Abstract
Lymphoid and myeloid lineage segregation is a major developmental step during early hematopoiesis from hematopoietic stem cells. It is not clear, however, whether multipotent progenitors (MPPs) adopt a lymphoid or myeloid fate through stochastic mechanisms, or whether this process can be regulated by extracellular stimuli. In this study, we show that lymphoid lineage specification occurs in MPPs before lymphoid lineage priming, during which MPPs migrate from the proximal to the distal region relative to the endosteum of the bone marrow. Lymphoid-specified MPPs have low myeloid differentiation potential in vivo, but potently differentiate into myeloid cells in vitro. When treated with pertussis toxin, an inhibitor of G protein–coupled receptor signaling, lymphoid-specified MPPs regain in vivo myeloid potential, and their localization is dispersed in the bone marrow. These results clearly demonstrate that specific microenvironments that favorably support lymphoid or myeloid lineage development exist at structurally distinct regions in the bone marrow.
Introduction
Hematopoietic stem cells (HSCs) can give rise to all classes of hematopoietic cells, which are categorized into 2 major groups, lymphoid and myeloid lineages.1 Lymphoid lineage cells, or lymphocytes, include T, B, and NK cells. Other hematopoietic cell types, such as megakaryocytes and erythrocytes (MegEs), as well as granulocytes and macrophages (GMs), belong to the myeloid lineage.1 The major divergence of lymphoid and myeloid lineages occurs at the multipotent progenitor (MPP) stage.2,3 However, regulatory mechanisms of this lineage choice by MPPs are not clear.
MPPs are derived from HSCs that have lost self-renewal ability. Subfractionation of MPPs has provided important insights into the hierarchy of lymphoid and myeloid lineage differentiation. Using the cell surface markers FMS-like tyrosine kinase 3 (Flt3) and vascular cell adhesion molecule-1 (VCAM-1), MPPs were separated into 3 distinct subsets: Flt3lowVCAM-1+, Flt3highVCAM-1+, and Flt3highVCAM-1− fractions (Figure 1A).2,4 Characterization of these 3 MPP subsets indicates a sequential loss of MegE and GM lineage differentiation potential before lymphoid lineage commitment at the common lymphoid progenitor (CLP) stage in the bone marrow or double-negative 3 stage in the thymus.3-6 Flt3highVCAM-1− MPPs have high lymphoid potential, but much weaker myeloid potential in vivo compare with more primitive VCAM-1+ MPP fractions. This lymphoid-biased differentiation potential suggests that although Flt3highVCAM-1− MPPs have not committed into the lymphoid lineage, they are programmed or instructed to become lymphocytes. This transition process with reducing myeloid potential before the lymphoid lineage commitment is denoted by lymphoid lineage specification. Subsequent lymphoid lineage commitment occurs when all myeloid differentiation potential becomes silenced.
Gene expression analysis of MPPs indicated that these uncommitted progenitors have already initiated the expression of several lymphoid and myeloid lineage-specific genes.4,7,8 Such promiscuous expression of lineage-specific genes before lineage commitment is known as lineage priming, a phenomenon thought to be involved in maintaining the plasticity of the differentiation potential of MPPs.9-11 Of the different MPP subsets, lymphoid lineage priming first occurs in Flt3highVCAM-1− MPPs,2,4 indicating that the lymphoid lineage differentiation program is initiated at this stage. Flt3highVCAM-1− MPP significantly overlaps with lymphoid-primed MPP (LMPP) defined by Jacobsen's group,12 in which lymphoid and myeloid promiscuous gene expression is observed.3,12,13 It remains unclear, however, whether all cells in LMPP or Flt3highVCAM-1− MPP populations are homogeneously primed for the lymphoid lineage. It is also unclear whether lymphoid specification and lymphoid priming occur simultaneously or whether they could represent separate developmental stages during lymphoid differentiation. In addition, it is not well understood whether extracellular factors play a role in triggering lymphoid lineage specification, priming, and/or commitment in MPPs.
Accumulated evidences have demonstrated the importance of the microenvironments of bone marrow in the maintenance of HSC activity and in B-cell development.14 These microenvironments are mainly formed by nonhematopoietic cells, such as reticular cells that produce SDF1 and osteoblasts.14 Although precise mechanisms are not completely understood, G protein–coupled receptors (GPCRs), such as chemokine receptors expressed on hematopoietic progenitors, play a critical role in regulating the localization and interaction between hematopoietic progenitors and bone marrow stromal cells.14 In particular, CXC chemokine receptor 4 and its ligand SDF1 are indispensable for the retention of HSCs in the bone marrow and/or formation of the HSC niche in adult mice.15-17 In addition to chemokine receptors, other types of GPCRs also critically regulate the localization of hematopoietic cells. For example, sphingosine-1-phosphate (S1P) receptor 1 (S1PR1) is indispensable for the egress of single-positive thymocytes from thymus to the periphery.18 In contrast to this dynamic movement of developing T cells regulated by S1PR1 as well as multiple chemokine receptors on thymocytes at the different maturational stages, it is not clear whether MPPs migrate to different microenvironments of the bone marrow that can affect to their lymphoid and myeloid lineage choice.
In this study, we found that Flt3highVCAM-1− MPPs (or simply VCAM-1− MPPs hereafter), which have high lymphoid and low myeloid potential in vivo, regain high myeloid differentiation potential when treated with a G protein inhibitor, pertussis toxin (PTX), before in vivo injection. Compared with the more primitive VCAM-1+ MPPs, VCAM-1− MPPs preferentially localize at a more distal region in the bone marrow from the endosteum where osteoblasts reside. However, upon treatment with PTX, VCAM-1− MPPs lose such specific localization capacity and home to the area closer to the endosteal region, where VCAM-1+ MPPs and myeloid-committed GM progenitors (GMPs) are also localized. Further subfractionation of the VCAM-1− MPP population suggests that migration away from the endosteum/outer region occurs before the onset of lymphoid lineage priming. These results indicate that lymphoid lineage specification occurs during the migration of MPPs to a new location in the bone marrow at the transition from the VCAM-1+ MPP to VCAM-1− MPP stage, which is regulated by G protein–mediated signals. In addition, conceptual lymphoid and myeloid niches might be enriched at different regions in the bone marrow.
Methods
Mice
C57Bl/Ka-Thy1.1 (CD45.2), C57Bl/Ka-Thy1.1-Ly5.1 (CD45.1), and C57Bl/Ka-Thy1.1 (CD45.1/CD45.2) mice maintained at the Duke University Medical Center Animal Care Facility were used as wild-type (WT) mice.4 β-Actin green fluorescent protein (GFP) transgenic mice (GFP mice) and recombination-activating gene (RAG)1/GFP knockin (KI) mice,19 both of which are on C57Bl/Ka-Thy1.1 background, were also used. All studies and procedures were approved by the Duke University Animal Care and Use Committee.
FACS, in vitro cultures, and in vivo injections of MPPs
Fluorescence-activated cell sorter (FACS) sorting and analysis, gene expression analysis, and characterization of in vitro and in vivo differentiation potential of MPPs were done as previously reported.2,4,20 Briefly, doubly sorted cells (103) in each MPP subset (CD45.2) were intravenously injected into lethal dose (920 rad)–irradiated host (CD45.1/CD45.2) with 2 × 105 whole bone marrow cells (CD45.1). Peripheral blood was obtained from reconstituted mice 2 weeks after injection for FACS analysis. Percentage of chimerism after in vivo injections was calculated by [% donor-derived population/(% donor derived + % rescue bone marrow derived)] × 100.2,4
To determine the T- and B-cell differentiation potential of MPP subsets, single MPPs were cultured in each well of 96-well plates with either OP9 (for B cell) or OP9-DL1 (for T cell) cells in the presence of Flt3 ligand and interleukin (IL)-7, as previously described.21 After 10 to 14 days in culture, cells were harvested from each well and analyzed by FACS for the presence of B cells (B220+CD19+) or T cells (Thy1+CD25+). For clonal analysis of GM and B-cell differentiation, single MPPs from each fraction were cultured on OP9 stromal cell layers in the presence of stem cell factor (SCF) and Flt3 ligand for 2 days and further cultured for another 7 to 9 days with additional cytokines, including IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-7. Readout patterns were analyzed by FACS. Methylcellulose cultures to determine the GM differentiation potential of MPPs were performed, as previously described.2,4
Gene expression analysis in MPP subsets
Quantitative reverse transcription–polymerase chain reaction (RT-PCR) analysis of lymphoid- and myeloid-specific gene expression was performed, as previously described.4 Multiplex single-cell RT-PCR was carried out by sorting single MPPs directly into 96-well polymerase chain reaction (PCR) plate containing Cells to Signal Lysis buffer (Ambion, Austin, TX). Reverse transcription was performed using Moloney murine leukemia virus reverse transcriptase with gene-specific 3′ primers (hypoxanthine phosphoribosyltransferase (HPRT), IL-7 receptor (IL-7R)α, and GM-CSF receptor (GM-CSFR)α). A 50-μL PCR reaction was subsequently carried out with the addition of 5′-specific primers. A second round of nested PCR was performed to amplify each gene separately using 2.5 μL of DNA amplified from the first PCR reaction. Only wells positive for HPRT signal were scored for expression of IL-7Rα or GM-CSFRα.
PTX treatment and in vivo homing of MPPs
To investigate the role of GPCRs on MPPs, we purified 5 × 104 VCAM-1+ and VCAM-1− MPPs from GFP mice, incubated the cells at 37°C for 2 hours in RPMI 1640 with 10% FCS in the presence or absence of PTX (100 ng/mL), and injected the cells intravenously into irradiated (920 rads) or nonirradiated WT mice. To examine the location of MPPs in the bone marrow, we removed femurs from the host mice at 48 hours after injection. GMPs were purified from GFP mice using cell surface markers, as previously described.22 When examining the homing capacity of VCAM-1−RAG1− and VCAM-1−RAG1+ MPPs, the cells were purified from RAG1/GFP KI mice and incubated with 0.5 nM 5- (and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE) for 10 minutes at 37°C before injection into recipient mice. The bones were fixed with 1% paraformaldehyde and embedded in OCT medium. Longitudinal cryostat sections (5 μm thick) were stained with DAPI (4,6 diamidino-2-phenylindole), and the image under a fluorescence microscope (Zeiss, Oberkochen, Germany) was captured with a charge-coupled device camera.
Results
Lymphoid lineage specification occurs before lymphoid lineage priming
RAG1 is one of the earliest known lymphoid-related genes up-regulated in VCAM-1− MPPs.2 Therefore, RAG1 expression can be a marker of MPPs in which lymphoid lineage priming occurs. In earlier studies, Kincade's group prospectively isolated and characterized early lymphoid progenitors (ELPs), which are defined as RAG1+ cells from the c-Kit+lineage−Sca-1+ bone marrow fraction in RAG1-GFP KI mice.7 Although both VCAM-1− MPPs and ELPs have high lymphoid and limited myeloid differentiation potential,2,4,7 the relationship between VCAM-1− MPPs and ELPs has not been clarified. Therefore, we examined RAG1 expression in the MPP population derived from RAG1-GFP KI mice.19
The majority of MPPs that express RAG1 from RAG1-GFP KI mice resided in the VCAM-1− fraction (Figure 1B), which we have previously shown to have a lymphoid-biased differentiation potential in vivo.4 Very few VCAM-1+ MPPs expressed RAG1 regardless of the Flt3 expression level (Figure 1B). Therefore, virtually no lymphoid lineage priming marked by the RAG1 expression occurs before the VCAM-1− MPP stage.
The discrepancy between the in vitro and in vivo myeloid differentiation potential of VCAM-1−RAG1− MPPs. (A) Hierarchical relationship of hematopoietic progenitors. The MPP population is subdivided into 3 fractions based on Flt3 (F) and VCAM-1 (V) expression.4 Lymphoid (L), GM, and MegE (E) potential of each population is also indicated. This scheme is based on the conceptual in vivo contribution of the populations to the various hematopoietic lineages; other models have been proposed as well.41 CMP, common myeloid progenitor; GMP, granulocyte/macrophage progenitor; MEP, megakaryocyte/erythroid progenitor. (B) Analysis of RAG1 (GFP) expression in Flt3lowVCAM-1+, Flt3highVCAM-1+, and Flt3highVCAM-1− MPPs4 in RAG1-GFP KI mice by FACS. FACS plots shown are pregated on parameters defining each MPP subset, as previously described.4 (C) In vitro GM differentiation potential of VCAM-1+RAG1−, VCAM-1−RAG1−, and VCAM-1−RAG1+ MPPs in methylcellulose culture in the presence of SCF, IL-3, IL-6, and with (□) or without (■) GM-CSF. (D) In vivo differentiation potential of VCAM-1+RAG1−, VCAM-1−RAG1−, and VCAM-1−RAG1+ MPPs into GM cells. (E) The frequency of MPPs in each subset giving rise to B (B220+CD19+) and T (Thy-1+CD25+) cells in OP9 or OP9-DL1 cocultures. (F) Clonal analysis of GM (Mac-1+) and B-cell (B220+CD19+) differentiation potential.2 *P < .05 (statistical significance) by Student t test.
The discrepancy between the in vitro and in vivo myeloid differentiation potential of VCAM-1−RAG1− MPPs. (A) Hierarchical relationship of hematopoietic progenitors. The MPP population is subdivided into 3 fractions based on Flt3 (F) and VCAM-1 (V) expression.4 Lymphoid (L), GM, and MegE (E) potential of each population is also indicated. This scheme is based on the conceptual in vivo contribution of the populations to the various hematopoietic lineages; other models have been proposed as well.41 CMP, common myeloid progenitor; GMP, granulocyte/macrophage progenitor; MEP, megakaryocyte/erythroid progenitor. (B) Analysis of RAG1 (GFP) expression in Flt3lowVCAM-1+, Flt3highVCAM-1+, and Flt3highVCAM-1− MPPs4 in RAG1-GFP KI mice by FACS. FACS plots shown are pregated on parameters defining each MPP subset, as previously described.4 (C) In vitro GM differentiation potential of VCAM-1+RAG1−, VCAM-1−RAG1−, and VCAM-1−RAG1+ MPPs in methylcellulose culture in the presence of SCF, IL-3, IL-6, and with (□) or without (■) GM-CSF. (D) In vivo differentiation potential of VCAM-1+RAG1−, VCAM-1−RAG1−, and VCAM-1−RAG1+ MPPs into GM cells. (E) The frequency of MPPs in each subset giving rise to B (B220+CD19+) and T (Thy-1+CD25+) cells in OP9 or OP9-DL1 cocultures. (F) Clonal analysis of GM (Mac-1+) and B-cell (B220+CD19+) differentiation potential.2 *P < .05 (statistical significance) by Student t test.
Because VCAM-1− MPPs preferentially give rise to lymphocytes, but still retain some myeloid differentiation potential (Figure 1A),2,4 we asked whether the GM differentiation potential in this population is enriched in the RAG1− fraction. We compared the in vitro and in vivo GM potential in VCAM-1+RAG1−, VCAM-1−RAG1−, and VCAM-1−RAG1+ MPPs. Previously, we showed that both Flt3lowVCAM-1+ and Flt3highVCAM-1+ MPPs are equally potent in giving rise to GM lineage cells.4 Therefore, we combined these 2 MPP fractions as VCAM-1+(RAG1−) MPP population for these comparative analyses. As shown in Figure 1C, VCAM-1−RAG1+ MPPs only gave rise to GM colonies on methylcellulose cultures at the plating efficiency less than 10%. VCAM-1−RAG1− MPPs, in contrast, potently formed GM colonies in a similar fashion to the more primitive VCAM-1+RAG1− MPPs at 30% to 40% plating efficiency (Figure 1C). Despite its high in vitro GM potential, VCAM-1−RAG1− MPPs minimally gave rise to GM cells when injected in vivo (Figure 1D). Both VCAM-1−RAG1− and VCAM-1−RAG1+ MPPs accounted for, at most, 4% of the observed GM chimerism in vivo, compared with 20% to 32% GM chimerism by VCAM-1+RAG1− MPPs (Figure 1D). In contrast, we did not observe any significant difference in lymphocyte development potential among VCAM-1+RAG1−, VCAM-1−RAG1−, and VCAM-1−RAG1+ MPPs in vivo (data not shown). Indeed, almost all VCAM-1+RAG1−, VCAM-1−RAG1−, and VCAM-1−RAG1+ MPPs had the potential to give rise to T and B cells in in vitro stromal cell cultures (Figure 1E). These results suggest that most VCAM-1− MPPs are lymphoid specified in vivo regardless of their RAG1 priming status or intrinsic GM differentiation ability.
GM-CSF is dispensable for GM lineage commitment in vivo, even though GM-CSF is one of the major cytokines that support GM colony formation by bone marrow cells in in vitro cultures.23,24 However, if GM-CSF is available, hematopoietic progenitors with GM-CSFRs may be actively instructed to differentiate toward the GM lineage by GM-CSF stimulation.20 In fact, the addition of GM-CSF enhanced the GM colony formation of VCAM-1−RAG1+ MPPs by approximately 5-fold (Figure 1C). However, the ability of VCAM-1−RAG1− MPPs in forming GM colonies was the same regardless of the presence of GM-CSF (Figure 1C). This indicates that the GM potential in VCAM-1−RAG1− MPPs is not the result of stimulation of cells with cytokines that may trigger a latent differentiation potential in a nonphysiologic setting. Rather, it should be assumed that such intrinsic GM potential is maintained at a high level in VCAM-1−RAG1− MPPs, which cannot be triggered and/or supported in vivo.
Lymphoid-specified VCAM-1−RAG1− MPPs maintain high levels of GM-related gene expression
Because VCAM-1−RAG1− MPPs are lymphoid specified, as shown above, we investigated whether these MPPs expressed any lymphoid-related genes. In addition to RAG1, we have previously shown that IL-7Rα and early B-cell transcription factor (EBF), 2 known lymphoid-related genes, are up-regulated in VCAM-1− MPPs.2 Therefore, we compared the expression level of these genes in VCAM-1+RAG1−, VCAM-1−RAG1−, and VCAM-1−RAG1+ MPPs. As shown in Figure 2B, only VCAM-1−RAG1+ MPPs, but not VCAM-1+RAG1− or VCAM-1−RAG1− MPPs, up-regulated appreciable levels of lymphoid-related IL-7Rα and EBF genes. VCAM-1−RAG1− MPPs, although also lymphoid specified, lacked lymphoid-related gene expression (Figure 2B). In addition, we did not observe any down-modulation of myeloid-related genes such as GM-CSFRα and CCAAT/enhancer-binding protein α in lymphoid-specified VCAM-1−RAG1− MPPs (Figure 2A). Upon the onset of lymphoid priming in VCAM-1−RAG1+ MPPs, however, GM-related genes became down-regulated and silenced upon further differentiation to CLPs (Figure 2A), where lymphoid lineage commitment is established. VCAM-1−RAG1− MPPs, which had low in vivo and high in vitro GM potential (Figure 1C,D), expressed even higher levels of GM-affiliated GM-CSFRα and C/EBPα genes compared with VCAM-1+RAG1− MPPs (Figure 2A). The high expression of GM-related genes most likely contributes to the high intrinsic myeloid differentiation potential in VCAM-1−RAG1− MPPs (Figure 1C-F) despite their propensity to differentiate into lymphocytes in vivo (Figure 1D). These results suggest that lymphoid specification occurs before the attenuation of myeloid differentiation potential in MPPs as early as the VCAM-1−RAG1− MPP stage.
Lack of lymphoid lineage priming in VCAM-1−RAG1− MPPs that are specified to the lymphoid lineage in vivo. Quantitative RT-PCR analysis of myeloid (A)- and lymphoid (B)-related gene expression in HSCs, MPPs, and CLPs. Relative expression level of each gene is normalized to GAPDH expression level. Expression level shown is the average value from triplicate samples from each cell population. The expression level of each gene in HSCs is arbitrarily set as 1. (C) Multiplex single-cell RT-PCR of lymphoid (IL-7Rα)- and myeloid (GM-CSFRα)-affiliated gene expression in MPP subsets. At least 50 HPRT+ wells were analyzed for the presence or absence of IL-7Rα and GM-CSFRα expression for each cell population.
Lack of lymphoid lineage priming in VCAM-1−RAG1− MPPs that are specified to the lymphoid lineage in vivo. Quantitative RT-PCR analysis of myeloid (A)- and lymphoid (B)-related gene expression in HSCs, MPPs, and CLPs. Relative expression level of each gene is normalized to GAPDH expression level. Expression level shown is the average value from triplicate samples from each cell population. The expression level of each gene in HSCs is arbitrarily set as 1. (C) Multiplex single-cell RT-PCR of lymphoid (IL-7Rα)- and myeloid (GM-CSFRα)-affiliated gene expression in MPP subsets. At least 50 HPRT+ wells were analyzed for the presence or absence of IL-7Rα and GM-CSFRα expression for each cell population.
We also examined the expression of lymphoid- and myeloid-related genes in MPPs on a single-cell level. Among the samples we examined, no VCAM-1+RAG1− MPPs expressed lymphoid-associated genes, such as IL-7Rα (Figure 2C). Approximately 20% of VCAM-1−RAG1− MPPs up-regulated IL-7Rα, but the majority of these IL-7Rα+ cells also expressed GM-CSFRα (Figure 2C). In contrast, significantly more VCAM-1−RAG1+ MPPs expressed IL-7Rα (∼60%; Figure 2C). The low frequency of cells expressing IL-7Rα in VCAM-1−RAG1− MPPs indicates that most of these cells are not primed for the lymphoid lineage, even though lymphoid specification has already been established at this stage. We do not rule out the possibility, however, that some unknown lymphoid-related genes other than the IL-7Rα, EBF, and RAG1 genes might be expressed in VCAM-1−RAG1− MPPs.
VCAM-1−RAG1− MPPs regain in vivo GM potential by PTX treatment
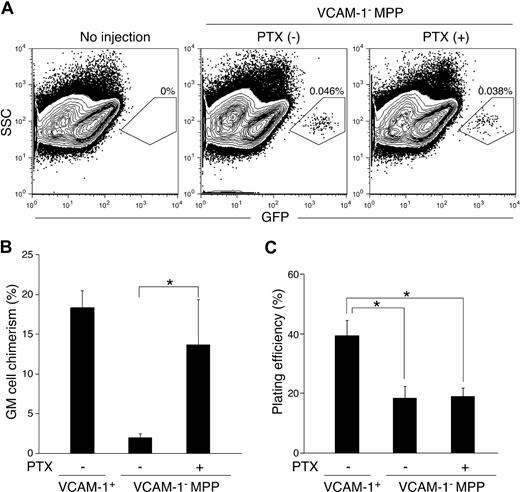
The existence of specialized niches for HSCs and B-cell progenitors in the bone marrow has been demonstrated.14,25 The discrepancy between in vitro and in vivo myeloid differentiation potential of VCAM-1−RAG1− MPPs led us to hypothesize that these lymphoid-specified MPPs are localized in a microenvironment in which the cue to initiate myeloid differentiation and/or production of myeloid growth factors is defective. The lack of myeloid stimuli, in turn, leads to preferential differentiation into lymphocytes from MPPs. We reason that if lymphoid-specified VCAM-1−RAG1− (and VCAM-1−RAG1+) MPPs are localized in a distinct microenvironment, it is likely that they are recruited there and home to this niche through a GPCR-dependent manner similar to the regulatory mechanisms of organogenesis of various organs26 or differential T- and B-cell localization in the spleen.27,28 To test this hypothesis, we evaluated the GM differentiation potential of VCAM-1− MPPs treated with PTX, which can uncouple GPCR signaling by inhibiting the interaction of GPCRs with G proteins.29 In this experiment, we purified VCAM-1− MPPs from enhanced GFP transgenic mice (simply GFP mice hereafter) and injected them into host so that we could detect donor-derived cells in a highly sensitive manner.4 Because GFP expression is regulated by the β-actin promoter, all cells derived from this GFP mouse line are positive for GFP. Therefore, we did not subdivide the VCAM-1− MPP population by RAG1 expression in this study. As shown in Figure 3A, treatment of VCAM-1− MPPs with PTX resulted in similar bone marrow engraftment efficiencies, judging by the percentage of donor-derived GFP+ cells in the recipient bone marrow 48 hours after injection. This result suggests that GPCRs do not have a significant role in MPP homing to the bone marrow from peripheral blood.
Myeloid potential of MPPs after PTX treatment. (A) FACS analysis of bone marrow cells from irradiated recipient WT mice 48 hours after intravenous injection of GFP+ VCAM-1− MPPs with (right panel) or without (middle) PTX treatment. Bone marrow from mice that did not receive an injection of cells is also shown as a control (left). Shown FACS plots are pregated on the Lin− fraction. The percentages represent the frequency of GFP+-injected cells in whole bone marrow. In vivo (B) and in vitro (C) GM differentiation potential of VCAM-1+ and VCAM-1− MPPs with or without PTX treatment. After 2-hour culture in the presence or absence of PTX, the cells were subjected to the experiments as described in Figure 1C,D. *P < .05 (statistical significance) by Student t test.
Myeloid potential of MPPs after PTX treatment. (A) FACS analysis of bone marrow cells from irradiated recipient WT mice 48 hours after intravenous injection of GFP+ VCAM-1− MPPs with (right panel) or without (middle) PTX treatment. Bone marrow from mice that did not receive an injection of cells is also shown as a control (left). Shown FACS plots are pregated on the Lin− fraction. The percentages represent the frequency of GFP+-injected cells in whole bone marrow. In vivo (B) and in vitro (C) GM differentiation potential of VCAM-1+ and VCAM-1− MPPs with or without PTX treatment. After 2-hour culture in the presence or absence of PTX, the cells were subjected to the experiments as described in Figure 1C,D. *P < .05 (statistical significance) by Student t test.
Whereas PTX treatment did not change the GM differentiation potential of VCAM-1− MPPs in in vitro methylcellulose cultures (Figure 3C), VCAM-1− MPPs treated with PTX resulted in a significantly increased GM differentiation in vivo (Figure 3B). These results indicate the presence of 2 different bone marrow microenvironments: the first is where myeloid differentiation is actively triggered in MPPs, which we refer to as the myeloid niche; the second is the place in which myeloid differentiation potential of VCAM-1− MPPs may not be initiated/supported or could be blocked, leading VCAM-1− MPPs to differentiate into lymphocytes (lymphoid niche). Furthermore, this localization of VCAM-1− MPPs to the lymphoid niche should be directly or indirectly regulated by actions of GPCRs, of which actions are blocked by PTX.
Localization of VCAM-1+ MPPs and VCAM-1− MPPs to the distinct regions in the bone marrow
If specialized niches do exist for lymphoid and myeloid differentiation, VCAM-1+ MPPs and VCAM-1− MPPs should localize to different regions within the bone marrow. To test this hypothesis, we purified VCAM-1+ MPPs and VCAM-1− MPPs from GFP mice and injected them into host mice to examine the MPP homing location in the bone marrow. Initially, we attempted to detect GFP+ cells in irradiated mice. However, because the bone marrow structure was severely destroyed by irradiation, it was almost impossible for us to obtain consistent results to determine the location of GFP+ cells in the bone marrow in the experimental setting used in Figure 3A,B (data not shown). Instead, we injected GFP+ MPPs into nonirradiated mice. In this case, injected MPPs could home to the bone marrow in nonirradiated mice (300-430 GFP+ cells/femur by FACS analysis) at similar or even higher efficiency than in irradiated mice (90-130 GFP+ cells/femur; Figure 4C) irrespective of VCAM-1 expression on MPPs and PTX treatment, and were detected in the bone marrow sections from host mice 48 hours after injection (Figure 4B).
Localization of MPP subsets in structurally distinct regions of bone marrow. (A) Spatial designation of bone marrow in femurs. Endosteal region is determined, as previously described.30 The central marrow region30 between the endosteum and central sinus is further divided into 2 regions, as shown in this figure. The borders of each region are indicated with white dotted lines. The section was stained with DAPI (blue). Original magnification of the image is ×20. (B) Three different localization patterns of injected MPPs to the inner region (section 1), outer region (section 2), and endosteal region (section 3). Arrows indicate the injected GFP+ cells (green). (C) Localization of VCAM-1+ and VCAM-1− MPPs to the distinct regions in bone marrow. At least 30 cells from each population were counted. Representative results from 3 independent experiments were shown. *P < .05 (statistical significance) in the distribution of injected cells to the 3 regions by χ2 test. (D) VCAM-1− MPPs and GMPs localize to distinct regions in the bone marrow after intravenous injections into nonirradiated wild-type mice. Localization of injected cells was examined, as in panel C. One result from 2 replicate experiments was shown. *P < .05 (statistical significance) by χ2 test.
Localization of MPP subsets in structurally distinct regions of bone marrow. (A) Spatial designation of bone marrow in femurs. Endosteal region is determined, as previously described.30 The central marrow region30 between the endosteum and central sinus is further divided into 2 regions, as shown in this figure. The borders of each region are indicated with white dotted lines. The section was stained with DAPI (blue). Original magnification of the image is ×20. (B) Three different localization patterns of injected MPPs to the inner region (section 1), outer region (section 2), and endosteal region (section 3). Arrows indicate the injected GFP+ cells (green). (C) Localization of VCAM-1+ and VCAM-1− MPPs to the distinct regions in bone marrow. At least 30 cells from each population were counted. Representative results from 3 independent experiments were shown. *P < .05 (statistical significance) in the distribution of injected cells to the 3 regions by χ2 test. (D) VCAM-1− MPPs and GMPs localize to distinct regions in the bone marrow after intravenous injections into nonirradiated wild-type mice. Localization of injected cells was examined, as in panel C. One result from 2 replicate experiments was shown. *P < .05 (statistical significance) by χ2 test.
Nilsson et al defined 2 different regions in the bone marrow of longitudinal sections of femurs: the endosteal and central marrow regions (Figure 4A).30 The endosteal region is arbitrarily defined as within 12 cells of the endosteum and includes the HSC niche formed by osteoblasts.14,30 The rest of the bone marrow is referred to as the central marrow region that is between the endosteum and central sinus. In this region, the B-cell niches composed of SDF1+ reticular cells and other HSC niches, namely the vascular niches, are sparsely present.14,17,31,32 We further divided this central marrow region into 2 halves: the outer (near endosteum) and inner (near central sinus) regions (Figure 4A). Forty-eight hours after intravenous injection of VCAM-1+ MPPs or VCAM-1− MPPs, we examined the location of donor-derived GFP+ cells in the femurs of the host mice. As shown in Figure 4D, VCAM-1+ MPPs localized to the endosteal and outer regions of the central marrow, whereas most VCAM-1− MPPs were localized within the inner regions of the central marrow. This specific localization of VCAM-1− MPPs at the inner region was perturbed by PTX treatment (Figure 4D).
Finally, we addressed whether both RAG1− and RAG1+ subfractions in the VCAM-1− MPP population preferentially localized to the inner region of the central marrow. For this purpose, we purified the 2 VCAM-1− MPP populations from RAG1-GFP KI mice, then labeled them with CFSE and injected them into nonirradiated mice. Some MPPs might have become damaged during the CFSE labeling as the number of cells we could detect in the bone marrow was less (90-140 cells/femur by FACS) than that in the experiments shown in Figure 4C. Nevertheless, the result shown in Figure 4D demonstrated that lymphoid-specified MPPs (VCAM-1−RAG1−) as well as lymphoid-primed MPPs (VCAM-1−RAG1+) preferentially locate to the inner region. Our results in this study suggest that the endosteal and/or outer regions of the central marrow may contain specific microenvironments that can actively initiate and support myeloid differentiation in MPPs.
More importantly, VCAM-1− MPPs are located at the inner region of central marrow, and thus may be protected from stimulation that promotes differentiation of MPPs toward the myeloid lineage. The sensitivity of this specific localization of VCAM-1− MPPs to PTX treatment further suggests a mechanistic role of GPCRs in mediating the homing of these cells to the inner region of central marrow, which might play a critical role in initiation of lymphocyte differentiation at the MPP stage.
Discussion
Several studies in recent years suggested a role for extrinsic factors in influencing cell fate decisions in hematopoietic progenitors.20,33 However, the role of cytokines or other extrinsic factors in influencing lymphoid versus myeloid lineage differentiation has remained unclear. Our study provides evidence that the bone marrow microenvironment does play a critical role in lineage diversification from MPPs toward the lymphoid and myeloid lineages. The results of this study and our previous studies suggest that the myeloid developmental program seems to be dominant over the lymphoid differentiation program.20,34 Therefore, lack of stimulation that leads HSCs and MPPs to myeloid lineage might be prerequisite for lymphoid lineage priming and commitment.
Nonetheless, the findings in this study do not address whether certain extrinsic factors instruct MPPs toward the myeloid lineage or simply play a supportive role like many other myeloid growth factors.35-37 Recently, we demonstrated that the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway plays an important role in myeloid differentiation, most likely at the onset of myeloid lineage commitment during hematopoiesis.34 This suggests that extracellular stimuli are necessary for proper myeloid cell development from HSCs. Our data suggest that most GMPs are located at the endosteal region in the bone marrow (Figure 4D), implying that the endosteal region includes HSC and myeloid niches.
Heterogeneity of bone marrow stromal cells has been suggested because of the difference in phenotype, cytokine production pattern, and ability to support hemato/lymphopoiesis.38 However, unlike the spleen, which has well-defined structures, such as red/white pulps and periarterial lymphatic sheath (known as the T-cell zone) and can be identified by simple hematoxylin and eosin staining of the sections, little is known about the structure of the bone marrow.30 Scadden and colleagues recently demonstrated that HSCs locate more proximal to the endosteal region than more mature hematopoietic progenitors using live imaging techniques.39 Li's group showed that proximal localization of HSCs to the endosteum is enhanced in irradiated mice, but not evident in nonirradiated mice.32 In this study, we simply divided the central marrow region, which is the rest of the distinctive endosteal region,30 into outer half (outer region) and inner half (inner region), and found that the majority of VCAM-1− MPPs localized at the inner region (Figure 4C,D). Therefore, unique distribution patterns of bone marrow stromal cells that preferentially support lymphoid and myeloid specification and commitment might be present in the bone marrow (Figure 5). Thus, it is important in the future to identify bone marrow stromal cells that produce the extrinsic factors responsible for supporting myeloid differentiation.
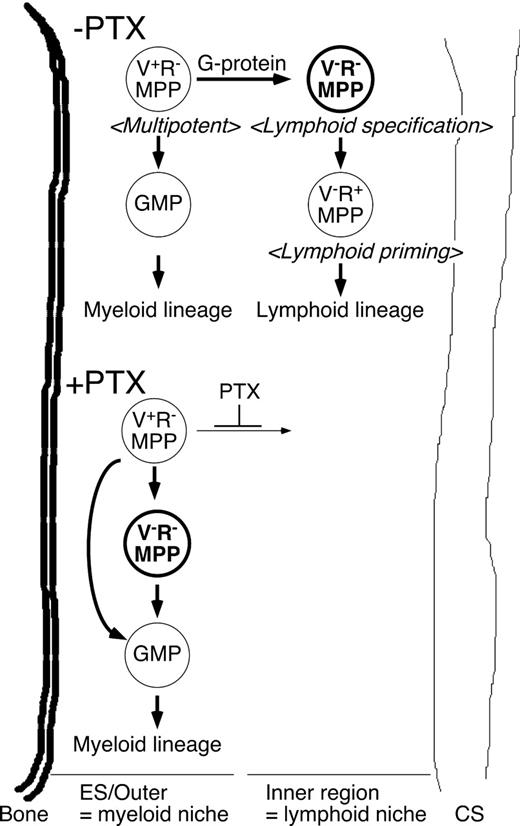
A proposed model of the regulation of lymphoid and myeloid lineage choice by MPPs. Schematic representation of GPCR-dependent regulation of lymphoid lineage specification at the VCAM-1−RAG1− MPP population based on the results in this study. We propose that relocation of VCAM-1−RAG1− MPPs marks the onset of lymphoid specification, and is necessary for proper lymphocyte development before the lymphoid lineage commitment stage.
A proposed model of the regulation of lymphoid and myeloid lineage choice by MPPs. Schematic representation of GPCR-dependent regulation of lymphoid lineage specification at the VCAM-1−RAG1− MPP population based on the results in this study. We propose that relocation of VCAM-1−RAG1− MPPs marks the onset of lymphoid specification, and is necessary for proper lymphocyte development before the lymphoid lineage commitment stage.
In conclusion, the results in this study provide important insights into the regulatory mechanisms of how lymphoid specification is determined at the VCAM-1−RAG1− MPP stage during the hemato/lymphopoiesis. These results further demonstrate the importance to clarify the nature of bone marrow microenvironments to understand the regulation of lymphoid and myeloid lineage choice not only in the steady state hematopoiesis, but also in special circumstances such as inflammation, in which granulopoiesis is enhanced at the expense of B-cell development, as previously demonstrated.40 Another important next issue to address is to determine which GPCR(s) is involved in homing lymphoid-specified progenitors to their specialized microenvironment. Because other bacteria components, such as cholera toxin, also target the function of GPCRs, it will be interesting to see whether treatment of MPPs with cholera toxin also affects their preferential localization in the bone marrow. These experiments, along with gene expression profiling of MPP subsets and characterization of bone marrow microenvironments, will help to narrow down the types of GPCRs involved in the homing process of lymphoid-specified progenitors. These next studies will be a key in advancing our understanding of lymphocyte development in adults at the earliest stage.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Sakaguchi, Kuwahara, and Zuniga-Pflucker for RAG1-GFP KI mice and OP9-DL1 cells, respectively.
This work was supported by the Duke Stem Cell Research Program Annual Award, National Institutes of Health grants AI056123 and CA098129 (M.K.), and National Institutes of Health grant AI52077 (A.Y.L.). M.K. is a scholar of the Leukemia & Lymphoma Society.
National Institutes of Health
Authorship
Contribution: A.Y.L. performed the research and wrote the paper; A.W. and T.O. performed the research; and M.K. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Motonari Kondo, 101 Jones Bldg, DUMC 3010, Research Dr, Durham, NC 27710; e-mail: motonari.kondo@duke.edu.