Abstract
Immunophenotypic classification of acute lymphoblastic leukemia (ALL) has well-recognized prognostic implications. The significance of CD20 expression has been evaluated in childhood precursor B-lineage ALL with conflicting results. We retrospectively analyzed the influence of CD20 expression on outcome in 253 adults with de novo precursor B-lineage ALL treated with either conventional (VAD/CVAD) or intensive (hyper-CVAD) frontline chemotherapy regimens in the pre-rituximab era. Overall, CD20 positivity of at least 20% was associated with lower 3-year rates of complete remission duration (CRD; 20% vs 55%, P < .001) and overall survival (OS; 27% vs 40%, p = .03). In the CD20 negative subset, the 3-year rates for CRD (58% vs 42%, p = .04) and OS (60% vs 28%, P < .001) were superior for hyper-CVAD compared with VAD/CVAD; rates were particularly favorable for the CD20 negative younger age group (68% and 85%, respectively). In contrast, 3-year CRD and OS rates were uniformly poor for the CD20-positive group regardless of therapy (27% or less). Multivariate analysis for event-free survival identified older age, leukocyte count higher than 30 × 109/L, presence of Philadelphia chromosome, high systemic risk classification, and CD20 positivity as independent predictors of worse outcome. In conclusion, CD20 expression in de novo adult precursor B-lineage ALL appears to be associated with a poor prognosis. Incorporation of monoclonal antibodies directed against CD20 into frontline chemotherapy regimens warrants investigation.
Introduction
Immunophenotypic classification of adult acute lymphoblastic leukemia (ALL) has great import with respect to characterization of the disease, prognostication, and delineation of therapy. The close association between phenotypic subgroups of ALL with particular cytogenetic and molecular aberrancies accounts in part for the prognostic significance. An example includes myeloid marker co-expression (eg, CD13, CD33) in an older patient with precursor B-cell ALL, which could indicate presence of the Philadelphia chromosome (Ph). The addition of the tyrosine kinase inhibitor imatinib to chemotherapy for patients with Ph positive ALL has dramatically improved outcome.1,2 The incorporation of other targeted agents, such as monoclonal antibodies directed against specific hematopoietic cell surface antigens, has also been explored. Rituximab is a chimeric monoclonal antibody directed at surface CD20, which induces complement-dependent cytolysis, antibody-dependent cell-mediated cytotoxicity, and apoptosis. Rituximab has significant single agent activity in previously treated indolent non-Hodgkin lymphoma (NHL) and hairy cell leukemia.3,4 The incorporation of rituximab into chemotherapy regimens has significantly improved disease-free survival (DFS) rates for certain subsets of NHL and other B-lineage lymphoproliferative disorders such as Burkitt-type leukemia/lymphoma and mantle cell leukemia/lymphoma.5,6 This favorable impact of chemoimmunotherapy regimens on outcome has also extended to diseases such as chronic lymphocytic leukemia, where CD20 expression is even lower than normal B cells.7,8
The CD20 molecule is a B-lineage specific antigen expressed on both normal and malignant cells during nearly all stages of B-cell differentiation, with the exception of stem (precursor) cells and plasma cells. It is a 33 kDa to 37 kDa nonglycosylated transmembrane phosphoprotein that forms tetramers and functions as a calcium channel, playing an important role in cell-cycle progression and differentiation via downstream signaling pathways. Its role in the apoptosis pathways including regulation of the proapoptotic proteins SERCA3 and Bax/Bak by alterations in intracellular Ca++ metabolism has been established.9 The constitutive activation of the survival pathways involving NF-κB and ERK1/2 by CD20 results in overexpression of Bcl-2–and Bcl-2–related gene, which in turn confer drug resistance.10 Thus, CD20 expression may be of prognostic relevance in addition to serving as a therapeutic target for monoclonal antibody therapy.
Heterogeneity in expression of CD20 among various B-cell malignancies has been well described.7 CD20 expression (defined as ≥ 20% leukemia cells positive) occurs in approximately 40% to 50% of ALL cases with precursor B-cell immunophenotype. The prognostic implications of CD20 expression has been investigated in childhood precursor B-cell ALL with conflicting results. Borowitz et al11 identified a worse event-free survival (EFS) with a higher fluorescence intensity of CD20, whereas Jeha et al12 found that CD20 expression conferred a slightly favorable prognosis (5-year EFS rate of 84% ± 2.9% versus 78% ± 3.1%, p = .08), suggesting that the intensity of therapy might influence the prognostic significance of CD20 expression. Herein we report the prognostic significance of CD20 expression in de novo precursor B cell adult ALL treated with frontline conventional or intensive therapy in the pre-rituximab era.
Methods
Study group
From 1985 to 2000, 508 adolescents and adults with newly diagnosed ALL or lymphoblastic lymphoma were treated with either VAD (vincristine, Adriamycin, dexamethasone) or hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, Adriamycin, and dexamethasone alternating with high-dose methotrexate and cytarabine).13,14 Diagnoses of Burkitt-type ALL, T-lineage ALL, and lymphoblastic lymphoma were excluded from the analysis. Of the remaining 304 patients, 253 (80%) had complete flow cytometry results and serve as the basis for this report. The protocols were reviewed by the Institutional Review Board at the M. D. Anderson Cancer Center. Informed consent was obtained from patients in accordance with institutional guidelines and the Declaration of Helsinki.
Eligibility criteria were age at least 15 years and absence of other co-existing active malignancy with expected consequent death within 12 months. Active concomitant infections were not prohibitive of study entry except for positivity for human immunodeficiency virus-1. There were no exclusions by performance status, cardiac status, or hepatorenal function.
Therapy
Frontline chemotherapy with the VAD (1985-1992) or hyper-CVAD (1992-2000) regimens have previously been described in detail.13,14 The study period antedated the use of imatinib for Ph-positive ALL or rituximab for CD20-positive ALL. The components of the regimens are summarized below. VAD consisted of vincristine (VCR) 0.4 mg intravenously daily days 1 to 4, doxorubicin 12 mg/m2 days 1 to 4, dexamethasone 40 mg days 1 to 4, 9 to 12, 17 to 20 followed by course 2 with same therapy and cyclophosphamide 1 g/m2 intravenously day 1 (CVAD). Four weekly courses of methotrexate (MTX) 40 to 160 mg/m2 intravenously day 1 and L-asparaginase 20 000 international units intravenously day 2 followed. Intensification with doxorubicin 60 mg/m2 intravenously day 1 and ara-C 3 g/m2 intravenously every 12 hours times 6 doses days 1 to 3 was followed by 3 courses of M-DOMP (MTX 400 mg/m2 intravenously day 1 with escalation to 1600 mg/m2, daunorubicin 60 mg/m2 intravenously day 15, VCR 2 mg intravenously day 15, 6-mercaptopurine [6-MP] 75 mg/m2 orally days 15 to 19, prednisone 120 mg orally days 15 to 19). For patients younger than 50 years of age, autologous stem cell transplant (SCT) was then performed after CBV (cyclophosphamide 1.5 g/m2 intravenously days 1 to 4, BCNU 300 mg/m2 intravenously day 1, VP-16 250 mg/m2 intravenously daily for 3 days). Older patients received intensification with AdOAP (doxorubicin 20 mg/m2 intravenously day 1, VCR 2 mg intravenously day 1, ara-C 30 mg/m2 daily days 1 to 7 continuous intravenous infusion, prednisone 100 mg daily days 1 to 5). Three more courses of M-DOMP were administered after the CBV plus SCT or AdOAP. The entire regimen (except for SCT) was repeated an additional 2 times to complete the regimen.
Hyper-CVAD consisted of an intensive phase with standardized CNS prophylaxis followed by a maintenance phase. The intensive phase included 8 alternating courses given every 21 days or earlier if adequate count recovery (at least 14 days apart). Odd courses (1, 3, 5, 7) were hyper-CVAD: hyper-fractionated cyclophosphamide 300 mg/m2 intravenously every 12 hours for 6 doses days 1 to 3 with Mesna 600 mg/m2 daily via continuous intravenous infusion days 1 to 3; VCR 2 mg intravenously days 4, 11; doxorubicin 50 mg/m2 intravenously over 2 or 24 hours via central venous catheter day 4; and dexamethasone 40 mg daily days 1 to 4, 11 to 14. To prevent tumor lysis syndrome, course 1 included intravenous hydration with alkalinization and allopurinol (or urate oxidase if high tumor burden). Oral sodium bicarbonate was also given days 1 to 3. Even courses (2, 4, 6, 8) were MTX and ara-C: MTX 200 mg/m2 intravenously over 2 hours, then MTX 800 mg/m2 intravenously over 22 hours day 1, ara-C 3 g/m2 (1 g/m2 if age ≥ 60 years) intravenously every 12 hours for 4 doses days 2, 3 (once serum MTX level at end of infusion [0 hour] ≤ 20 μM), and Solu-Medrol 50 mg intravenously every 12 hours days 1 to 3 for 6 doses. Intravenous alkalinization was used to promote excretion of MTX in all courses. Calcium leucovorin 50 mg intravenous was given 12 hours after completion of MTX, then 15 mg intravenously every 6 hours for 8 doses or until MTX levels were less than 0.1 μM. An algorithm of additional leucovorin rescue (50-100 mg intravenously every 4-6 hours) was implemented if MTX levels were elevated (0 hour [repeated if elevated] ≥ 20 μM, 24 hour ≥ 1 μM, 48 hour > 0.1 μM). Oral acetazolamide was given for urine pH less than 7.0.
CNS prophylaxis included alternating intrathecal therapy with 12 mg MTX (6 mg if via Ommaya reservoir) on day 2 and 100 mg of ara-C on day 7 or 8 of each course for total of 4, 8, or 16 intrathecal treatments, depending on either low, indeterminate, or high risk for CNS relapse (based on serum lactate dehydrogenase (LDH) level ≥ 1400 U/L and/or proliferative index %S + G2M ≥ 14%).15 Therapy for active CNS leukemia at presentation included an increase in the frequency of the alternating intrathecal therapy during the induction course to twice weekly until the CSF cell count normalized and cytologic examination was negative for evidence of malignant cells on 2 assessments. Intrathecal therapy was then administered weekly for 4 weeks, then according to the prophylactic schedule (2 intrathecals per course) for the remaining courses of intensive chemotherapy. No prophylactic cranial irradiation was given. Therapeutic radiation (24-30 Gy directed at the base of the skull administered in 10 to 12 fractions) was given if indicated at presentation, for example, for CNS disease characterized by cranial nerve palsies.
Maintenance included 6-MP, MTX, VCR, and prednisone (POMP) for 24 months. Between 1992 and 1995, oral POMP was given with 6-MP 50 mg orally 3 times daily, MTX 20 mg/m2 orally or intravenously weekly, VCR 2 mg intravenously monthly, and prednisone 200 mg daily for 5 days monthly, starting with VCR. From 1995 to 1999, intravenous POMP was given with 6-MP 1 g/m2 over 1 hour daily times 5 every month, MTX 10 mg/m2 intravenously over 1 hour daily times 5 every month, and VCR with prednisone monthly as just described. Oral POMP was resumed after 1999 when further development of IV 6-MP ceased. Two intensification courses were administered during the POMP maintenance during months 9 and 12 with VP-16 100 mg/m2 intravenously daily days 1 to 5 and pegylated asparaginase 2500 U/m2 intravenously day 1. After July 2000, these intensifications were changed to MTX 100 mg/m2 intravenously day 1 and L-asparaginase 20 000 units intravenously day 2 weekly times 4 weeks during months 7 and 11 of maintenance therapy.
Assessments
Pretreatment evaluations included history with physical examination and documentation of extramedullary disease (EMD) by imaging and/or appropriate cytological/histological evaluations; complete blood count with differential; hepatic and renal function studies with lactate dehydrogenase (LDH) and uric acid levels; and bone marrow aspirate/biopsy for morphology, immunohistochemical stains, flow cytometric immunophenotyping, and karyotyping. Analysis of cerebrospinal fluid (CSF) was performed concomitantly with prophylactic intrathecal (IT) treatment. Abnormal chest radiographs were evaluated further by imaging with computed tomography (CT). Magnetic resonance imaging and/or CT of the brain were performed for cranial nerve palsies or positive CSF cytology. Abnormal radiographic studies were repeated to document CR. Bone marrow aspirations were repeated on days 14 and 21 of course 1 to determine response.
Immunophenotypic analyses were performed on RBC-lysed whole bone marrow aspirate samples. A total of 2 × 106 cells per tube were stained, lysed, and then washed before acquisition. For the detection of cytoplasmic antigens, fixation and permeabilization steps were taken before staining using the InterPrep kit (Beckman-Coulter, Fullerton, CA) for TdT, and the Fix & Perm kit (Caltag Laboratories, Burlingame, CA) for myeloperoxidase. Three or four color flow cytometric immunophenotypic analysis was conducted using FACScan or FACSCalibur instruments (BD Biosciences, San Jose, CA). The antigens assayed included CD10, CD19, CD20, CD22, CD34, CD38, TdT, surface Igκ, surface Igλ, CD13, CD15, CD33, CD117, HLA-DR, myeloperoxidase, CD2, CD3 (surface and cytoplasmic), CD5, and CD7. All antibodies were obtained from BD Biosciences, except those directed against myeloperoxidase (Caltag) and TdT (Supertechs, Rockville, MD). Irrelevant monoclonal antibody reagents of the same isotypes, conjugated with the same fluorochromes (changed once during the study period when procedure was modified from 3- to 4-color flow cytometry), were used as negative controls. Data were analyzed using CellQuest (BD Biosciences). An arbitrary cut-off of 20% or more analyzed events brighter than the negative control stain was required for an antigen to be considered positive. An immunoglobulin κ/λ ratio of less than 0.5 or more than 5 was considered evidence of monoclonality. Conventional G-band karyotype analysis was performed as described previously.12
Response criteria and statistical methods
CR was defined as at least 5% blasts in a normocellular or hypercellular marrow with granulocyte count greater than 109/L and platelet count greater than 100 × 109/L. Complete resolution of EMD was required for CR. Other response outcomes were defined as induction death if it occurred after the start of therapy without meeting the definition of CR or resistant disease, and as resistant disease if the patient survived the treatment period but the leukemia persisted. Relapse was defined as disease recurrence at any site after achievement of CR. High risk for systemic relapse was defined as presence of one or more of the following disease features: pretreatment leukocyte count more than 5 × 109/L, Ph positivity, CNS disease at start, or requirement for more than 1 course to achieve CR.
Survival (OS) was measured from initiation of therapy until death. Disease-free survival (DFS) was measured from CR until relapse or death. Event-free survival (EFS) was measured from start of therapy until failure to respond, relapse, or death. Complete remission duration (CRD) was measured from CR until relapse. Unadjusted OS, CRD, and DFS analyses were performed using Kaplan-Meier plots16 with differences among them analyzed by the log-rank test.17 Goodness of fit was assessed by Martingale residual plots.18 The Cox proportional hazards model19 was used to assess CD20 expression and patient characteristics in predicting EFS. Factors significant for EFS outcomes by univariate analysis were analyzed further by stepwise regression using the assumption of proportional hazards as suggested by Cox.
Results
Study group
Two-hundred fifty-three patients with precursor B-cell ALL were treated with either VAD/CVAD (n = 110) or hyper-CVAD (n = 143). Allogeneic stem cell transplant (SCT) was performed in first CR for 7 of the 52 (13%) patients with Ph-positive ALL; 3 others underwent allogeneic SCT in first CR, owing to adverse feature of t(4;11) karyotype.
The median age of the group overall was 41 years (range, 15-80); 19% were aged 60 or older. One-hundred twenty patients (47%) expressed CD20 at a level of at least 20%. Distribution of pretreatment characteristics was similar by CD20 status (positive vs negative) except for performance status, thrombocytopenia, and presence of lymphadenopathy (Table 1). There was a higher incidence of thrombocytopenia, but a lower incidence of lymphadenopathy, in the CD20-positive group. Worse performance status (Zubrod ≥ 2) was observed in a higher proportion of the CD20-positive group (vs CD20 negative group) for the following subsets: age 31 to 59 years treated with hyper-CVAD (41% vs 21%, p = .07) and age 59 years or younger treated with VAD/CVAD (21% vs 0%, p = .03). The incidence of the Ph chromosome was similar regardless of CD20 expression, but none of the patients with the t(4;11) translocation expressed CD20. Co-expression of other surface antigens was examined on a limited basis. There was a higher incidence of CD19 positivity and lower incidence of myeloid marker expression (eg, CD13, CD33) in the CD20-positive group (Table 1).
Outcome
CR rate was similar irrespective of CD20 status (negative vs positive, 68% vs 83% with VAD/CVAD and 95% vs 94% with hyper-CVAD; Table 2). However, the incidence of disease recurrence was higher in the CD20-positive group (71% vs 53% with VAD/CVAD, p = .08, 61% vs 37% with hyper-CVAD, p = .005). The overall incidence of isolated (n = 11) or concurrent (n = 6) CNS relapse was 8%. The incidence of CNS relapse was lower for the hyper-CVAD regimen compared with VAD/CVAD (4% vs 13%, p = .02), but there was no significant difference in CNS relapse within treatment arms by CD20 status (CD20-positive vs -negative, 5% vs 4% or 16% vs 11%, respectively).
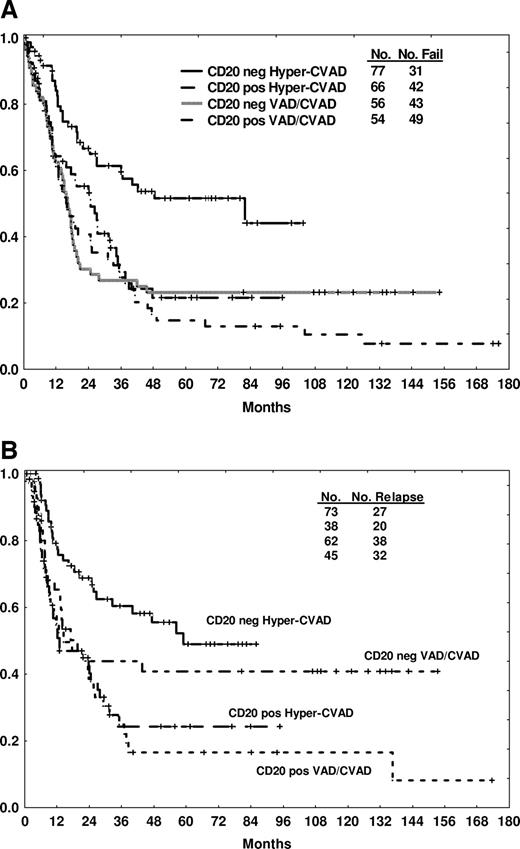
Three-year CRD and OS rates were significantly worse with CD20 expression (20% vs 55%, P < .001 and 27% vs 40%, p = .03, respectively). The benefit of intensification with the hyper-CVAD regimen was apparent in the CD20-negative group, with improvements in 3-year CRD (58% vs 42%, p = .04) and OS (60% vs 28%, P < .001) rates compared with VAD/CVAD (Table 2, Figure 1A,B). In contrast, the 3-year CRD (22% vs 18%) and OS (27% vs 26%) rates were similar for the CD20-positive group regardless of therapy (Table 2, Figure 1A,B).
Outcome by CD20 expression (positive or negative by traditional cut point of 20%) and by therapy. Survival (A) and CR duration (B) are depicted.
Outcome by CD20 expression (positive or negative by traditional cut point of 20%) and by therapy. Survival (A) and CR duration (B) are depicted.
Pretreatment characteristics were assessed for prognostic influence. Multivariate analysis (MVA) of pretreatment characteristics for EFS included age, performance status, gender, French-American-British (FAB) subtype, leukocyte count, hemoglobin, platelet count, percent peripheral blasts, percent marrow blasts, karyotype (Ph vs others), LDH, beta-2 microglobulin, splenomegaly, hepatomegaly, lymphadenopathy, CNS disease, systemic risk, CNS relapse risk, and therapy (VAD/CVAD vs hyper-CVAD). Factors which correlated with worse outcome by MVA included the traditional prognostic factors of older age, leukocyte count 30 × 109/L or more, presence of the Ph chromosome, and high systemic risk classification. CD20 positivity was also an independent predictor of outcome, albeit with relatively wide confidence intervals surrounding the hazard ratio (Table 3).
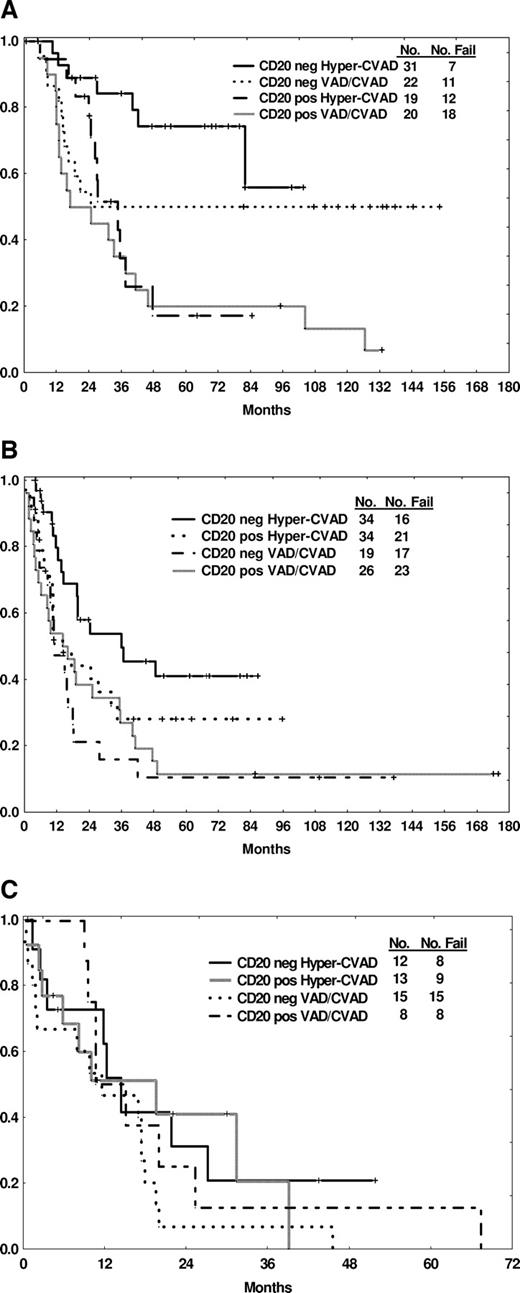
To examine further the prognostic significance of CD20 status by age, a subset analysis comparing OS by therapy was conducted (Table 4, Figure 2A-C). CD20 positivity conferred a worse survival in the group aged 30 years or less regardless of therapy (85% vs 35% with hyper-CVAD, p = .009, 50% vs 35% with VAD/CVAD, p NS; Figure 2A), whereas 3-year OS rates for the elderly group aged 60 years or older were similar (20% or less) regardless of CD20 expression or therapy (Figure 2C). In the intermediate age group, the most favorable 3-year OS rate was observed in the CD20-negative group treated with the hyper-CVAD regimen (Table 4, Figure 2B). Three-year CRD rates were also influenced by CD20 expression: favorable outcome was observed for the CD20-negative groups within the younger and intermediate age subgroups treated with hyper-CVAD and within the younger group treated with VAD/CVAD (Table 4).
Outcome by CD20 expression and therapy according to age subgroups. Age 30 or less (A), age 31 to 59 (B), and age at least 60 (C) years.
Outcome by CD20 expression and therapy according to age subgroups. Age 30 or less (A), age 31 to 59 (B), and age at least 60 (C) years.
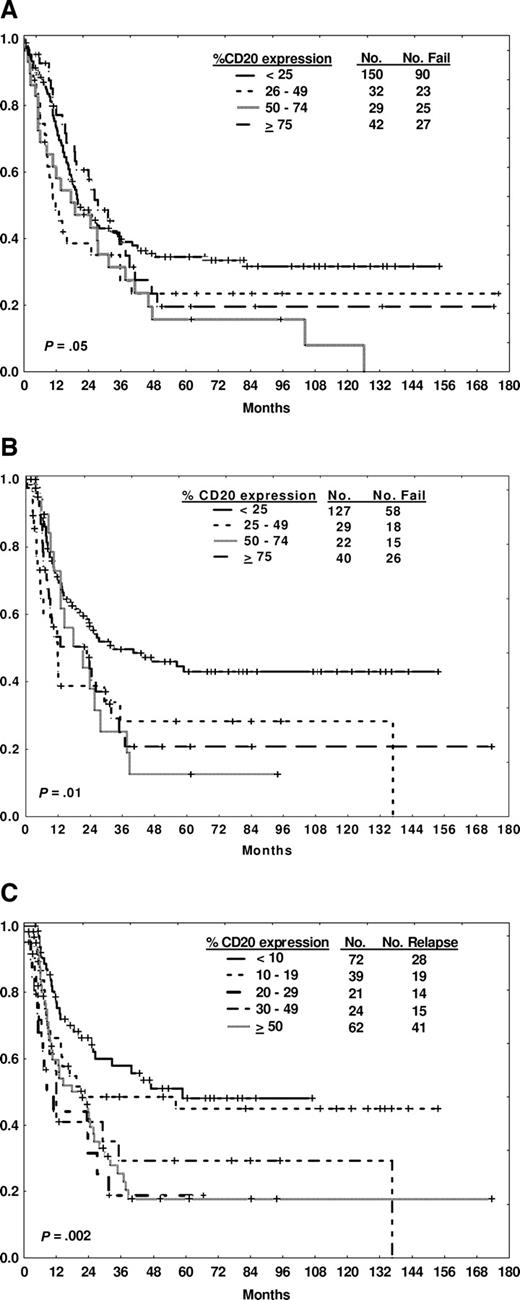
The traditional arbitrary expression rate of at least 20% was used to determine CD20 positivity based on established practice. An analysis of alternative cut points was conducted to determine whether a lower level of CD20 expression might also be of prognostic relevance, accounting for potential variability in the measurement of CD20 expression. The median CD20 expression of the group overall was 18%; the mean CD20 expression was 30%. A window of plus or minus 10% was therefore used for further analysis, for example, comparison of outcome by CD20 expression cut-offs of 10% or 30% (Table 5). Overall, 67% of the cases expressed CD20 of at least 10%; whereas 40% expressed CD20 of at least 30%. The 10% cut-off yielded reproducible findings with respect to CRD and OS when compared with the 20% cut-off, whereas the 30% cut-off appeared less discriminatory. An analysis of outcome by increasing levels of CD20 expression as assessed by quartiles (Figure 3A,B) or by centiles surrounding the 10% cut point (Figure 3C) was performed. These analyses showed clustering beyond the lowest cut point rather than a direct correlation between increasing levels of CD20 expression and poorer outcome.
Outcome by degree of CD20 expression. Survival (A) and CR duration (B) by quartiles of CD20 expression. CR duration (C) by centiles of CD20 expression.
Outcome by degree of CD20 expression. Survival (A) and CR duration (B) by quartiles of CD20 expression. CR duration (C) by centiles of CD20 expression.
Discussion
Incorporation of targeted therapy agents into chemotherapy regimens has improved the outcome for several lymphoproliferative disorders. In mature B-lineage (Burkitt-type) ALL, where high-intensity CD20 expression is a universal feature, the incorporation of rituximab into hyper-CVAD has significantly improved outcome.5 This was particularly notable for the elderly subgroup for which further intensification of chemotherapy was not feasible. The prognostic significance of CD20 expression in the more heterogeneous subgroup of precursor B-cell ALL has not been as well delineated. Based on interactions with apoptosis and survival pathways, CD20 expression could theoretically confer increased drug resistance, resulting in persistence of leukemia subclones, which eventually resurge and lead to disease recurrence.11 Targeting the CD20 molecule in conjunction with pharmacological agents could reverse these resistance mechanisms and potentially avert disease recurrence.
In childhood precursor B-cell ALL, the influence of CD20 expression was evaluated by the traditional arbitrary cut-off of 20% and by fluorescence intensity in a subset of patients treated on risk-adapted Pediatric Oncology Group (POG) protocols.11 An inferior EFS was observed with CD20 expression by either methodology. This impact on outcome was independent of other traditional risk factors, including age and adverse karyotypic aberrancies. Jeha et al12 assessed the significance of CD20 expression in children with precursor B-cell ALL treated with sequential St Jude Total Therapy protocols. The incidence of CD20 expression at least 20% was 48%; an age-associated decrease in frequency was noted at the extremes of the age spectrum (younger than 1 year and older than 10 years). As in the previous study, there was no correlation between CD20 expression and known prognostic factors, although certain disease features (eg, hepatomegaly, splenomegaly) were associated with a higher incidence of CD20 positivity. In contrast to the POG experience, CD20 expression was associated with a slightly more favorable outcome (5-year EFS rate of 84% ± 2.9% vs 78% ± 3.1%, p = .08; 5-year OS rate of 88% ± 2.5% vs 83% ± 2.8%, p = .13) and was not an independent predictor of outcome by multivariate analysis. It was postulated that the disparate results could be accounted for by differences in intensity and/or efficacy of the POG and St Jude chemotherapy programs.
We evaluated the significance of CD20 expression in adults with precursor B-cell ALL treated with 2 sequential regimens of increasing intensity (VAD/CVAD then hyper-CVAD) in the pre-rituximab era. Overall frequency of CD20 expression of at least 20% was similar to the St Jude experience (47%). There was no difference in frequency by the traditional prognostic factors, which determine systemic risk in adult ALL (eg, age, leukocyte count, karyotype). CD20 positivity was associated with a higher incidence of relapse (65% vs 42%, P < .001), lower 3-year CRD rate (20% vs 50%, P < .001), and lower 3-year OS rate (27% vs 40%, p = .03) overall. Whereas intensifying the chemotherapy (from VAD/CVAD to hyper-CVAD) significantly improved the outcome for the CD20-negative group (3-year CRD rates improved from 42% to 58%, p = .04 and OS rates from 28% to 60%, P < .001), the outcome for the CD20-positive group was similar regardless of chemotherapy regimen (3-year CRD rates, 18% to 22% and OS rates, 26% to 27%, respectively). This suggests that further intensifying the chemotherapy for CD20-positive precursor B-cell ALL in a manner other than incorporating monoclonal antibodies (eg, rituximab) or other targeted agents would be unlikely to improve outcome. The one potential caveat is the incorporation of asparaginase, since it was not a substantial component of either regimen.
The independent adverse influence of CD20 expression on EFS in this cohort was observed in a multivariate analysis, which also identified the traditional prognostic factors of older age, higher leukocyte count, Ph positivity, and high systemic relapse risk as predictors of inferior outcome. Based on these findings, an age-based subset analysis was conducted. Surprisingly, the adverse impact of CD20 expression was most apparent in the youngest age group; the primary cause of failure was related to a higher incidence of systemic disease recurrence in the CD20-positive group aged 30 years or younger regardless of therapeutic regimen (68% vs 33% for hyper-CVAD, p = .02 80% vs 35% for VAD/CVAD, p = .01). This is particularly relevant given that the current standard approach is to administer pediatric regimens (which traditionally do not incorporate rituximab or other monoclonal antibodies) to the younger patients, given the inferior outcomes observed with conventional adult ALL regimens. In the elderly patients, the 3-year CRD and OS rates did not appear to be influenced by CD20 expression, although CRD rates were slightly higher in the CD20-negative subgroup.
The traditional arbitrary selection of 20% as the cut point for CD20 positivity was based on established practice. Additional analyses of absolute CD20 expression were performed to determine whether alternate cut points could also be informative. Surprisingly, the 10% cut point for CD20 expression appeared equally discriminatory with respect to CRD and OS outcomes (Table 5). Interestingly, quartile and centile analysis of absolute CD20 expression showed clustering of outcomes with CD20 expression levels exceeding either 20% (centile analysis) or 25% (quartile analysis), suggesting that the prognostic influence of CD20 expression may be an “all or nothing” phenomenon.
In conclusion, CD20 expression appears to be a poor prognostic feature of precursor B-cell adult ALL, particularly in the younger subsets. Based on this finding, and the early promising results observed in mature B-cell (Burkitt-type) ALL, rituximab was incorporated into the hyper-CVAD regimen for adults with CD20-positive precursor B-cell ALL.5 Preliminary results suggest improved 3-year DFS and OS rates with the chemoimmunotherapy regimen compared with hyper-CVAD without rituximab, particularly for the younger age group (62% vs 28%, p = .005 and 73% vs 50%, p = .07, respectively).20 In contrast to the mature B-cell (Burkitt-type) ALL experience, the addition of rituximab has not yet proven beneficial for the elderly subset.20 Hoelzer et al21 reported similar preliminary findings of improvements in outcome with rituximab-based chemoimmunotherapy for CD20-positive precursor B-cell ALL. In this study, stratification of outcome by a lower cut point of CD20 expression (10%) has implications with respect to the current practice of allocation to rituximab-based chemotherapy and needs to be confirmed prospectively. Differences in the intensity of CD20 expression (rather than absolute expression) by age could account for the lack of clinical benefit of rituximab observed to date for the elderly patients with nonmature B-cell (Burkitt-type) ALL. This hypothesis needs to be investigated prospectively. If a lower CD20 intensity was observed in the elderly relative to the younger age groups, the use of more dose intensive rituximab dosing or the newer engineered anti-CD20 monoclonal antibodies (eg, of atumumab) could be explored.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported in part by National Institutes of Health grant 5K12 CA88084-2.
National Institutes of Health
Authorship
Contribution: D.A.T. designed and performed the research, analyzed the data, and wrote the paper; S.O.B. performed the research and revised the paper; J.L.J., J.C., S.F., G.G.-M., S.V., C.K., S.P., Y.H., W.W., and M.J.K. performed the research; S.P. analyzed the data; and H.M.K. performed the research and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Deborah A. Thomas, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: debthomas@mdanderson.org.