We have quantified the relative contribution of donor antigen-presenting cell populations to alloantigen presentation after bone marrow transplantation (BMT) by using transgenic T cells that can respond to host-derived alloantigen presented within the donor major histocompatibility complex. We also used additional transgenic/knockout donor mice and/or monoclonal antibodies that allowed conditional depletion of conventional dendritic cells (cDCs), plasmacytoid DC (pDCs), macrophages, or B cells. Using these systems, we demonstrate that donor cDCs are the critical population presenting alloantigen after BMT, whereas pDCs and macrophages do not make a significant contribution in isolation. In addition, alloantigen presentation was significantly enhanced in the absence of donor B cells, confirming a regulatory role for these cells early after transplantation. These data have major implications for the design of therapeutic strategies post-BMT, and suggest that cDC depletion and the promotion of B-cell reconstitution may be beneficial tools for the control of alloreactivity.
Introduction
Recognition of host alloantigen (alloAg) by donor T cells commonly results in graft-versus-host disease (GVHD), which is a key contributor to the high mortality associated with bone marrow transplantation (BMT). GVHD is initiated by residual host antigen-presenting cells (APCs) that directly present host antigen (Ag) to donor T cells.1 Subsequent Ag presentation is mediated by donor APCs, which present host Ag to donor T cells via the indirect pathway of antigen presentation, predominantly via major histocompatibility complex (MHC) class II to CD4 T cells. The APC populations contributing to this effect and the rate at which this process occurs after BMT are unclear. Using novel reagents, we demonstrate that donor conventional dendritic cells (cDCs) are the primary APCs responsible for indirect presentation of alloantigen after BMT, and this process commences almost immediately after transplantation.
Methods
Mice
Female C57BL/6 (B6, H-2b, CD45.2+), B6.Ptprca (H-2b, CD45.1+), and BALB/c (H-2d, CD45.2+) mice were purchased from the Animal Resource Center (Perth, Australia). B cell–deficient (B6.mMT; H-2b, CD45.2+) mice were bred at the Queensland Institute of Medical Research (QIMR; risbane, Australia). B6.CD11c.DTR transgenic (Tg) mice (where the diphtheria toxiin [DT] receptor and enhanced green fluorescent protein [EGFP] are driven off the CD11c promoter) and congenic BALB/c mice (H-2d, CD45.1+) were bred at the Herston Medical Research Centre (Brisbane, Australia). Macrophage-Fas–induced apoptosis (MAFIA) Tg (B6.MAFIA, H-2b, CD45.2+; where Fas and EGFP are driven off the c-fms promoter) were provided by A.R.P. In the CD11c.DTR mouse, administration of DT leads to systemic depletion of donor cDCs in treated animals, as evidenced by examination of spleen, peripheral/mesenteric lymph nodes, skin, and lung.2,,–5 We have also confirmed cDC depletion in liver (data not shown). In the MAFIA mouse, the systemic depletion of macrophages and dendritic cells (DCs) can be achieved with administration of the AP20187 dimerizing ligand. Administration of ligand results in activation of cytoplasmic elements of the Fas protein and induces apoptosis in colony-stimulating factor-1 receptor–positive [CSF-1R+] cells. The original paper6 describing these mice demonstrated that the resulting depletion is systemic, with depletion of DCs and macrophages occurring within 24 hours of the third injection when ligand (at 10 mg/kg) was administered daily. As such, to deplete reconstituting Tg donor macrophages and DCs, we commenced AP20187 treatment 3 days before injection of TEa Tg T cells, and continued injecting until the day of analysis. TEa Tg animals were bred in the Herston Medical Research Center (Brisbane, Australia). TEa Tg mice (on a B6 background) were provided by J.S.B. All animal studies in this manuscript were carried out in accordance with and with approval of our institute's (QIMR) animal ethics committee.
Bone marrow transplantation
APC depletion
DT (Sigma-Aldrich, St Louis, MO) administration for the depletion of cDCs was performed, as reported elsewhere.10,11 To ensure rigorous and persistent depletion of cDCs throughout the period in which Tg T cells were present, 100 ng/dose DT was administered to recipients of CD11c.DTR Tg BM grafts on day 8, 10, and 12 posttransplant to maintain depletion throughout the period of TEa T-cell response in vivo.11 When only a single injection of DT was administered (at day 8), cDCs were confirmed to be reconstituting by day 13, the day of analysis of T-cell proliferation. Consistent with this, alloantigen presentation was reduced to an intermediary level between that seen in control mice and mice in which cDCs were persistently depleted (data not shown). The 120G8 monoclonal antibody (mAb; 1 mg/dose), was administered on days 8, 10, and 12 to deplete plasmacytoid DCs (pDCs).12 To deplete CSF-1R+ cells in MAFIA Tg donor mice, AP20187 ligand (a Fas dimerizer; gifted from ARIAD Pharmaceuticals, Cambridge, MA) was administered daily at 10 mg/kg intravenously (I.V.) on days 8-12 posttransplant.6 Controls were recipients of MAFIA Tg BM receiving vehicle or recipients of wild-type (WT) BM receiving ligand. Splenocytes were enriched for APCs using density-gradient centrifugation, as previously described,10 before fluorescence-activated cell sorter (FACS) staining to confirm depletions.
TEa Tg cell preparation and Modfit analysis
Spleens and peripheral lymph nodes were harvested, and Tg cells were FACS sorted on the basis of Vα2 and Vβ6 T-cell receptor (TCR) expression to more than 99% purity. Data from spleen and lymph nodes were congruent. Carboxyfluorescein succinimidyl ester (CFSE) labeling was performed, as previously described,13,14 and cells were adoptively transferred by intravenous injection. T-cell proliferation was measured by CFSE dilution and analyzed using Modfit software (Verity Software House, Topsham, ME). Calculated proliferation index = Σ all cells/computed number of parent cells.
Antibodies
Fluorescein isothiocyanate (FITC)–conjugated CD3 (17A2); phycoerythrin (PE)–conjugated CD45.2 (104), anti-F4/80 (HB198), and CD19 (6D5); PE-Cy7–conjugated streptavidin; and allophycocyanin-conjugated CD11c (N418), CD45.2 (104), and CD4 (GK1.5) were purchased from BioLegend (San Diego, CA). FITC-conjugated and biotinylated anti-Vβ6 (RR4-7) and PE-conjugated anti-Vα2 (B20.1) were purchased from BD Pharmingen (San Diego, CA). PE-conjugated antibody against mouse plasmacytoid dendritic cell antigen-1r (PDCA-1; JF05-1C2.4.1) was purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). The 120G8 monoclonal antibody (AbCys, Paris, France) was FITC-conjugated in-house.
Cytokine analysis
Serum interferon (IFN)–γ was determined 3 days after the adoptive transfer of TEa T cells (at the time of CFSE determination), using the BDCytometric Bead Array system (BD Pharmingen), according to the manufacturer's protocol. Ex vivo splenocytes were stimulated with soluble anti-CD3 (2 μg/mL; 2C11; produced in-house) and brefeldin A (1/1000 dilution; BioLegend) for 4 hours. Cells were processed for intracellular cytokine staining (ICC), per the manufacturer's protocol (BD Cytofix/Cytoperm Kit; BD Pharmingen).
Statistical analysis
Column graphs shown represent mean, with error bars demonstrating the SEM. SEM was shown, as all the data presented are reflective of both biologic and experimental variation. Statistical significance was determined using 2-tailed Mann-Whitney U tests, unless otherwise stated, with a P value cutoff of .05. Statistical analyses were performed using GraphPad Prism software (GraphPad, San Diego, CA).
Results
We used the B6 (I-Ab/I-E−/−) → BALB/c (I-Ad/I-Ed) model of MHC-mismatched allogeneic BMT to investigate the contribution of donor APC subsets to posttransplant alloreactivity. To quantify indirect alloantigen presentation in vivo, we used TEa Tg T cells, which possess a TCR specific for a host I-Ed–derived peptide when presented within the donor I-Ab molecule.15,–17
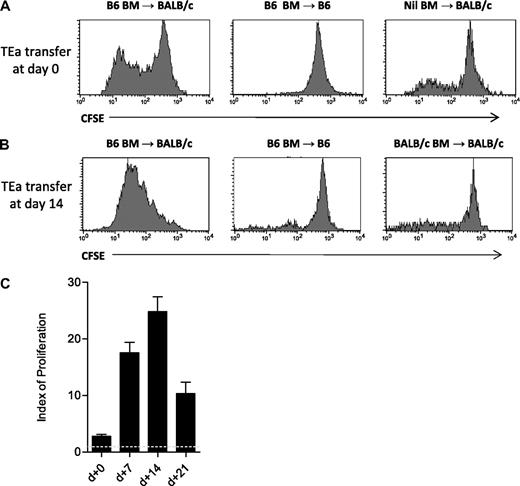
CFSE-labeled TEa T cells were adoptively transferred with B6 BM into BALB/c allogeneic recipients at the time of transplantation, and 7, 14, and 21 days after BMT. As shown in Figure 1A, presentation of host Ag by donor APCs had commenced within 72 hours of BMT, and was maximal 14 days later (Figure 1B,C). Negative controls confirm the specificity of the TEa response to donor MHC/host peptide complexes, as minimal proliferation was seen in both the B6 → B6 syngeneic, and BALB/c → BALB/c allogeneic (to the TEa T cell) setting.
Quantification of indirect alloAg presentation using TEa Tg T cells. (A) Lethally irradiated CD45.1+ BALB/c or B6 recipient mice were transplanted at day 0 with 1-2 × 106 CFSE-labeled CD45.2+ TEa T cells, with or without CD45.1+ B6 BM. Proliferation of TEa T cells (gated on CD45.2+Vα2+Vβ6+) was assessed by CFSE dilution 72 hours after transplantation, as shown. (B) Animals received primary grafts (day 0) as labeled. Fourteen days after transplant, 2-5 × 106 TEa Tg cells were adoptively transferred and proliferation (gated on CD4+Vα2+Vβ6+) was assessed by CFSE dilution 72 hours later. Data shown are representative of a minimum of 3 replicate experiments. (C) TEa Tg cells were adoptively transferred at the time points indicated and CFSE dilution was analyzed using Modfit software. Indices of proliferation are shown (combined data from 3-6 replicate experiments; n = 5-9 for each group). The dotted line represents the proliferation index in syngeneic recipients where there is no alloantigen presentation. Error bars represent SEM. All indices of proliferation are significantly different from each other (P ≤ .03), except day 7 versus day 14, where P = .07.
Quantification of indirect alloAg presentation using TEa Tg T cells. (A) Lethally irradiated CD45.1+ BALB/c or B6 recipient mice were transplanted at day 0 with 1-2 × 106 CFSE-labeled CD45.2+ TEa T cells, with or without CD45.1+ B6 BM. Proliferation of TEa T cells (gated on CD45.2+Vα2+Vβ6+) was assessed by CFSE dilution 72 hours after transplantation, as shown. (B) Animals received primary grafts (day 0) as labeled. Fourteen days after transplant, 2-5 × 106 TEa Tg cells were adoptively transferred and proliferation (gated on CD4+Vα2+Vβ6+) was assessed by CFSE dilution 72 hours later. Data shown are representative of a minimum of 3 replicate experiments. (C) TEa Tg cells were adoptively transferred at the time points indicated and CFSE dilution was analyzed using Modfit software. Indices of proliferation are shown (combined data from 3-6 replicate experiments; n = 5-9 for each group). The dotted line represents the proliferation index in syngeneic recipients where there is no alloantigen presentation. Error bars represent SEM. All indices of proliferation are significantly different from each other (P ≤ .03), except day 7 versus day 14, where P = .07.
We next examined the relative contribution of cDCs, pDCs, macrophages, and B cells to the presentation of host Ag by donor APCs after BMT. We adoptively transferred TEa cells at day 10 after transplantation, because this time point coincided with donor APC reconstitution10 and optimal alloantigen presentation (Figure 1C). We used T cell–depleted allogeneic grafts in these systems to avoid the corruption in DC maturation induced by acute GVHD.12
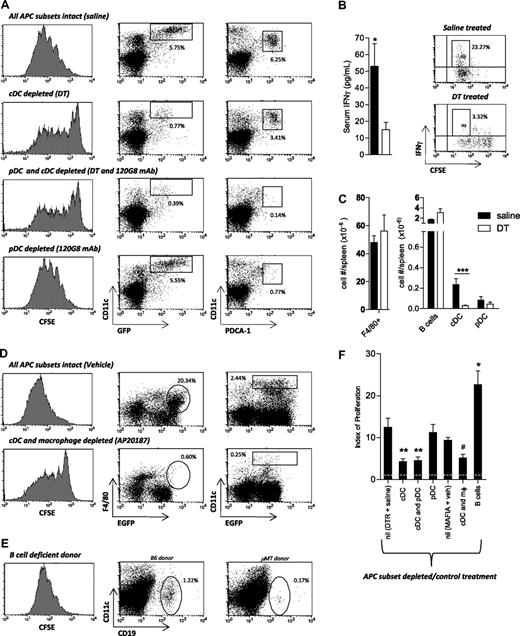
To examine the role of cDCs, we used Tg donors (B6.CD11c.DTR) in which DT administration leads to the specific ablation of donor cDCs.10,11 In the absence of donor cDCs, alloantigen presentation was markedly diminished compared with that in recipients where all APC subsets were intact (Figure 2A). Quantification of indirect alloantigen presentation confirmed that the diminished presentation in the absence of donor cDCs was highly significant (P = .001; DT treatment vs saline). Importantly, there was no decrease in alloAg presentation in recipients of WT marrow receiving DT (mean index of proliferation 18.72 ± 1.1). We also examined IFN-γ production in mice receiving DT or saline treatment as another hallmark of T-cell activation. As would be predicted by the proliferation data, serum IFN-γ was reduced in DT-treated animals compared with controls (P = .038), and the generation of IFN-γ from TEa T cells was similarly reduced when cDCs were absent (Figure 2B).
Contribution of APC subsets to alloAg presentation after BMT. (A) T cell–depleted B6.CD11c.DTR Tg BM was transplanted into irradiated BALB/c mice. Recipients were treated with saline, DT, and/or 120G8 mAb on days 8, 10, and 12, as described in “Methods.” CFSE-labeled TEa Tg cells were adoptively transferred 10 days after transplantation, and alloantigen presentation was quantified by assessment of CFSE dilution in cells recovered from spleen 72 hours later (gated on CD4+Vα2+Vβ6+). FACS dot plots demonstrate the proportions of cDCs and pDCs in splenic light-dense fractions remaining after each treatment. EGFP is driven off the CD11c promoter in B6.CD11c.DTR Tg mice, and as such, donor cDCs are defined as CD11chigh/EGFP+ in this system. Data are representative of 5 replicate experiments. (B) Serum IFN-γ levels were measured 3 days after adoptive transfer of TEa T cells by cytokine bead array in BALB/c recipients of B6.CD11c.DTR grafts that were treated with saline or DT. P = .038 (1-tailed), saline versus DT. Error bars represent SEM, n = 9 per group from 2 experiments. FACS plots demonstrate IFN-γ production by day 13 Tg TEa cells ex vivo, as measured by ICC staining after restimulation with soluble CD3 for 4 hours. Representative plots shown from n = 5. (C) Absolute numbers of splenic APCs in DT versus saline-treated recipients at day 13 after transplantation. Error bars represent SEM. For cDCs, n = 8; P < .001, saline versus DT treatment. No significant alteration in F4/80+ macrophages, B cells, or pDCs (n = 3-9 per group combined from 2 experiments). (D) Recipients of T cell–depleted MAFIA Tg BM were treated with vehicle (n = 3) or AP20187 ligand (n = 7), as described. Ten days after transplantation, CFSE-labeled TEa Tg cells were adoptively transferred, and alloantigen presentation was quantified, as described in panel A. FACS dot plots demonstrate the proportions of splenic macrophages (F4/80+/EGFP+) and cDCs (CD11chigh/EGFP+) in splenic light-dense fractions remaining after each treatment. MAFIA Tg cells express EGFP driven off the c-fms promoter, thus allowing identification of donor cells after transplantation. (E) T cell–depleted μMT BM was transplanted into irradiated BALB/c recipients. CFSE-labeled TEa Tg cells were adoptively transferred, and alloantigen presentation was examined, as described in panel A. FACS dot plots demonstrate the proportions of donor B cells in whole spleen at day 13 after transplantation. (F) Indices of adoptively transferred TEa T-cell proliferation in allograft recipients as calculated using Modfit software. The dotted line represents the proliferation index in syngeneic recipients where there is no alloantigen presentation. Error bars represent SEM, combined from 7 experiments. Saline treated, n = 10; cDC depleted, n = 12; cDC/pDC and pDC depleted only, n = 8; cDC/macrophage (mφ) depleted, n = 7; B-cell deficient, n = 4. **P < .007; *P = .02 versus saline-treated controls. #P = .03 versus MAFIA recipients treated with vehicle.
Contribution of APC subsets to alloAg presentation after BMT. (A) T cell–depleted B6.CD11c.DTR Tg BM was transplanted into irradiated BALB/c mice. Recipients were treated with saline, DT, and/or 120G8 mAb on days 8, 10, and 12, as described in “Methods.” CFSE-labeled TEa Tg cells were adoptively transferred 10 days after transplantation, and alloantigen presentation was quantified by assessment of CFSE dilution in cells recovered from spleen 72 hours later (gated on CD4+Vα2+Vβ6+). FACS dot plots demonstrate the proportions of cDCs and pDCs in splenic light-dense fractions remaining after each treatment. EGFP is driven off the CD11c promoter in B6.CD11c.DTR Tg mice, and as such, donor cDCs are defined as CD11chigh/EGFP+ in this system. Data are representative of 5 replicate experiments. (B) Serum IFN-γ levels were measured 3 days after adoptive transfer of TEa T cells by cytokine bead array in BALB/c recipients of B6.CD11c.DTR grafts that were treated with saline or DT. P = .038 (1-tailed), saline versus DT. Error bars represent SEM, n = 9 per group from 2 experiments. FACS plots demonstrate IFN-γ production by day 13 Tg TEa cells ex vivo, as measured by ICC staining after restimulation with soluble CD3 for 4 hours. Representative plots shown from n = 5. (C) Absolute numbers of splenic APCs in DT versus saline-treated recipients at day 13 after transplantation. Error bars represent SEM. For cDCs, n = 8; P < .001, saline versus DT treatment. No significant alteration in F4/80+ macrophages, B cells, or pDCs (n = 3-9 per group combined from 2 experiments). (D) Recipients of T cell–depleted MAFIA Tg BM were treated with vehicle (n = 3) or AP20187 ligand (n = 7), as described. Ten days after transplantation, CFSE-labeled TEa Tg cells were adoptively transferred, and alloantigen presentation was quantified, as described in panel A. FACS dot plots demonstrate the proportions of splenic macrophages (F4/80+/EGFP+) and cDCs (CD11chigh/EGFP+) in splenic light-dense fractions remaining after each treatment. MAFIA Tg cells express EGFP driven off the c-fms promoter, thus allowing identification of donor cells after transplantation. (E) T cell–depleted μMT BM was transplanted into irradiated BALB/c recipients. CFSE-labeled TEa Tg cells were adoptively transferred, and alloantigen presentation was examined, as described in panel A. FACS dot plots demonstrate the proportions of donor B cells in whole spleen at day 13 after transplantation. (F) Indices of adoptively transferred TEa T-cell proliferation in allograft recipients as calculated using Modfit software. The dotted line represents the proliferation index in syngeneic recipients where there is no alloantigen presentation. Error bars represent SEM, combined from 7 experiments. Saline treated, n = 10; cDC depleted, n = 12; cDC/pDC and pDC depleted only, n = 8; cDC/macrophage (mφ) depleted, n = 7; B-cell deficient, n = 4. **P < .007; *P = .02 versus saline-treated controls. #P = .03 versus MAFIA recipients treated with vehicle.
The administration of DT to recipients of CD11c.DTR Tg grafts depleted cDCs by more than 90%, but did not significantly deplete absolute number of pDCs (Figure 2C), consistent with previously published data from our own group10,18 and others.19 Thus, we additionally depleted pDCs using the 120G8 mAb, as previously described,12 which did not lead to a further reduction in alloantigen presentation (Figure 2A,F). As might be predicted from this, the specific depletion of donor pDCs in isolation had no effect on alloAg presentation (P = .63; Figure 2A,F). Importantly, the mean index of proliferation in recipients treated with isotype control antibody (MAC49) was 7.71 plus or minus 2.63 and was not increased relative to mice undergoing pDC depletion with the 120G8 Ab (index = 11.22 ± 1.9), or saline-treated controls (index = 12.42 ± 2.2).
We next addressed the role of donor macrophages in alloAg presentation using B6.MAFIA Tg donors, in which administration of the AP20187 ligand leads to conditional depletion of CSF-1R+ cells (ie, cells of the mononuclear phagocyte system, including monocytes-macrophages and DCs6 ). Surprisingly, donor macrophages were not engaged in alloAg presentation because the depletion of both populations reduced presentation to the same levels as cDC depletion alone (Figure 2D,F; P = .29). AP20187 treatment in recipients of MAFIA grafts led to a similar level of cDC depletion as DT treatment in the recipients of CD11c.DTR grafts (average depletion efficiency of 91% and 88%, respectively; Figure 2A,C,D). Alloantigen presentation in recipients of WT BM receiving AP20187 and MAFIA BM receiving vehicle was equivalent (index = 11.50 ± 1.63 for recipients of WT grafts treated with toxin, and 9.33 ± 0.69 for recipients of MAFIA grafts treated with vehicle only), confirming effects were due to cellular depletion rather than ligand administration.
Finally, the role of B cells in antigen presentation was investigated using B cell–deficient donors (B6.μMT). Indirect antigen presentation was significantly increased in the absence of donor B cells (Figure 2E,F; P = .024), consistent with the known regulatory role of B cells in acute, CD4-dependent GVHD.9 No increase in cDC number was observed in recipients of μMT grafts compared with WT (data not shown).
Discussion
These systems demonstrate that, of the classical APC subsets examined, only cDCs are involved in indirect alloAg presentation, which is capable of stimulating a naive donor T-cell response early post-BMT. This is the first study to examine the relative contribution of donor APC subsets to alloreactivity after BMT, and has been made possible by the TEa Tg mouse to measure alloantigen presentation in vivo. Whereas other groups have identified alloantigen-presenting APCs using the YAe antibody,15 in these BMT models the YAe mAb against the I-Ab/I-Ed peptide complex was not informative because, as reported by Viret and Janeway, APCs from B6 mice can self-assemble a structurally related complex under inflammatory conditions.17
Previous studies in the field have demonstrated that the non–T-cell (ie, bone marrow–derived) component of the donor graft plays a role in driving GVHD, but these studies have not examined which APC populations are involved. Using MHC class I–deficient donors, in a CD8 T cell–mediated model of GVHD, Matte et al20 demonstrated a reduction in GVHD severity when marrow-derived cells were MHC class I deficient. The key conclusion of this study was that the donor bone marrow–derived compartment plays an important role in GVHD. Whereas the authors reported the presence of donor-derived cDCs, no specific assessment of the presence or functional capacity of other potentially contributing APC subsets was undertaken. The role of the bone marrow–derived component of the graft has also been assessed in CD4-dependent models of both acute and chronic GVHD, with the clear conclusion that the capacity of donor BM-derived cells to deliver costimulatory signals21 and respond to LPS22 allows the full penetrance of GVHD. Whereas a previous study by our group demonstrated that donor cDC depletion can reduce the severity of GVHD,10 there were limitations to this work because of the restricted period of DC depletion due to DT toxicity, and the lack of an antigen-specific donor T-cell function as a readout. This has been overcome in these studies, and to our knowledge, this study represents the first to attempt specific examination of the relative contribution of the classical donor APC subsets to GVHD.
In contrast, previous studies have examined the role of host APC subsets in experimental transplantation, although these are by no means definitive. Recipient cDCs23 and pDCs24 in isolation are able to induce allogeneic T-cell responses after transfer into MHC class II−/− recipients in vivo after conditioning. Although elegant, these studies demonstrate only that cDCs and pDCs in isolation can initiate GVHD, but their relative contribution to this process when all APC fractions are intact remains unclear. Conversely, recipient B cells do not appear to invoke alloantigen-dependent donor T-cell responses, and instead appear to have a regulatory role that is IL-10–dependent.9 Finally, the elegant studies from Merad et al demonstrate the importance of host Langerhans cells, as their depletion can prevent GVHD within the skin.25
Although our data demonstrate that cDCs are the critical APCs for indirect, donor-mediated alloantigen presentation, it is important to note that this study has focused on the ability to induce allogeneic responses in naive T cells. It is indeed possible (and likely) that other APC subsets contribute to alloantigen presentation to induce tolerance instead. Our data demonstrating that B cells act in this fashion to diminish indirect alloantigen presentation are intriguing. However, the results are consistent with previous studies demonstrating a regulatory role for recipient B cells in attenuating GVHD via IL-10–mediated effects on T cell–APC interactions.9 Indeed, regulatory B cells are increasingly identified as an entity in multiple disease states (reviewed in Mizoguchi and Bhan26 ). Because donor B-cell reconstitution is markedly impaired during acute (T helper cell 1–dependent) GVHD,27 strategies to enhance reconstitution after transplant may prove useful in the prevention and/or treatment of acute GVHD.
It is important to note that the depletion of cDCs in these studies did not eliminate alloAg presentation altogether. This most likely reflects both the incomplete depletion and the partial reconstitution of cDCs during the depletion regimens used. However, we cannot exclude an additional minor contribution by an additional, nonclassical donor APC after transplantation (eg, marrow-derived myeloid precursors and/or endothelial cells). Taken together, these data have major implications for the design of therapies aimed at preventing pathogenic alloresponses after BMT. They suggest that the depletion of donor cDCs and promotion of donor B-cell expansion may be useful therapeutic addition to GVHD management, particularly because this approach would be predicted to leave graft-versus-leukemia effects largely intact.20,28
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council (Canberra, Australia), and the Leukemia Foundation of Australia (Brisbane, Australia).
Authorship
Contribution: K.A.M. designed and performed experiments and wrote the manuscript; T.B. contributed useful discussion; R.D.K., S.D.O., A.L.J.D., N.C.R., and Y.A.W. assisted with experimental work; L.J.R. contributed a vital reagent; A.R.P. provided a vital reagent and useful discussion; J.S.B. provided a vital reagent and contributed to the manuscript; G.R.H. designed experiments and wrote the manuscript; and K.P.A.M. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Kelli P. A. MacDonald, Bone Marrow Transplantation Laboratory, Queensland Institute of Medical Research, 300 Herston Rd, Herston, QLD, 4006 Australia; e-mail: Kelli.MacDonald@qimr.edu.au.