RNA or protein isolation, ΔNGFR+ cells were purified by MACS separation to enrich the cells with a transcriptional response to doxycycline. (C) Expression of Notch1-IC and Notch2-IC in stably transfected Notch1-IC/EREB (Notch1-IC), Notch2-IC/EREB (Notch2-IC), and CAT/EREB (CAT) cell lines was analyzed by Western blotting. Cells were treated as outlined in panel B. Proteins were harvested at the indicated time points after doxycycline addition. Notch1-IC and Notch2-IC were detected by specific antibodies raised against the intracellular part of Notch1 (left part) or Notch2 (right part). Equal protein loading was controlled by an α-actin antibody. The weak signal of approximately the same molecular weight as Notch2-IC in Notch2-IC/EREB and in CAT/EREB cells in the absence of doxycycline results from endogenous Notch2 expression in B cells and corresponds to the intracellular membrane-anchored part of the Notch2 receptor (Notch2™).
Establishment of Notch1-IC-, Notch2-IC-, and EBNA2-expressing EREB2-5 cells. (A) Expression vectors for Notch1-IC, Notch2-IC, and CAT (negative control) were stably transfected in EREB2-5 cells, a conditionally immortalized lymphoblastoid cell line, in which EBNA2 function depends on the presence of estrogen. Notch1-IC, Notch2-IC, and CAT were cloned downstream of a bidirectional doxycycline-dependent (Tet-O7) promoter. In the opposite orientation, truncated ΔNGFR was cloned to detect or sort Notch1/2-IC– and CAT-expressing cells. The tet-on cassette is coding for the reverse transactivator and the KRAB repressor. The hygromycin expression cassette (Hyg) allows selection of stably transfected cell clones, and the EBNA1 gene ensures episomal maintenance of the expression plasmids. Transfection of the expression plasmids into EREB cells resulted in the following cell lines: Notch1-IC/EREB, Notch2-IC/EREB, and CAT/EREB. Addition of estrogen results in the activation of EBNA2, and addition of doxycycline leads to the expression of Notch1-IC, Notch2-IC, and CAT as well as ΔNGFR. (B) Experimental design that was valid for all kinetic experiments: (i) before induction, cells were deprived of estrogen for 3 days. Cells were harvested directly after estrogen withdrawal (0 hours), 8 hours and 24 hours after doxycycline addition (Notch1-IC, Notch2-IC, CAT) and 4 hours and 24 hours after estrogen addition to CAT/EREB cells (EBNA2). (ii) To examine long-term effects of Notch1/2-IC expression, doxycycline was added immediately after estrogen depletion and cells were harvested 3 days later. The control cell line CAT/EREB was treated in the same way as Notch1-IC/EREB and Notch2-IC/EREB cells. Before the preparation of RNA or protein isolation, ΔNGFR+ cells were purified by MACS separation to enrich the cells with a transcriptional response to doxycycline. (C) Expression of Notch1-IC and Notch2-IC in stably transfected Notch1-IC/EREB (Notch1-IC), Notch2-IC/EREB (Notch2-IC), and CAT/EREB (CAT) cell lines was analyzed by Western blotting. Cells were treated as outlined in panel B. Proteins were harvested at the indicated time points after doxycycline addition. Notch1-IC and Notch2-IC were detected by specific antibodies raised against the intracellular part of Notch1 (left part) or Notch2 (right part). Equal protein loading was controlled by an α-actin antibody. The weak signal of approximately the same molecular weight as Notch2-IC in Notch2-IC/EREB and in CAT/EREB cells in the absence of doxycycline results from endogenous Notch2 expression in B cells and corresponds to the intracellular membrane-anchored part of the Notch2 receptor (Notch2™).
First, we compared the kinetics of doxycycline and estrogen induction. Before estrogen and doxycycline addition, cells were deprived of estrogen for 3 days to inactivate the EBNA2 function. At this time point, cells were arrested in cell cycle and the viral proteins LMP1 and LMP2A ceased to be expressed (see Figure 4), but most of the cells were still viable (see Figure 5). Cells were analyzed at different time points after estrogen and doxycycline addition. The induction of the TetO7 promoter was traced by analyzing the ΔNGFR expression on the cell surface. The regained ability of EBNA2 for DNA interaction was followed by a gel-retardation assay. ΔNGFR could be detected starting 2 hours after induction and reached its full expression level after 4 hours (Figure S1A). In contrast, the EBNA2 protein was functionally active and translocated to the nucleus already 20 minutes after the addition of estrogen (Figure S1B). Based on these results, we designed the following experimental approaches (Figure 1B). The stably transfected cell lines were kept in the absence of estrogen for 3 days, before doxycycline or estrogen was added. Doxycycline-induced cells were harvested 8 hours and 24 hours, and estrogen-induced cells 4 hours and 24 hours after induction (Figure 1B approach i). To analyze the effects of constitutive Notch signaling over a period of 3 days, cells were induced immediately after estrogen withdrawal and kept in the presence of doxycycline for 3 days before the cells were harvested or analyzed (Figure 1B approach ii).
Next, we tested the expression and functionality of Notch1-IC and Notch2-IC in the absence of EBNA2 in the stably transfected cell lines. Notch1-IC and Notch2-IC could clearly be detected by Western blotting (Figure 1C). The highest protein levels of Notch1/2-IC were obtained when cells were kept in the presence of doxycycline for 3 days, suggesting an accumulation of Notch1/2-IC over time. Two known common Notch1-IC and EBNA2 target genes, CD21 (CR2) and Igμ (IGHM),27 were regulated as expected after doxycycline addition to Notch1-IC/EREB and Notch2-IC/EREB cells. Thus CD21 (CR2) was up-regulated and Igμ (IGHM) was down-regulated after induction of Notch1-IC and Notch2-IC to a similar extent as after activation of EBNA2 on the RNA as well as on the protein level (Figure S2A,B). These data indicate that Notch1-IC and Notch2-IC are functionally active in the stably transfected cell lines to an extent that can be compared with EBNA2 activity.
EBV genes and classical Notch target genes are regulated with different efficiencies by Notch1-IC, Notch2-IC, and EBNA2
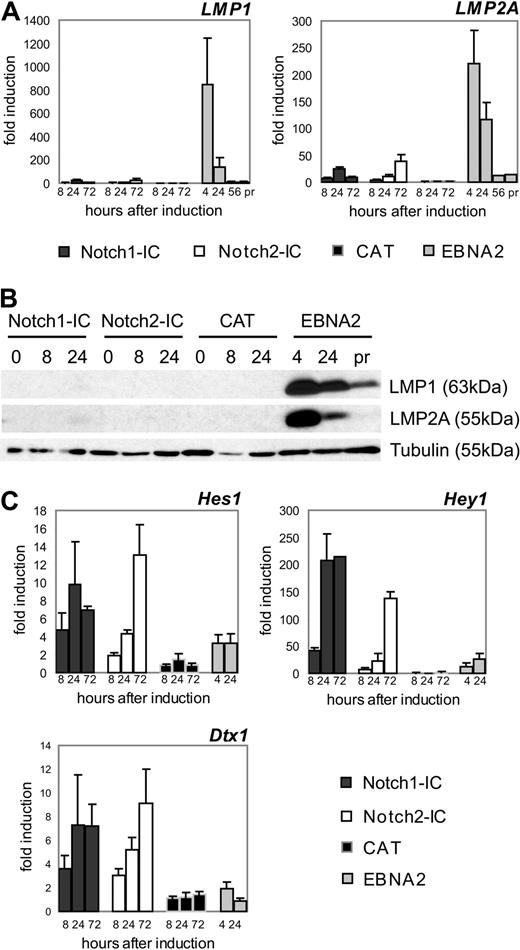
Next, we compared the induction of classical EBNA2 and Notch target genes in EBNA2/EREB and Notch1/2-IC/EREB cells by qRT-PCR.2,27 EBNA2 led to a robust induction of LMP1 (850-fold) and LMP2A (220-fold) mRNA expression, resulting in significantly increased protein levels 4 hours after activation (Figure 2A,B). However, Notch1-IC and Notch2-IC could induce the transcription of these 2 viral genes only approximately 10- to 30-fold, leading to marginal or undetectable LMP1 and LMP2A protein levels. In contrast, classical Notch target genes such as HES1, HEY1, and DTX1 were more potently induced by Notch1-IC and Notch2-IC than by EBNA2 (Figure 2C). These data indicated that EBNA2 and Notch1/2-IC activate target genes with different efficiencies.
Notch1-IC, Notch2-IC, and EBNA2 exhibit profound differences in the regulation of the viral genes LMP1 and LMP2A and the classical Notch target genes HES1, HEY1, and DTX1. Cells were treated as outlined in Figure 1B and harvested at the indicated time points. (A) The regulation of LMP1 and LMP2A on mRNA level in the stably transfected cell lines was investigated by qRT-PCR. To compare LMP1 and LMP2A levels early after EBNA2 induction with that in proliferating EREB cells, RNA was also prepared 56 hours after estrogen addition and from cells that were continuously growing in the presence of estrogen (pr). mRNA levels of LMP1 and LMP2A were normalized to the expression of ribosomal protein genes and standardized to the value at the time point of induction (0 hours) to obtain fold inductions. Values are representative for 3 independent qRT-PCRs of 3 biologic replicates. Error bars represent SDs. (B) LMP1 and LMP2A protein levels at different time points after induction of Notch1-IC, Notch2-IC, or CAT, after EBNA2 reactivation or in proliferating CAT/EREB cells. Whole-cell lysates were harvested directly after estrogen withdrawal (0 hours), at the indicated time points after doxycycline or estrogen addition or from CAT/EREB cells continuously proliferating in the presence of estrogen (pr). Membranes were stained with α-LMP1 or α-LMP2A antibodies. Equal protein loading was controlled by tubulin staining. (C) The regulation of HES1, HEY1, and DTX1 in the stably transfected cell lines was investigated by qRT-PCR. Total RNA was harvested at the indicated time points after doxycycline or estrogen addition. mRNA levels of HES1, HEY1, and DTX1 were normalized to the expression of ribosomal protein genes and standardized to the value at the time of induction (0 hours) to obtain fold inductions. Values are representative of 3 independent qRT-PCRs of at least 2 biologic replicates.
Notch1-IC, Notch2-IC, and EBNA2 exhibit profound differences in the regulation of the viral genes LMP1 and LMP2A and the classical Notch target genes HES1, HEY1, and DTX1. Cells were treated as outlined in Figure 1B and harvested at the indicated time points. (A) The regulation of LMP1 and LMP2A on mRNA level in the stably transfected cell lines was investigated by qRT-PCR. To compare LMP1 and LMP2A levels early after EBNA2 induction with that in proliferating EREB cells, RNA was also prepared 56 hours after estrogen addition and from cells that were continuously growing in the presence of estrogen (pr). mRNA levels of LMP1 and LMP2A were normalized to the expression of ribosomal protein genes and standardized to the value at the time point of induction (0 hours) to obtain fold inductions. Values are representative for 3 independent qRT-PCRs of 3 biologic replicates. Error bars represent SDs. (B) LMP1 and LMP2A protein levels at different time points after induction of Notch1-IC, Notch2-IC, or CAT, after EBNA2 reactivation or in proliferating CAT/EREB cells. Whole-cell lysates were harvested directly after estrogen withdrawal (0 hours), at the indicated time points after doxycycline or estrogen addition or from CAT/EREB cells continuously proliferating in the presence of estrogen (pr). Membranes were stained with α-LMP1 or α-LMP2A antibodies. Equal protein loading was controlled by tubulin staining. (C) The regulation of HES1, HEY1, and DTX1 in the stably transfected cell lines was investigated by qRT-PCR. Total RNA was harvested at the indicated time points after doxycycline or estrogen addition. mRNA levels of HES1, HEY1, and DTX1 were normalized to the expression of ribosomal protein genes and standardized to the value at the time of induction (0 hours) to obtain fold inductions. Values are representative of 3 independent qRT-PCRs of at least 2 biologic replicates.
DTX1 expression is tightly regulated by Notch2 signaling in B cells in vivo.4 Compared with other B-cell subsets, DTX1 mRNA is prominent and approximately 5-fold increased in marginal zone B cells, the generation of which depends on Notch2 signaling. Because DTX1 mRNA expression is induced to a similar extent by Notch1-IC and Notch2-IC in our experimental system, we suggest that the Notch signaling strength is comparable with Notch ligand stimulation in vivo.
Genome-wide expression analysis to compare genes regulated by Notch1-IC, Notch2-IC, and EBNA2
These profound differences between Notch-IC and EBNA2 in the regulation of their respective target genes prompted us to analyze the similarities and differences of Notch1-IC, Notch2-IC, and EBNA2 in gene regulation in a genome-wide approach. We performed Affymetrix GeneChip analysis with the human HGU133 Plus 2.0 chip at 4 different time points (0 hour, 8 hours, 24 hours, and 3 days) after Notch1-IC, Notch2-IC, or CAT induction and at 3 different time points (0 hour, 4 hours, and 24 hours) after EBNA2 activation, as outlined in Figure 1B. Total RNA was isolated from ΔNGFR+ cells (Figure S3). Three independent experiments for each time point were performed to ensure a statistically relevant dataset.
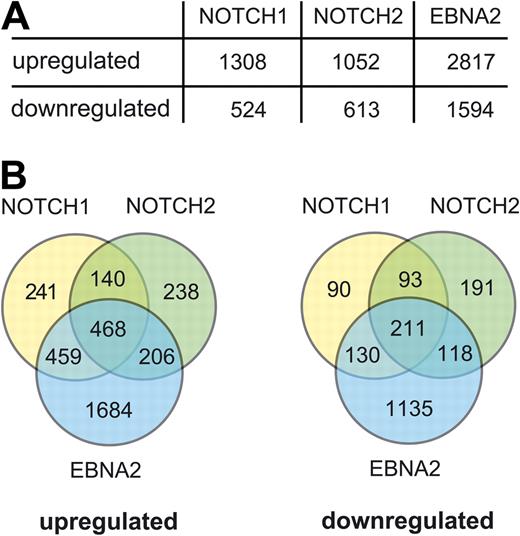
In total, approximately 55 000 probe sets were analyzed, representing roughly 30 000 genes. Permutation-based SAM procedure with a 1% false detection rate cutoff led to 2506, 3735, and 6910 genes differentially expressed in Notch1-IC–, Notch2-IC–, and EBNA2-expressing cells, respectively. The numbers of genes, which were activated or repressed at least 1.5-fold by Notch1-IC, Notch2-IC, or EBNA2 are summarized in Figure 3A. The compilation of Venn diagrams for all up-regulated and down-regulated genes (Figure 3B) revealed that a substantial number of genes were only regulated by EBNA2. Many of them might be indirect target genes induced by the strong and rapid expression of LMP1 and LMP2A. In addition, the comparison of gene sets, which were up- and down-regulated by Notch1-IC and Notch2-IC, revealed considerable differences.
Up- and down-regulated genes in Notch1-IC/EREB, Notch2-IC/EREB, and EBNA2/EREB cells. Affymetrix GeneChip analysis was performed with the human HGU133 Plus 2.0 chip. Notch1-IC/EREB, Notch2-IC/EREB, and CAT/EREB cells were induced and harvested, as outlined in Figure 1B. Before the preparation of total RNA, ΔNGFR+ cells were purified by MACS separation to enrich the cells with a transcriptional response to doxycycline (Figure S3). As negative control, CAT/EREB cells in the absence of estrogen and presence of doxycycline were treated in the same way as Notch1/2-IC/EREB cells. To study EBNA2-regulated genes, CAT/EREB cells were induced by estrogen (EBNA2/EREB). Three independent experiments for each time point were performed to ensure a statistically relevant dataset. Only 17 probe sets were found to be differentially expressed in CAT/EREB cells, excluding a considerable background activity of the tetracycline expression system. (A) Numbers of significantly regulated genes with an at least 1.5-fold induction or repression within any point during the kinetics by Notch1-IC, Notch2-IC, or EBNA2. (B) Significantly regulated genes with an at least 1.5-fold regulation were used for compilation of Venn diagrams to depict genes regulated solely or commonly by Notch1-IC, Notch2-IC, or EBNA2.
Up- and down-regulated genes in Notch1-IC/EREB, Notch2-IC/EREB, and EBNA2/EREB cells. Affymetrix GeneChip analysis was performed with the human HGU133 Plus 2.0 chip. Notch1-IC/EREB, Notch2-IC/EREB, and CAT/EREB cells were induced and harvested, as outlined in Figure 1B. Before the preparation of total RNA, ΔNGFR+ cells were purified by MACS separation to enrich the cells with a transcriptional response to doxycycline (Figure S3). As negative control, CAT/EREB cells in the absence of estrogen and presence of doxycycline were treated in the same way as Notch1/2-IC/EREB cells. To study EBNA2-regulated genes, CAT/EREB cells were induced by estrogen (EBNA2/EREB). Three independent experiments for each time point were performed to ensure a statistically relevant dataset. Only 17 probe sets were found to be differentially expressed in CAT/EREB cells, excluding a considerable background activity of the tetracycline expression system. (A) Numbers of significantly regulated genes with an at least 1.5-fold induction or repression within any point during the kinetics by Notch1-IC, Notch2-IC, or EBNA2. (B) Significantly regulated genes with an at least 1.5-fold regulation were used for compilation of Venn diagrams to depict genes regulated solely or commonly by Notch1-IC, Notch2-IC, or EBNA2.
To ascertain whether genes that are mainly regulated by Notch1/2-IC or EBNA2 belong to different functional groups, we clustered all genes that were up-regulated stronger by Notch1/2-IC than by EBNA2 and vice versa. DTX1, which was induced only by Notch1/2-IC, but not by EBNA2 (see Figure 2C), was chosen as a representative gene for the correlation of all genes mainly regulated by Notch1/2-IC. These genes clustered to functional groups like cell communication, cell differentiation, and development (Figure S4A-C).
Vice versa, we also clustered all genes with a similar expression pattern to PIK3R1, which was strongly induced by EBNA2, but not by Notch1/2-IC. A large number of target genes was induced 4 hours after EBNA2 activation, which were not or only marginally regulated by Notch1/2-IC (Figure S5A,B). These genes clustered to functional groups such as apoptosis, cell activation, cell proliferation, and chemotaxis (Figure S5C).
Only a small group of genes was similarly up-regulated by EBNA2 and Notch-IC, as shown by correlation of genes with an expression pattern similar to CR2 (Figure S6A,B). No functional group could be assigned significantly to these genes, due to their low number. These data indicate that EBNA2 and Notch1/2-IC differ considerably in their efficiency to activate target genes.
Cell cycle–associated genes regulated by Notch1-IC, Notch2-IC, and EBNA2
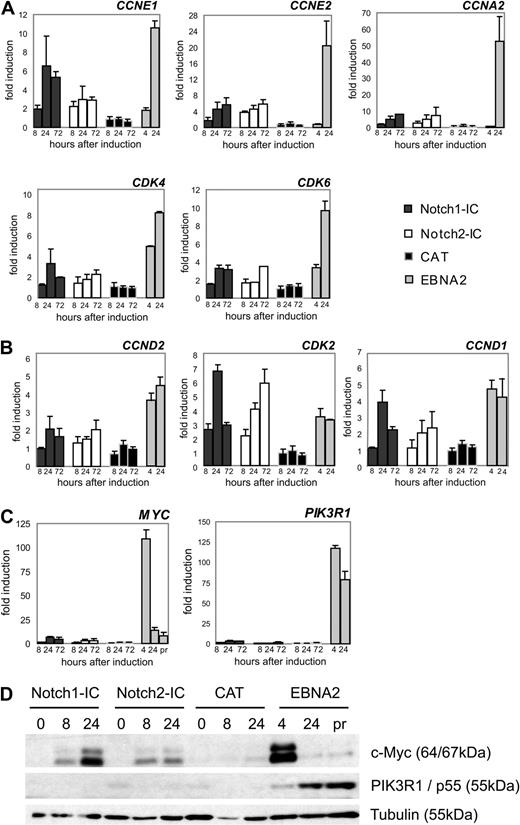
The group of genes similarly regulated by Notch1/2-IC and EBNA2 contained cyclin (CCN) D1, and cyclin-dependent kinase (CDK) 2 (Figure S6A,B), both involved in the G1-S transition of the cell cycle. To gain further insight into the regulation of cell cycle– and proliferation-associated genes, we clustered all differentially expressed genes that were assigned to these functional groups (Figure S7). Genes were arranged in 4 clusters. Genes in clusters 1 and 2 were up-regulated late by EBNA2, suggesting an indirect regulation. Notch1/2-IC was able to induce most of these genes, but to a lower extent than EBNA2. We selected CCNE1 (cyclin E1), CCNE2 (cyclin E2), CCNA2 (cyclin A2), and CDK6 from cluster 1 and CDK4 from cluster 2, to confirm their regulation by qRT-PCR (Figure 4A).
Cell cycle– and proliferation-associated genes differentially expressed by Notch1-IC, Notch2-IC, and EBNA2. Cells were treated as outlined in Figure 1B and harvested at the indicated time points. mRNA levels were quantified by qRT-PCR. mRNA levels were normalized to the expression of ribosomal protein genes and standardized to the value at the time of induction (0 hours). Values represent fold induction of mRNA levels compared with the mRNA levels at 0 hours, which was set to 1. Values are representative for 3 independent qRT-PCRs of at least 2 biologic replicates. (A) Cell cycle–associated genes, which were up-regulated late by EBNA2. These genes were in all cases up-regulated stronger by EBNA2 than by Notch1/2-IC. Cyclins (CCN); cyclin-dependent kinases (CDK). (B) CCN and CDK, which were up-regulated early by EBNA2 and were regulated similarly by Notch1/2-IC. (C) qRT-PCR analysis of the proliferation-associated genes phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) and MYC, which were up-regulated early and strongly by EBNA2, and only marginally by Notch1/2-IC. (D) Protein expression of MYC and PIK3R1 by Notch1/2-IC and EBNA2: Notch1-IC/EREB, Notch2-IC/EREB, and CAT/EREB cells were deprived of estrogen for 3 days. Whole-cell lysates were harvested from growth-arrested cells (0 hours), from cells that were stimulated with doxycycline for 8 hours or 24 hours (Notch1-IC, Notch2-IC, CAT) or from CAT/EREB cells, which were stimulated with estrogen for 4 hours or 24 hours (EBNA2). In addition, whole-cell extracts were prepared from CAT/EREB cells continuously growing in the presence of estrogen (pr). Membranes were stained with antibodies raised against human Myc protein and PIK3R1 (recognizing p85α, p55α, and p50α). Equal protein loading was controlled by staining with an α-tubulin antibody. Error bars in panels A through C represent SDs.
Cell cycle– and proliferation-associated genes differentially expressed by Notch1-IC, Notch2-IC, and EBNA2. Cells were treated as outlined in Figure 1B and harvested at the indicated time points. mRNA levels were quantified by qRT-PCR. mRNA levels were normalized to the expression of ribosomal protein genes and standardized to the value at the time of induction (0 hours). Values represent fold induction of mRNA levels compared with the mRNA levels at 0 hours, which was set to 1. Values are representative for 3 independent qRT-PCRs of at least 2 biologic replicates. (A) Cell cycle–associated genes, which were up-regulated late by EBNA2. These genes were in all cases up-regulated stronger by EBNA2 than by Notch1/2-IC. Cyclins (CCN); cyclin-dependent kinases (CDK). (B) CCN and CDK, which were up-regulated early by EBNA2 and were regulated similarly by Notch1/2-IC. (C) qRT-PCR analysis of the proliferation-associated genes phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) and MYC, which were up-regulated early and strongly by EBNA2, and only marginally by Notch1/2-IC. (D) Protein expression of MYC and PIK3R1 by Notch1/2-IC and EBNA2: Notch1-IC/EREB, Notch2-IC/EREB, and CAT/EREB cells were deprived of estrogen for 3 days. Whole-cell lysates were harvested from growth-arrested cells (0 hours), from cells that were stimulated with doxycycline for 8 hours or 24 hours (Notch1-IC, Notch2-IC, CAT) or from CAT/EREB cells, which were stimulated with estrogen for 4 hours or 24 hours (EBNA2). In addition, whole-cell extracts were prepared from CAT/EREB cells continuously growing in the presence of estrogen (pr). Membranes were stained with antibodies raised against human Myc protein and PIK3R1 (recognizing p85α, p55α, and p50α). Equal protein loading was controlled by staining with an α-tubulin antibody. Error bars in panels A through C represent SDs.
Clusters 3 and 4 contained genes that were strongly activated already 4 hours after EBNA2 activation. Among them were some known EBNA2 target genes such as CCND2 (cyclin D2), MYC, and PIK3R1 in cluster 3 and CDK2 in cluster 4. Some of these genes in clusters 3 and 4 were comparably up-regulated by EBNA2 and Notch1/2-IC as CCND1, CCND2, and CDK2, whereas others as MYC and PIK3R1 were preferentially up-regulated by EBNA2, as confirmed by qRT-PCR (Figure 4B,C). In accordance with the mRNA expression data, PIK3R1 protein could be detected in EBNA2- but not in Notch1/2-IC-expressing cells. (Figure 4D). MYC protein expression was induced by Notch2-IC and to a higher extent by Notch1-IC, but did not reach the high peak of MYC protein levels as observed early after EBNA2 induction (Figure 4D).
Notch1-IC and Notch2-IC lead to limited S-phase entry and induction of apoptosis
Notch1-IC can only partially substitute for EBNA2 in B-cell immortalization.21,22 For Notch2, the major Notch family member in mature B cells, no experimental approach addressing this topic has been performed to date. Because Notch1/2-IC induced cell cycle-associated genes and induced, in addition, considerable MYC protein levels, we readdressed the question as to whether Notch1-IC or Notch2-IC can replace EBNA2 in its function to maintain B-cell immortalization.
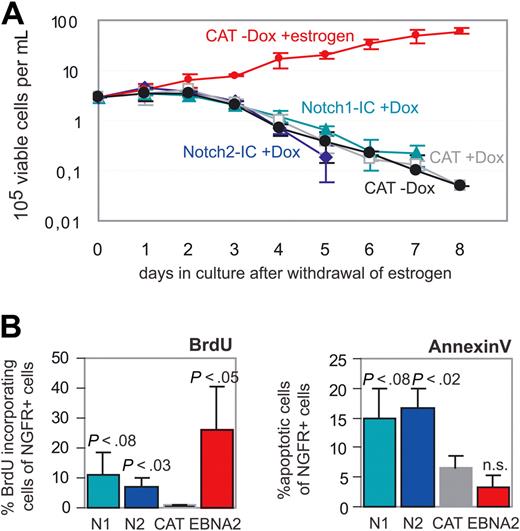
Cells were deprived of estrogen, and immediately reinduced either by doxycyline (Notch1-IC/EREB, Notch2-IC/EREB, and CAT/EREB) or by estrogen (EBNA2/EREB). Living cells were counted over a period of 8 days. Whereas the number of EBNA2/EREB cells increased over this period, the cell numbers declined in Notch1-IC- and Notch2-IC-expressing cells with similar kinetics as in the negative control CAT/EREB (Figure 5A). In comparison with CAT/EREB cells, the percentage of ΔNGFR+ cells rather declined than increased in Notch1/2-IC/EREB cells, assuming a negative selection by Notch1/2-IC expression (data not shown). However, 3 days after estrogen withdrawal, unlike the negative control CAT/EREB, Notch1-IC/EREB and Notch2-IC/EREB cells were still able to incorporate BrdU (Figure 5B), indicating that Notch1/2-IC can drive some cells into the S phase, although cell numbers did not increase. By annexin staining, we tested whether this is due to an apoptotic effect of Notch1/2-IC expression. The higher percentage of apoptotic cells in Notch1/2-IC (15%-17%)– compared with CAT (7%)– or EBNA2 (3%)–expressing cells suggests that in the absence of EBNA2, Notch1/2-IC actively induces apoptosis in EBV-infected B cells (Figure 5B).
Neither Notch1-IC nor Notch2-IC can maintain B-cell immortalization in the absence of EBNA2. Cells were deprived of estrogen, and Notch1-IC, Notch2-IC, CAT, and EBNA2 were immediately induced by addition of doxycyline and estrogen, respectively. (A) The maintenance of immortalization in Notch1-IC/EREB (Notch1-IC + Dox), Notch2-IC/EREB (Notch2-IC + Dox), and CAT/EREB (CAT + Dox; CAT-Dox) cells in the absence of estrogen was investigated by counting living cells over a period of 8 days after withdrawal of estrogen. CAT/EREB cells after readdition of estrogen (CAT-Dox + estrogen) were used as positive control. Fresh culture medium was added every other day. CAT/EREB cells in the presence of estrogen were diluted when the cells reached a density of 106 cells/mL. These dilutions were included in the calculation of the depicted cell numbers. (B) BrdU incorporation was used as a marker for proliferation. Three days after estrogen withdrawal, the indicated cell lines Notch1-IC/EREB (N1), Notch2-IC/EREB (N2), and CAT/EREB (CAT) were incubated with BrdU for 4 hours. BrdU incorporation into ΔNGFR+ cells was analyzed by FACS. Apoptotic cells were determined 3 days after estrogen withdrawal by annexin V/7-AAD staining. Percentage of early apoptosis was measured by analyzing annexin V+7-AAD− cells in ΔNGFR+ cells of Notch1-IC/EREB (N1), Notch2-IC/EREB (N2), and CAT/EREB (CAT). As positive control, CAT/EREB cells, which were reinduced by estrogen immediately after estrogen withdrawal, were analyzed (EBNA2). Both diagrams show mean values of 4 independent experiments. P values were determined by Student t test comparing Notch1-IC/EREB (N1), Notch2-IC/EREB (N2) in the absence of estrogen and CAT/EREB in the presence of estrogen (EBNA2) with the control CAT/EREB (CAT) in the absence of estrogen.
Neither Notch1-IC nor Notch2-IC can maintain B-cell immortalization in the absence of EBNA2. Cells were deprived of estrogen, and Notch1-IC, Notch2-IC, CAT, and EBNA2 were immediately induced by addition of doxycyline and estrogen, respectively. (A) The maintenance of immortalization in Notch1-IC/EREB (Notch1-IC + Dox), Notch2-IC/EREB (Notch2-IC + Dox), and CAT/EREB (CAT + Dox; CAT-Dox) cells in the absence of estrogen was investigated by counting living cells over a period of 8 days after withdrawal of estrogen. CAT/EREB cells after readdition of estrogen (CAT-Dox + estrogen) were used as positive control. Fresh culture medium was added every other day. CAT/EREB cells in the presence of estrogen were diluted when the cells reached a density of 106 cells/mL. These dilutions were included in the calculation of the depicted cell numbers. (B) BrdU incorporation was used as a marker for proliferation. Three days after estrogen withdrawal, the indicated cell lines Notch1-IC/EREB (N1), Notch2-IC/EREB (N2), and CAT/EREB (CAT) were incubated with BrdU for 4 hours. BrdU incorporation into ΔNGFR+ cells was analyzed by FACS. Apoptotic cells were determined 3 days after estrogen withdrawal by annexin V/7-AAD staining. Percentage of early apoptosis was measured by analyzing annexin V+7-AAD− cells in ΔNGFR+ cells of Notch1-IC/EREB (N1), Notch2-IC/EREB (N2), and CAT/EREB (CAT). As positive control, CAT/EREB cells, which were reinduced by estrogen immediately after estrogen withdrawal, were analyzed (EBNA2). Both diagrams show mean values of 4 independent experiments. P values were determined by Student t test comparing Notch1-IC/EREB (N1), Notch2-IC/EREB (N2) in the absence of estrogen and CAT/EREB in the presence of estrogen (EBNA2) with the control CAT/EREB (CAT) in the absence of estrogen.
Differentially expressed proapoptotic and antiapoptotic genes in Notch1-IC-, Notch2-IC-, and EBNA2-expressing cells
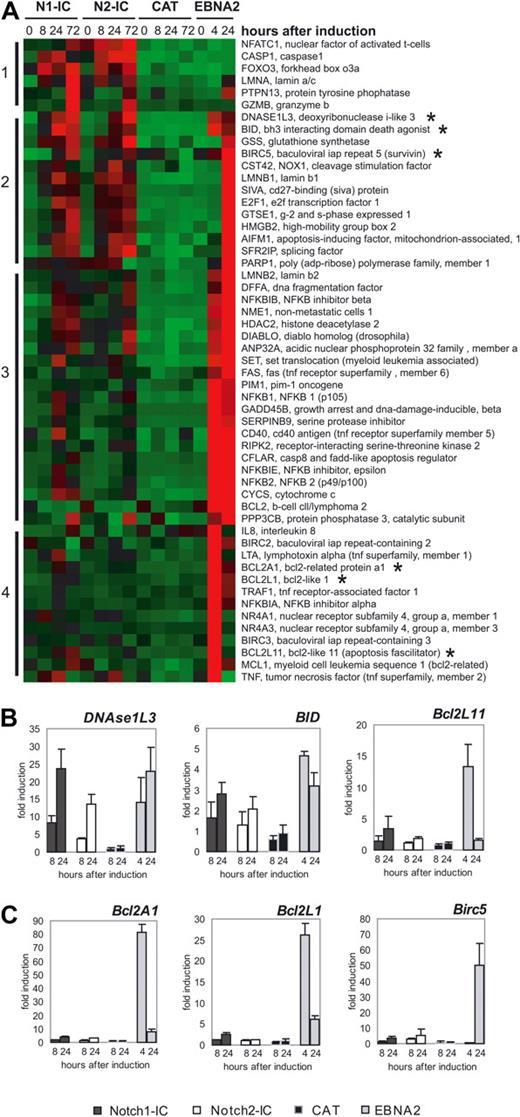
Because we observed an increased percentage of apoptotic cells in Notch1/2-IC/EREB cells compared with CAT/EREB cells, we examined chip data for genes associated with apoptosis. The cluster analysis revealed 4 different clusters (Figure 6). Cluster 1 contained genes that are mainly induced by Notch1/2-IC, cluster 2 genes that are regulated by Notch1/2-IC and EBNA2, and cluster 3 and 4 genes that are mainly regulated by EBNA2. Whereas cluster 1 contained mainly proapoptotic genes such as FOXO3 and GZMB, we found pro- as well as antiapoptotic genes in clusters 2, 3, and 4. We identified several proapoptotic genes as DNASE1L3, BID, SIVA, AIFM1, GADD45B, and BCL2L11 (BIM) that are comparably induced by Notch1/2-IC and EBNA2. In contrast, most antiapoptotic genes such as BCL2A1 (BFL-1), BCL2L1, BIRC5 (Survivin), BCL2, BIRC2, BIRC3, and MCL1 were found to be more intensely up-regulated by EBNA2 than by Notch1/2-IC. We confirmed the regulation of the proapoptotic genes DNASE1L3, BID, and BCL2L11 (BIM) (Figure 6B), as well as the antiapoptitic genes BCL2A1, BCL2L1, and BIRC5 (Figure 6C) by qRT-PCR.
Differentially expressed proapoptotic and antiapoptotic genes in Notch1-IC/EREB, Notch2-IC/EREB, CAT/EREB, and EBNA2/EREB cells. (A) The heat map displays genes associated with anti- and proapoptotic functions, which were regulated at least 2-fold. Absolute expression values are compressed for better demonstration to −2 to +2. Red squares indicating a high (up to +2) expression, green (up to −2) a low expression, and black the mean (0) expression over the whole kinetics of all cell lines. Numbers and bars on the left side describe the clusters after hierarchical clustering. Gene symbols and gene names are depicted on the right side of the heat map. The cell lines are indicated above the heat map, as follows: N1-IC (Notch1-IC/EREB), N2-IC (Notch2-IC/EREB), CAT (CAT/EREB), and EBNA2 (CAT/EREB after addition of estrogen). The transcripts of which the expression was validated by quantitative RT-PCR are indicated by asterisks. Quantitative RT-PCR analysis of apoptotic (B) and antiapoptotic (C) genes: cells were deprived of estrogen for 3 days before doxycycline (Notch1-IC, Notch2-IC, CAT) or estrogen (EBNA2) addition. Total RNA was prepared from growth-arrested cells 0 hours, 8 hours, and 24 hours after doxycycline addition, and 4 hours and 24 hours after estrogen induction. mRNA expression was measured by qRT-PCR. mRNA levels are normalized to the transcriptional level of ribosomal protein genes and standardized to the time point of induction (0 hours), to calculate the fold induction at the different time points after doxycycline or estrogen addition. Values are representative of 3 independent qRT-PCRs of 3 biologic replicates. The analyzed genes are indicated above the diagrams; the coding of the graphs is elucidated beside the diagram: Notch1-IC (Notch1-IC/EREB), Notch2-IC (Notch2-IC/EREB), CAT (CAT/EREB), and EBNA2 (CAT/EREB after addition of estrogen).
Differentially expressed proapoptotic and antiapoptotic genes in Notch1-IC/EREB, Notch2-IC/EREB, CAT/EREB, and EBNA2/EREB cells. (A) The heat map displays genes associated with anti- and proapoptotic functions, which were regulated at least 2-fold. Absolute expression values are compressed for better demonstration to −2 to +2. Red squares indicating a high (up to +2) expression, green (up to −2) a low expression, and black the mean (0) expression over the whole kinetics of all cell lines. Numbers and bars on the left side describe the clusters after hierarchical clustering. Gene symbols and gene names are depicted on the right side of the heat map. The cell lines are indicated above the heat map, as follows: N1-IC (Notch1-IC/EREB), N2-IC (Notch2-IC/EREB), CAT (CAT/EREB), and EBNA2 (CAT/EREB after addition of estrogen). The transcripts of which the expression was validated by quantitative RT-PCR are indicated by asterisks. Quantitative RT-PCR analysis of apoptotic (B) and antiapoptotic (C) genes: cells were deprived of estrogen for 3 days before doxycycline (Notch1-IC, Notch2-IC, CAT) or estrogen (EBNA2) addition. Total RNA was prepared from growth-arrested cells 0 hours, 8 hours, and 24 hours after doxycycline addition, and 4 hours and 24 hours after estrogen induction. mRNA expression was measured by qRT-PCR. mRNA levels are normalized to the transcriptional level of ribosomal protein genes and standardized to the time point of induction (0 hours), to calculate the fold induction at the different time points after doxycycline or estrogen addition. Values are representative of 3 independent qRT-PCRs of 3 biologic replicates. The analyzed genes are indicated above the diagrams; the coding of the graphs is elucidated beside the diagram: Notch1-IC (Notch1-IC/EREB), Notch2-IC (Notch2-IC/EREB), CAT (CAT/EREB), and EBNA2 (CAT/EREB after addition of estrogen).
Because most of these antiapoptotic genes are known as LMP1 target genes,28 their high expression in EBNA2-expressing cells might be due to the high levels of LMP1 in EBNA2/EREB compared with Notch1/2-IC/EREB cells. To strengthen this assumption, we compared our chip data with chip data previously published by Vockerodt et al, studying genes that are differentially regulated by LMP1 in germinal center B cells.29 Approximately 10% of the EBNA2-regulated genes turned out to be LMP1 regulated, including the antiapoptotic genes BCL2A1 and BCL2L1. These data show that Notch1/2-IC as well as EBNA2 induce proapoptotic genes, implying that in B cells EBNA2 as well as Notch1/2-IC lead to the induction of apoptosis as long as antiapoptotic stimuli, for instance via LMP1 or CD40 signaling, are missing.
In B-cell lymphomas, DTX1 is highly expressed in combination with antiapoptotic genes
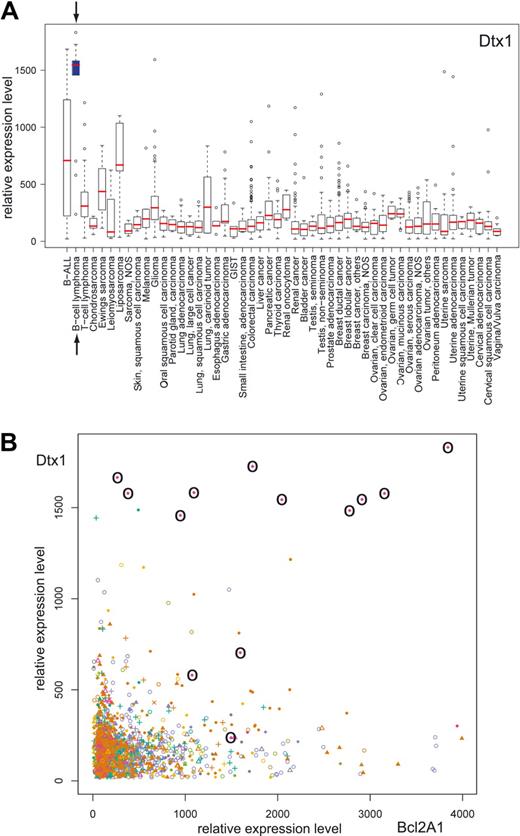
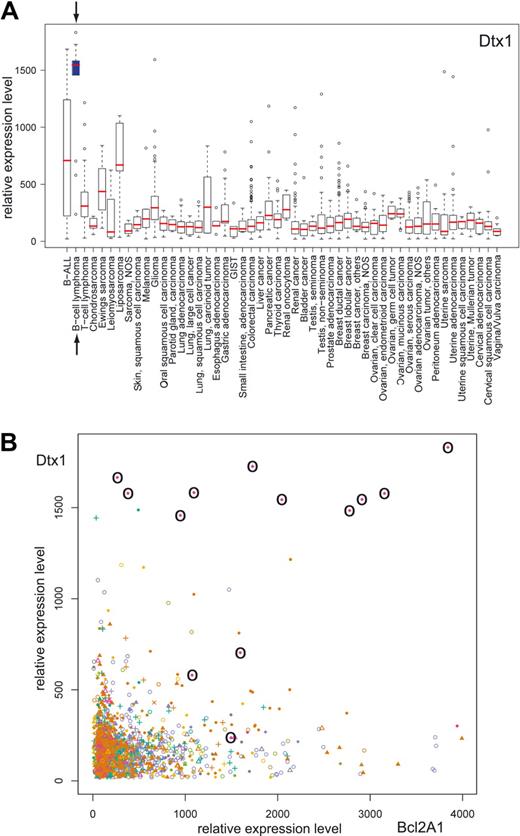
The inability of Notch signaling in B cells to provide antiapoptotic signals suggests the need for other pathways, leading to antiapoptotic signals, as a prerequisite for B-cell proliferation. To analyze whether Notch signaling is present and associated with the expression of antiapoptotic genes in B-cell lymphomas, we performed in silico transcriptomics with a recently published database of gene expression patterns in human normal and pathologic tissues30 (http://www.genesapiens.org/). As indicator of Notch signaling, we chose DTX1 expression, because DTX1 mirrors Notch activity in mature B cells most precisely.4 Expression analysis of DTX1 across a variety of 50 different groups of cancer tissues revealed the highest DTX1 expression levels in the group of B-cell lymphomas (represented by 13 samples; Figure 7A). High DTX1 expression in B-cell lymphomas was found to be associated by high expression levels of antiapoptotic genes such as BCL2A1 (Figure 7B) and BIRC5, which were strongly induced by EBNA2, but only marginally by Notch1/2-IC in our experimental setting (see Figure 6C). These data suggest that Notch signaling in B-cell lymphomas is always associated with the expression of antiapoptotic proteins, which are most likely induced by costimulatory signals like CD40 signals.
DTX1 is highly expressed in B-cell lymphomas and is associated with high BCL2A1 expression. The GeneSapiens database of gene expression patterns was analyzed for DTX1 expression. (A) Box-plot analysis of the DTX1 expression levels across a variety of cancer tissues. The box refers to the quartile distribution (25%-75%) range, with the median shown as a red horizontal line. In addition, the 95% range and individual outlier samples are shown. The group of B-cell lymphomas is highlighted by arrows and a blue box. (B) Correlation plot of DTX1 and BCL2A1 expression across a variety of cancer tissues. All samples from B-cell lymphomas are highlighted by circles.
DTX1 is highly expressed in B-cell lymphomas and is associated with high BCL2A1 expression. The GeneSapiens database of gene expression patterns was analyzed for DTX1 expression. (A) Box-plot analysis of the DTX1 expression levels across a variety of cancer tissues. The box refers to the quartile distribution (25%-75%) range, with the median shown as a red horizontal line. In addition, the 95% range and individual outlier samples are shown. The group of B-cell lymphomas is highlighted by arrows and a blue box. (B) Correlation plot of DTX1 and BCL2A1 expression across a variety of cancer tissues. All samples from B-cell lymphomas are highlighted by circles.
Discussion
It has been shown that Notch1-IC can only partially replace the function of EBNA2 in EBV-immortalized B cells. Only if Notch1-IC was expressed at extremely high levels or in combination with LMP1, proliferation of immortalized B cells could be rescued to some extent in the absence of EBNA2.21,22 However, it was still in question whether Notch2, the major Notch family member in mature B cells, is more potent in replacing EBNA2. In this study, we show that neither Notch1-IC nor Notch2-IC can maintain B-cell proliferation in the absence of EBNA2. Although Notch1/2-IC expression drove some cells into the S phase of the cell cycle, cell numbers did not increase.
To study whether the biologic differences between EBNA2 and Notch1/2-IC can be traced back to differences in gene regulation, we performed a genome-wide expression analysis, revealing profound differences among Notch1-IC, Notch2-IC, and EBNA2 in the regulation of target genes. EBNA2 turned out to be more efficient in up-regulating genes involved in proliferation, survival, and chemotaxis, whereas Notch1-IC and Notch2-IC were more potently inducing genes associated with development and differentiation.
The different mode of gene regulation could originate from the different structures of EBNA2 and Notch1/2-IC. This might result in (1) different binding affinities of EBNA2- and Notch-IC–containing complexes to certain RBPJ binding sites or in (2) qualitative different interactions with other transcription factors additionally binding within EBNA2- or Notch-responsive promoters. Thus, within the LMP1 promoter, Spi1/SpiB has to bind in addition to RBPJ to allow EBNA2-mediated transactivation.31 Besides, EBNA2 and Notch1/2-IC interact very exclusively with viral and cellular proteins that modulate their transcriptional activity.2
Because EBNA2 plays a central role in early cell-cycle progression driving resting B cells from G0 to G1, it was not surprising that a lot of genes up-regulated by EBNA2 clustered in the functional group “cell cycle and proliferation.” Early events after EBNA2 expression are the up-regulation of the cellular genes cyclin D2 and MYC, as well as the viral genes LMP1 and LMP2A.14–16,32 Of note, Notch1/2-IC also induced the expression of cyclins and CDKs. Although most of them were more intensely up-regulated by EBNA2, a small cluster of genes containing CDK5R1, CCND1, CCNH, and CDK2 was comparably regulated by Notch1/2-IC and EBNA2. This is in line with former observations that Notch1-IC can up-regulate CCND1 and CDK2, resulting in S-phase entry.33,34 In our experimental system, Notch1/2-IC was also able to drive some cells into the S phase, however, without resulting in permanent proliferation of the cells. The differences in the regulation of LMP1, LMP2A, as well as PI3KR1 and MYC that were strongly and rapidly up-regulated solely by EBNA2 might explain at least partially the biologic differences between EBNA2 and Notch1/2-IC. These genes are direct target genes of EBNA2 and play an essential role in the proliferation of immortalized cell lines.28,35 Although Notch1/2-IC could induce MYC expression to a certain degree, which is in line with observations showing that MYC is a direct target gene of Notch1-IC in T-ALLs,36–38 it could not induce the high peak of MYC levels, as observed early after EBNA2 induction. High MYC expression itself can induce hyperphosphorylation of Rb, activation of CDK2, and entry into the cell cycle.39 Thus, high MYC levels as induced by EBNA2 might be essential to drive EBV-infected cells into the cell cycle. As soon as EBV-infected cells express all viral latent genes, which drive in concert B-cell proliferation and activation, MYC levels significantly decrease and are even lower than in Notch1-IC–expressing cells.
A further reason for the biologic difference between Notch-IC and EBNA2 in EBV-infected cells might be the apoptotic effect mediated by Notch-IC in B cells. Strikingly, both EBNA2 and Notch1/2-IC induced the expression of proapoptotic genes. In contrast, antiapoptotic genes were strongly up-regulated solely in EBNA2-expressing cells. This observation might indicate that Notch1/2-IC induces proliferation of B cells only in the presence of antiapoptotic signals.
This finding could be important for the understanding of the influence of Notch signaling to the pathogenesis of B-cell lymphomas. It is still an open question as to whether deregulated Notch signaling contributes to the development of B-cell lymphomas. Notch2 is highly expressed in B-cell chronic lymphocytic leukemia (B-CLL), and both Notch1 and Notch2 are overexpressed in Hodgkin lymphoma.40–42 Stimulation of Notch receptors resulted in an increased proliferation of Hodgkin lymphoma and multiple myeloma cell lines.41,42 But also, opposite effects of deregulated Notch signaling in B cells have been described. Thus, Notch-IC inhibits growth and induces apoptosis in avian, murine, and human B-cell lines.43–45 The balance between proliferative and apoptotic signals, which may be influenced by the cellular background, the Notch signaling strength, and costimulatory signals, might determine the biologic outcome of Notch signaling. In B cells, Notch1/2-IC expression might rather induce apoptosis than proliferation as long as antiapoptotic signals like CD40 and LMP1 signals are missing. This is in line with recently published data showing a proliferative effect of Notch signaling in murine primary B cells in cooperation with BCR and CD40 stimulation, and with our own observation that Notch1-IC induces proliferation in combination with LMP1 in B cells.21,46
These findings suggest that Notch signaling is active in certain B-cell lymphomas, in which antiapoptotic signals are provided by other stimuli. In silico analysis of the recently established human transcriptome database GeneSapiens30 supported our hypothesis. An expression plot across the tumor samples revealed a very high DTX1 expression in B-cell lymphomas, which is accompanied by high expression of antiapoptotic genes such as BCL2A1 and BIRC5. High DTX1 expression in germinal center (GC) B cells and GC-derived B-cell lymphomas has been described previously.47 DTX1 is a reliable Notch target gene in vivo, which is highly induced in lymphoid subsets such as T-cell precursors and marginal zone B cells that both depend on active Notch signaling. In B cells, DTX1 perfectly mirrors Notch2 expression.4 In contrast to some cell systems, in which DTX1 has been described as a negative regulator of Notch signaling, overexpression of DTX1 does not negatively modulate Notch signaling in B cells.48 Thus, the high DTX1 expression might be taken as an indicator for active Notch signaling in B-cell lymphomas.
Strikingly, our in silico analysis revealed that HES1 is not significantly elevated in B-cell lymphomas (data not shown). Because HES1 has been shown to be the main mediator of Notch-induced apoptosis in B cells,43,44 the low HES1 expression might be due to negative selection processes in B-cell lymphoma. A recent publication, showing that γ-secretase inhibitors inhibit the growth of certain B-cell lymphomas without further inhibiting the already low HES1 expression,49 underlines our finding that Notch signaling is active in B-cell lymphomas, but does not lead to the up-regulation of HES1.
Further studies will be necessary to explore whether Notch signaling contributes mechanistically to B-cell lymphomagenesis. This will throw light on the question as to whether Notch may serve as a potential target for treatment of certain B-cell malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Elisabeth Kremmer for the α-LMP2A antibody 14B6, the α-ΔNGFR antibody, and her help in preparation of the α-Notch2 antibody; G. W. Bornkamm for providing the pRTS vector and helpful discussion; W. Hammerschmidt and B. Kempkes for helpful discussions; G. Marschall and U. Bär for technical assistance; and B. Jungnickel for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB684).
Authorship
Contribution: H.K. designed and performed experiments, and assisted in writing the manuscript; F.H. performed experiments and assisted in preparing the manuscript; R.H., H.B., U.H.W., and D.E. contributed to the Affymetrix GeneChip analysis; M.H. contributed to the analysis of gene expression patterns with the GeneSapiens database; U.Z.-S. designed research and wrote the manuscript; and L.J.S. designed research, evaluated data, and assisted in preparing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Ursula Zimber-Strobl, Institute of Clinical Molecular Biology and Tumor Genetics, Helmholtz Center Munich, Marchioninistrasse 25, D-81377 Munich, Germany; e-mail: strobl@helmholtz-muenchen.de.
References
Author notes
*U.Z.-S. and L.J.S. contributed equally to this study.