Abstract
Long-term T-cell reconstitution after hematopoietic stem cell transplantation (HSCT) is dependent on patient thymic function and affected by graft-versus-host disease (GVHD). To assess the impact of acute GVHD (aGVHD) on thymic function, we followed a cohort of 93 patients who received HSCT from a human histocompatibility leukocyte antigen-identical sibling, mainly for hematologic malignancies. Thymic output was measured by signal-joint T-cell receptor excision circles (sjTREC) real-time polymerase chain reaction. Absolute sjTREC number was lower at 6 months in patients with aGVHD (P = .014), associated with lower absolute counts of naive CD4 T cells at 6 and 12 months (P = .04 and .02), and persistent abnormalities in T-cell repertoire diversity. Age and aGVHD affected thymic function independently in multivariate analysis. In patients less than 25 years of age, thymic function recovered almost totally at 1 year. As a marker of thymocyte proliferation, we quantified the βTREC generated during the T-cell receptor β-chain recombination, in a group of 20 age-matched patients. Mean βTREC level was reduced at 6 months in patients with aGVHD, indicating an impact on early thymic differentiation rather than on intrathymic proliferation. These data show that aGVHD or its treatment has a transient impact on thymic function in younger patients in the first months after HSCT.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is widely used in the treatment of malignant and nonmalignant diseases. One of the main concerns is the profound and long-lasting immunodeficiency consecutive to this procedure. A potent immune reconstitution (IR) is essential to limit the infection risk and disease relapse. T-cell recovery after HSCT proceeds normally along 2 different pathways. The thymic-independent pathway includes transfer of graft-derived mature donor T cells, followed by antigen- or cytokine-driven expansions, and has limited T-cell receptor (TCR) diversity. The thymic-dependent pathway that involves generation of new naive T cells from donor-derived precursor cells accounts for a more durable reconstitution of the T-cell compartment and generates a more diverse TCR repertoire.1 During the past years, evaluation of the thymic function has been largely improved by the quantification, using real-time PCR, of the signal joint T-cell receptor excision circles (sjTREC), which result from the deletion of the TCRδ region during TCRα locus rearrangement.2,3 Using this technique together with T-lymphocyte subset phenotyping, we and others have shown that long-term T-cell reconstitution after HSCT is dependent on thymic function and is a slow process that can be influenced by several factors, including the following: age of patients, histocompatibility leukocyte antigen (HLA) mismatches, unrelated donors, source of stem cell, or occurrence of graft-versus-host disease (GVHD).1,4-8
GVHD, in its 2 different forms, acute and chronic, remains the major complication after allo-HSCT. Acute GVHD (aGVHD) is characterized by the alloreactivity of donor-derived T cells and mediates direct tissue injury through apoptosis, but also cytokine release and inflammatory response, whereas chronic GVHD (cGVHD) presents features of autoimmune disorders with chronic inflammation affecting multiple tissues.9,10 All of these mechanisms could target the host thymus rich in antigen-presenting cells. Indeed, in experimental murine models, GVHD is associated with both a reduction of thymic cellularity and a destruction of the thymic architecture.11-13 In humans, GVHD has also been linked to a decreased IR with low TREC counts.14-16 However, most studies have looked at long-term effects of cGVHD,6,16 but the impact of aGVHD on thymic function has not been extensively studied in humans. Moreover, age of the recipient, which is a critical parameter of thymic function, needs to be specifically taken into account in such studies.
In this study, we focused on the long-term impact of aGVHD on thymic-dependent immune recovery in a group of 93 patients who underwent HSCT from an HLA-identical sibling donor. IR was evaluated using a combination of T-cell naive/memory phenotyping by flow cytometry, sjTREC quantification, and T-cell repertoire analysis. Altogether, these data demonstrated a decrease of thymic function in aGVHD patients 6 months after transplantation, which is reversible in younger patients at 1 year after transplantation. To better define the mechanisms of this decreased thymic function, we analyzed intrathymic proliferation in an age-matched group of 20 patients using sj/βTREC quantification, showing a very early effect of aGVHD on thymocyte differentiation.
Methods
Patient characteristics
Patient characteristics, treatments, and posttransplant events are summarized in Table 1. The study population consisted of 93 consecutive transplant recipients who received a non–T cell–depleted HSCT from a HLA identical sibling at the Bone Marrow Transplantation Unit, Hôpital Saint-Louis (Paris, France) between March 2001 and June 2005. Cord blood transplants were excluded. Bone marrow (BM) and mobilized peripheral blood stem cells were used in 56 and 37 patients, respectively. Peripheral blood samples were collected before transplant and at 3, 6, 12, and 24 months after transplant, analyzed in flow cytometry, and stored for TREC and T-cell repertoire analysis. The investigation was approved by the Committee on Medical Ethics of the Hôpital Saint-Louis, and informed consent was obtained in accordance with the Declaration of Helsinki.
GVHD
aGVHD and cGVHD were diagnosed and graded according to published criteria.18 All patients were considered at risk for aGVHD as of day +1 after transplant. Occurrence of cGVHD was evaluated among patients who survived with sustained engraftment from day +100 after transplant. Incidence of aGVHD for grades I, II, III, and IV was 19.3%, 44.1%, 4.3%, and 5.4%, respectively. Incidence of cGVHD on evaluable patients was 44.4%. All of the patients with aGVHD, including grade I patients, received corticoid treatment (2 mg/kg/day for 14 days, then tapered). The 26 patients (33.8%) who progressed or in whom GVHD flared during steroid tapering were considered as steroid-resistant, and then received second-line treatment depending on protocols. Median length of corticoid treatment was 179 days.
Flow cytometry analysis
Lymphocyte immunophenotyping was performed on fresh whole blood samples on ethylenediaminetetraacetic acid (EDTA) by direct 2- or 4-color immunofluorescence. Measurements of forward and side scatter were combined with CD45 and CD14 determinations to identify lymphocytes and exclude monocytes. Lymphocyte gate purity was 98% or greater. The following monoclonal antibodies and combinations were used: CD45-fluorescein isothiocyanate (FITC), CD14-phycoerythrin (PE), CD62L-FITC, CD45RA-PE, CD4-peridinin chlorophyll protein (PerCP), and CD8-allophycocyanin (APC; all from BD Biosciences, Le Pont de Claix, France, except CD62L from Beckman Coulter, Villepinte, France). Absolute counts of total, CD4+, and CD8+ lymphocytes were determined with the Multitest CD3-FITC, CD8-PE, CD45-PerCP, and CD4-APC (BD Biosciences). Appropriate isotype-matched controls were carried out on each sample. Gated lymphocytes (104) were analyzed with a FACSCalibur analyzer (BD Biosciences).
sjTREC quantification
Peripheral blood mononuclear cells (PBMCs) were separated on lymphocyte separation medium (Eurobio, Les Ulis, France), and 5 to 10 × 106 cells were stored lysed in TriReagent solution (Molecular Research Center, Cincinnati, OH). RNA and genomic DNA were subsequently extracted from the same sample following manufacturer's instructions. Quantification of thymic sjTREC was done by real-time quantitative PCR (ABI PRISM7700; Applied Biosystems, Foster City, CA), as described.5 Values were normalized for the genomic copy number using albumin gene quantification. Data were expressed per 150 000 PBMC, or the total number of TREC per cubic millimeter of blood was calculated using the absolute quantification of cells by the Trucount system (BD Biosciences).
βTREC quantification
βTREC quantification was adapted from Dion et al.19 Briefly, a first PCR reaction was carried out in multiplex with 3 different outer primer mixes, each containing outer alb primers (see Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), 1 to 5 μg of genomic DNA, 200 μM each 2′-deoxynucleoside 5′-triphosphate (dNTP), 2.5 mM MgCl2, 1× buffer, and 1.25 U of platinium Taq polymerase (Invitrogen, Cergy-Pontoise, France) in 50 μL (10 minutes at 95°C, then 20 cycles of 95°C, 30 seconds; 60°C, 30 seconds; and 72°C, 2 minutes). Final quantification was made on ABI PRISM 7700, in duplicate with a second multiplex reaction that contains 5 μl 1/100 or 1/1000 dilution of the first PCR product, inner primers and probe for sjTREC or one of the Dβ-Jβ segments, inner primers and probe for albumin gene, 1.25 mM each dNTP, 3 mM MgCl2, 1× buffer, and 1.25 U platinium Taq polymerase in 25 μL (5 minutes at 95°C, then 40 cycles of 95°C, 15 seconds and 60°C, 1 minute). The sum of the 10 Dβ-Jβ segments was finally multiplied by 1.3 to extrapolate for all of the 13 existing Dβ-Jβ segments. All primers and probes have been ordered from Eurogentec (Seraing, Belgium), except the vic-labeled probe from Applied Biosystems.
T-cell repertoire
A total of 2 to 5 μg total RNA was reverse transcribed into cDNA using Superscript III, first-strand synthesis system for reverse transcription PCR (Invitrogen). TCRBV quantitative PCR amplification, BV-BC runoff using an internal BC fluorescent primer, gel running, and Immunoscope software analysis were performed, as previously described.20 Definition of the Immunoscope profiles as polyclonal, oligoclonal, or negative was done as in Talvensaari et al5
Statistical analysis
Statistical analyses were carried out at J0 and 3, 6, 12, and 24 months using 2 categories of nonparametric and parametric tests. Nonparametric Mann-Whitney test was used to compared effect of different clinical factors (aGVHD, cGVHD, age, source of cells, total body irradiation (TBI), malignant disease, or not) on the sjTREC and βTREC counts. Because data did not have a normal distribution, a logarithmic transformation was done to be able to perform different models of analyses of covariance (ANCOVA), including the factors that have an impact on thymus reconstitution by univariate analyses. ANCOVA is a general model with one continuous outcome variable and one or more qualitative or quantitative factors. ANCOVA tests whether certain factors have an effect on the outcome variable after removing the variance for which quantitative predictors (covariates) account. Differences in categorical variables between 2 groups were evaluated by χ2 test (with Yates correction if needed) or Fischer exact test using 2 × 2 contingency tables. All tests were 2-sided, with type I error rate fixed at 0.05. All statistical analyses were performed with SPSS15 and Stata 10 softwares.
Results
aGVHD delays the naive CD4 T-lymphocyte reconstitution
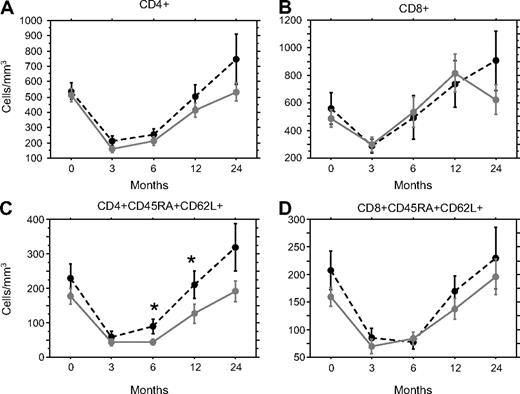
The role of aGVHD on T-cell reconstitution was assessed in a group of 93 patients receiving an HLA-identical sibling HSCT. Lymphocyte reconstitution assessed by flow cytometry showed, as expected, a drop in the absolute counts of total T (CD3+) per mm3 at 3 months posttransplantation (median 326 CD3+/mm3, range 40-2482) and a slow and progressive recovery toward normal value only at 1 year after transplant (median 924 CD3+/mm3, range 121-4500). Although slightly higher, the T-cell count was not significantly different between patients with aGVHD of grade I or higher and patients without aGVHD (median 370 vs 246; 724 vs 374; and 1041 vs 883 cells/mm3 at, respectively, 3, 6, and 12 months). Similarly, total CD4 and CD8 T-cell counts did not show significant difference according to the GVHD status of the patients (Figure 1A,B). The reconstitution of the CD45RA+ CD62L+ naive compartment was significantly slower in patients with aGVHD in the CD4+, but not CD8+ population at months 6 (P = .044) and 12 (P = .023; Figure 1C,D).
aGVHD delays the naive CD4 T-lymphocyte reconstitution. T-cell subset reconstitution was measured by flow cytometry before and 3, 6, 12, and 24 months after transplantation on patients with ( , plain gray lines, n = 68) or without aGVHD (●, dotted black lines, n = 25). Mean ± SE CD4+ (A), CD8+ (B), naive CD4+CD45RA+CD62L+ (C), or naive CD8+CD45RA+CD62L+ (D) T cells/mm3 were represented. *P < .05.
, plain gray lines, n = 68) or without aGVHD (●, dotted black lines, n = 25). Mean ± SE CD4+ (A), CD8+ (B), naive CD4+CD45RA+CD62L+ (C), or naive CD8+CD45RA+CD62L+ (D) T cells/mm3 were represented. *P < .05.
aGVHD delays the naive CD4 T-lymphocyte reconstitution. T-cell subset reconstitution was measured by flow cytometry before and 3, 6, 12, and 24 months after transplantation on patients with ( , plain gray lines, n = 68) or without aGVHD (●, dotted black lines, n = 25). Mean ± SE CD4+ (A), CD8+ (B), naive CD4+CD45RA+CD62L+ (C), or naive CD8+CD45RA+CD62L+ (D) T cells/mm3 were represented. *P < .05.
, plain gray lines, n = 68) or without aGVHD (●, dotted black lines, n = 25). Mean ± SE CD4+ (A), CD8+ (B), naive CD4+CD45RA+CD62L+ (C), or naive CD8+CD45RA+CD62L+ (D) T cells/mm3 were represented. *P < .05.
aGVHD delays the recovery of the thymic output
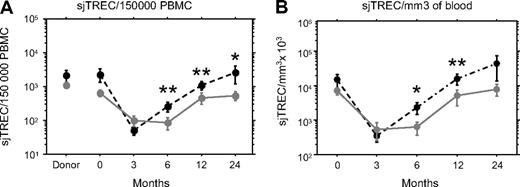
The slower recovery of naive CD4 T cell in aGVHD patients prompted us to look at thymic output using sjTREC quantification. The proportion of recent thymic emigrants assessed by the number of sjTREC per 150 000 PBMC (≈ 1 μg of DNA) was similar in the 2 groups of patients, before and up to 3 months after transplantation and for their respective donors as well (Figure 2A). At month 6, sjTREC/150 000 PBMC started to rise in the patients without aGVHD and became significantly higher than for patients with aGVHD of grade I or higher (P = .008). The median sjTREC level rose in both categories of patients thereafter, but stayed significantly higher in patients without aGVHD (P = .008, and P = .040 at, respectively, 12 and 24 months after graft).
aGVHD delays the recovery of the thymic output. Number of sjTREC was measured by quantitative PCR before and 3, 6, 12, and 24 months after transplantation in the PBMCs of patients with ( , plain gray lines) or without aGVHD (●, dotted black lines). Mean ± SE sjTREC/150 000 PBMC (A) or total number of sjTREC/blood mm3 (B) was represented. *P < .05; ** P < .01.
, plain gray lines) or without aGVHD (●, dotted black lines). Mean ± SE sjTREC/150 000 PBMC (A) or total number of sjTREC/blood mm3 (B) was represented. *P < .05; ** P < .01.
aGVHD delays the recovery of the thymic output. Number of sjTREC was measured by quantitative PCR before and 3, 6, 12, and 24 months after transplantation in the PBMCs of patients with ( , plain gray lines) or without aGVHD (●, dotted black lines). Mean ± SE sjTREC/150 000 PBMC (A) or total number of sjTREC/blood mm3 (B) was represented. *P < .05; ** P < .01.
, plain gray lines) or without aGVHD (●, dotted black lines). Mean ± SE sjTREC/150 000 PBMC (A) or total number of sjTREC/blood mm3 (B) was represented. *P < .05; ** P < .01.
Because the copy number of sjTREC/150 000 PBMC could be influenced by the proliferation of memory or effector T cells, we also expressed the sjTREC counts per mm3 of patient blood taking advantage of the absolute quantification of the T cells. In that case, total number of sjTREC displayed the same pattern as number of sjTREC/PBMC, with no difference until 3 months after transplantation and a faster increase in the absence of aGVHD (Figure 2B, P = .019, P = .010, and P = .269 at, respectively, 6, 12 and 24 months after graft). Thus, the lower thymic output in aGVHD was also observed for absolute sjTREC copy number, ruling out an indirect effect of higher T-cell proliferation in aGVHD.
aGVHD delays the recovery of a fully diverse T-cell repertoire
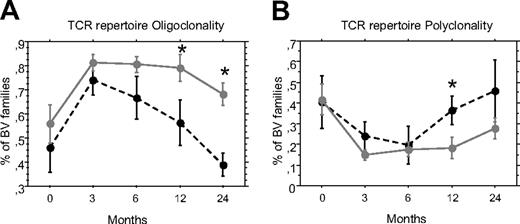
Reconstitution of a complete T-cell repertoire after allo-HSCT is dependent on the contribution of new naive T cells. We looked at the effect of aGVHD on T-cell repertoire diversity using quantitative spectratyping or Immunoscope,20 which quantifies the CDR3 length polymorphism of 25 BV families in 15 of the 93 patients (10 with aGVHD). The number of oligoclonal BV families reflecting a skewed repertoire decreased faster for patients without aGVHD to reach pretransplant level between 1 and 2 years after transplant (Figure 3). The frequency of oligoclonal families was significantly different between patients with and without aGVHD at 12 and 24 months (P = .046 and .015, respectively). Similarly, the number of polyclonal families increased faster in patients without aGVHD, the difference being also significant at 12 months posttransplant (P = .046). Thus, aGVHD was associated with a slower transition from oligoclonal to polyclonal families.
aGVHD delays the recovery of a fully diverse T-cell repertoire. Diversity of the T-cell repertoire was measured using Immunoscope before and 3, 6, 12, and 24 months after transplantation, in the PBMC of patients with ( , plain gray lines) or without aGVHD (●, dotted black lines). Mean percentage ± SE of oligoclonal (A) or polyclonal (B) BV families was represented. *P < .05.
, plain gray lines) or without aGVHD (●, dotted black lines). Mean percentage ± SE of oligoclonal (A) or polyclonal (B) BV families was represented. *P < .05.
aGVHD delays the recovery of a fully diverse T-cell repertoire. Diversity of the T-cell repertoire was measured using Immunoscope before and 3, 6, 12, and 24 months after transplantation, in the PBMC of patients with ( , plain gray lines) or without aGVHD (●, dotted black lines). Mean percentage ± SE of oligoclonal (A) or polyclonal (B) BV families was represented. *P < .05.
, plain gray lines) or without aGVHD (●, dotted black lines). Mean percentage ± SE of oligoclonal (A) or polyclonal (B) BV families was represented. *P < .05.
Age and aGVHD have an independent effect on thymic function recovery
According to the role of thymic output on T-cell recovery, we tested, in univariate analysis, the effect on the total sjTREC count of different factors. Age, aGVHD and cGVHD, stem cell source, TBI in the conditioning regimen, nonmyeloablative conditioning, or underlying disease that could influence T-cell reconstitution were tested using Mann-Whitney's U test before and at 3, 6, and 12 months after transplantation (Table 2). As expected, age had a strong impact on thymic function and sjTREC number whatever the time point before or after transplant was. As we already reported,21 older age, occurrence of a malignant disease, and use of PBSC were significantly associated with a lower value of sjTREC before transplant in the recipients (Table 2). Patients with malignant disease or patients grafted with PBSC had less sjTREC/blood mm3 than the one grafted with BM, but were also much older (median age 36 vs 14 years, P < .001), as were patients with malignant versus nonmalignant diseases (median age 47 vs 24 years, P < .0001 for PBSC vs BM). At month 6, only aGVHD and age had an impact on the patient sjTREC level. At month 12, we found an association between patient age, occurrence of aGVHD of grade I or higher, and a low value of sjTREC/mm3 blood, but also with cGVHD (limited or extensive) or the source of stem cell with less sjTREC/blood mm3 at 12 months in case of PBSC. Because many of these factors could be related, we performed an ANCOVA, including all factors found to be significant in univariate analyses. So, at month 6, the multivariate analysis included age (more or less than 25 years) and aGVHD (grade 0 vs I-IV). It confirmed that both age and aGVHD had a significant and independent impact on the recovery of thymic function at month 6 after HSCT (Table 2). Nonmyeloablative conditioning was not taken into account because it showed no effect in univariate analysis. Moreover, analysis including only the 79 patients who received a myeloablative conditioning still found a significantly lower absolute number of sjTREC at 6 months in patients of aGVHD overall (P = .035) and in patients younger than 25 years (P = .007).
aGVHD effect on thymic function is reversible in younger patients
To distinguish effects of aGVHD from age on sjTREC reconstitution, we analyzed separately younger and older patients. We chose 25 years of age as a cut-off because we and others showed previously that sjTREC decreased relatively slowly until that age before dropping more sharply thereafter.2,21 Accordingly, patients older than 25 years had the lowest sjTREC levels before transplantation, but showed also a minimal recovery in presence or absence of aGVHD up to 1 year after graft. Recovery of sjTREC level was much more efficient in younger patients whatever their aGVHD status was, thus confirming the role of age in sjTREC recovery (Figure 4A,B). The significant delay in sjTREC reconstitution was also more evident in younger patients. For recipients less than 25 years of age, the median count of sjTREC/blood mm3 at month 6 was significantly lower in aGVHD patients (P = .005). Within this subgroup, the median age was not significantly different for patients with and without aGVHD (8.8 and 13.9 years, respectively). Six months later, the number of sjTREC rose up in both groups and the difference was not significant anymore, evidencing that in younger patients (< 25 years of age), the thymic impact of an episode of aGVHD could be fully reversible.
Age, aGVHD, and cGVHD have an independent effect on thymic function recovery. Mean ± SE number of sjTREC/mm3 of blood was measured by quantitative PCR, before and 3, 6, and 12 months after transplantation, in the PBMC of patients younger (A) or older (B) than 25 years, with ( , plain gray lines) or without aGVHD (●, dotted black lines) or in all patients (C) with aGVHD (gray lines and symbols) and cGVHD (dotted lines, squares). *P < .05; ** P < .01.
, plain gray lines) or without aGVHD (●, dotted black lines) or in all patients (C) with aGVHD (gray lines and symbols) and cGVHD (dotted lines, squares). *P < .05; ** P < .01.
Age, aGVHD, and cGVHD have an independent effect on thymic function recovery. Mean ± SE number of sjTREC/mm3 of blood was measured by quantitative PCR, before and 3, 6, and 12 months after transplantation, in the PBMC of patients younger (A) or older (B) than 25 years, with ( , plain gray lines) or without aGVHD (●, dotted black lines) or in all patients (C) with aGVHD (gray lines and symbols) and cGVHD (dotted lines, squares). *P < .05; ** P < .01.
, plain gray lines) or without aGVHD (●, dotted black lines) or in all patients (C) with aGVHD (gray lines and symbols) and cGVHD (dotted lines, squares). *P < .05; ** P < .01.
aGVHD and cGVHD have a different impact on thymic function kinetics
There is a strong link between aGVHD and cGVHD, with, in this cohort, cGVHD occurring more frequently in patients who had previously aGVHD (P = .009, χ2 test). Overall, among the 86 patients with complete follow-up for cGVHD, 35 had suffered aGVHD and cGVHD, 17 neither of them, 29 had aGVHD only, and 5 cGVHD only. This allowed us to separate the effects of aGVHD from cGVHD. Patients with aGVHD had the lowest sjTREC levels at month 6 and could thereafter recover in case of a resolutive episode, whereas cGVHD was significantly associated with low sjTREC at month 12 (Figure 4C). Notably, patients developing cGVHD without a prior aGVHD episode had, at month 6, a thymic function indistinguishable from those patients without GVHD. This suggests an independent impact of GVHD, either acute or chronic, on thymic function, with no indication in this study that a defect in thymic function could precede the occurrence of cGVHD.
aGVHD-induced delay in thymic function recovery is not primarily due to a default in intrathymic proliferation
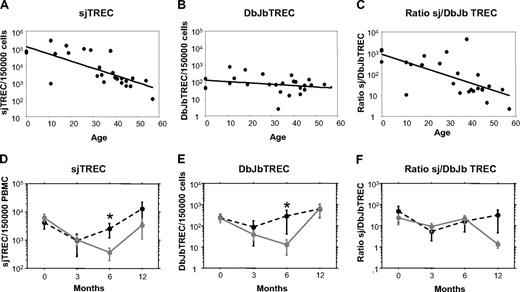
To study the mechanisms of aGVHD in thymic function recovery after allogeneic HSCT, we analyzed more in depth 20 patients, 14 of them having aGVHD. They were chosen because of a long complete follow-up (more than 1 year) and a matched mean age of 29.9 (range 8.8-60.1) and 30.5 (range 10.5-54.6) for patients with or without aGVHD, respectively. Thymic function was evaluated through the quantification of sjTREC and βTREC.19 This technique quantifies TCR Dβ–Jβ recombinations, the more primitive event in αβ T-cell differentiation before TCR α-chain recombination. So, sjTREC/βTREC ratio will reflect the αβ T-cell proliferation rate between β- and α-chain recombination. In a group of 25 normal blood donors, including 3 cord blood samples (age range 0-57 years), βTREC levels were stable with age, whereas sjTREC sharply decreased, indicating that the decrease of intrathymic proliferation could mostly explain the age-related decrease in thymic output (Figure 5A-C). In the 2 groups of age-matched HSCT patients, we confirmed the sjTREC decrease at month 6 in patients with aGVHD, as observed in the whole cohort (Figure 5D). Analysis of βTREC counts showed the same significant delay in patients with aGVHD (P < .05 at month 6), whereas the βTREC counts were much more stable for patients without GVHD (Figure 5E). As a result, the sjTREC/βTREC ratio remained quite stable between the 2 groups at month 3 and month 6, whereas a low ratio at month 12 was explained by a faster βTREC than sjTREC recovery in patients with aGVHD (Figure 5F). These results indicate that the delay in thymic function recovery in case of aGVHD could not be explained primarily by a default in intrathymic proliferation. This suggests that the impact of aGVHD at month 6 could mainly occur at an early thymocyte differentiation step before the TCR β-chain recombination.
aGVHD-induced delay in thymic function recovery is not primarily due to a default in intrathymic proliferation. Number of sjTREC/150 000 PBMC (A), Dβ-JβTREC/150 000 PBMC (B), or sj/βTREC ratio (C) was measured by quantitative PCR, in 25 normal blood donors, including 3 cord blood samples and plotted against age. Mean ± SE number of sjTREC/150 000 PBMC (D), Db-JbTREC/150 000 PBMC (E), or sj/βTREC ratio (F) was measured by quantitative PCR before and 3, 6, and 12 months after graft, in the PBMC of 20 patients with ( , plain gray lines) or without aGVHD (●, dotted black lines). * P < .05.
, plain gray lines) or without aGVHD (●, dotted black lines). * P < .05.
aGVHD-induced delay in thymic function recovery is not primarily due to a default in intrathymic proliferation. Number of sjTREC/150 000 PBMC (A), Dβ-JβTREC/150 000 PBMC (B), or sj/βTREC ratio (C) was measured by quantitative PCR, in 25 normal blood donors, including 3 cord blood samples and plotted against age. Mean ± SE number of sjTREC/150 000 PBMC (D), Db-JbTREC/150 000 PBMC (E), or sj/βTREC ratio (F) was measured by quantitative PCR before and 3, 6, and 12 months after graft, in the PBMC of 20 patients with ( , plain gray lines) or without aGVHD (●, dotted black lines). * P < .05.
, plain gray lines) or without aGVHD (●, dotted black lines). * P < .05.
Discussion
An impaired thymic function after HSCT has been largely documented in cGVHD,5-7,16,22 but the impact of aGVHD remains more controversial. Especially, it is not yet clear whether a past history of aGVHD further impacts thymic function and IR, as suggested by some,14,23 but not by other studies.6,24-26 Another issue that has not been solved yet and that could partly explain the discrepancy between previous studies is the respective influence of age and GVHD, which both have a major impact on thymic function and could be confounding factors. These apparently simple questions need to be answered before gaining insight into the mechanisms driving T-cell reconstitution in HSCT patients. We studied in this work a large cohort of non-T-cell–depleted HSCT from HLA-identical sibling with sequential samples stored every 3 months up to at least 1 year posttransplant. Multivariate analysis ruled out a role for stem cell source (BM vs peripheral blood hematopoietic stem cell), underlying disease (malignant or not), use of nonmyeloablative conditioning, and, notably, TBI in sjTREC reconstitution after HSCT (Table 2). Patients younger than 25 years of age showed a more potent thymic function recovery than older patients in agreement with previous studies.7,22 Notably, multivariate analysis showed an independent effect of age and aGVHD on sjTREC values at month 6. Age and aGVHD were also clearly separated regarding the long-term impact on thymic recovery. Among patients up to 25 years of age, those who suffered an episode of aGVHD could fully recover, 12 months after graft, a thymic function indistinguishable from patients without GVHD and from pretransplant values (Figure 4A). Conversely, cGVHD and age were closely linked at month 12, with a dominant effect of age on thymic function at this time point (Table 2). In total, we evidenced a role of aGVHD per se, in thymic defects at month 6, which is thereafter translated in low numbers of naive CD4+ T cells at months 6 and 12 and in persistent defects of T-cell diversity (Figures 1,2).
Another notable issue is the sensitivity of thymic dysfunction to aGVHD. In fact, when taking into account aGVHD patients with grades 0-I versus grade II and above, the difference in thymic output at month 6 was also significant in patients up to 25 years of age (P = .006), but less than when considering patients without any symptom of aGVHD compared with others (grades I-IV) (P = .0047; Figure 2). Actually, median sjTREC values in patients with grade I aGVHD (n = 25) were closer to that of those with grades II through IV than to patients without aGVHD (data not shown). This supports the idea that the thymus could be very sensitive to aGVHD and that thymic GVHD could not directly be reflected by usual aGVHD grading based on clinical and histologic criteria. However, because all aGVHD patients, including grade I, received steroids, it is possible that this treatment could impact thymic function. But, experiments in mice have shown that GVHD-induced apoptosis of thymocyte was independent of glucocorticoids,27 and in humans, patients receiving cord blood transplant who did not develop GVHD, but received steroids for GVHD prophylaxis, had a normal T-cell reconstitution.6
We then addressed issues regarding mechanisms of aGVHD-associated thymic impairment taking advantage of the development of βTREC assay.19 Because it has been shown that in mice the level of thymic output after HSCT is primarily determined by the intrathymic proliferation of precursor T cells,28 we decided to measure the impact of aGVHD on it, in a selected cohort of age-matched patients. During differentiation, αβ T cells rearrange first the TCR β-chain at the double-negative (DN) level, proliferate to the double-positive (DP) stage, and then rearrange their TCR α-chain.29,30 Quantifying the TRECs generated at these 2 checkpoints allowed estimation of the intrathymic proliferative history of the lymphocytes. Using this technique, a reduction of intrathymic proliferation in recently HIV-infected individuals and an age-related decreased of thymic output due to an age-dependent decrease in intrathymic proliferation have been shown.19 We confirmed, in a group of healthy donors, an age-related decrease in intrathymic proliferation, but not in T-lymphocyte β-chain commitment assessed by βTREC values (Figure 5A-C). However, aGVHD-associated decrease in thymic output could not have been explained primarily by a decrease in intrathymic proliferation (Figure 5D-F). The sjTREC decrease at month 6, reflecting a reduction in the number of lymphocytes that have rearranged their α-chain, was concomitant to a decrease in the Dβ-JβTREC level, which indicates a reduction in the number of lymphocytes that have rearranged their β-chain, resulting in a comparable sj/βTREC ratio in patient with or without aGVHD. This could indicate that aGVHD acts early before β-chain rearrangement. This result is consistent with experimental work in mice, in which reduction of DP thymocytes during aGVHD was secondary to a decreased commitment of resident pro-T and pre-T cells to enter the cell cycle.12 Several mechanisms could explain such an early action. Histologic analyses and functional studies have shown that thymic epithelial cells and thymocytes are targets of aGVHD in mice,31 but also in humans,32 and that results in a profound modification of thymic architecture. Although alloreactivity of donor T cells seems the more likely mechanism,13 thymic radiosensitivity has also been suggested.33 However, there was no effect of irradiation either on GVHD or on thymic output in our group of patients (Tables 1,2). The loss of these epithelial cells could result in a decreased Notch signal that has been shown as essential for the survival and the differentiation of DN thymocytes.34 Moreover, stromal and epithelial thymic cells produce locally growth factors; cytokines essential for thymocyte survival such as interleukin (IL)-7, stem cell factor, and Flt3L35,36 ; or chemokines such as C-C chemokine ligand (CCL) 21 and CCL25 involved in the T-cell precursor homing.37,38 We assessed, by enzyme-linked immunosorbent assay, the serum concentration of IL-7, Fms-like tyrosine3 Ligand (Flt3L), CCL21, and CCL25 among 18 of the 20 patients selected for the βTREC analysis, but none gave significant results between the 2 groups (data not shown). Obviously, we cannot exclude an in situ paracrine action of these molecules, as proposed for Flt3L.39 Lastly, aGVHD could decrease the number of multipotent precursor stem cells being periodically released from the BM and migrating into the thymus.40
Finally, we evidenced that the thymic impact of an episode of aGVHD could be fully reversible in younger patients. Age and thymic function are strongly linked, but the defect seems to be different in case of aGVHD, in which it occurs before the β-chain recombination and in aging, in which Dion et al19 demonstrated a decrease in thymocyte proliferation was mainly accountable for the decrease of thymic output with age. However, several other defects that involve T-cell progenitors, or cytokine or hormone production, have been linked to thymic involution and could explain the lower capacity of aged patients to restore an efficient thymic output after HSCT whether they had aGVHD or not.41
Excess morbidity due to a high incidence of opportunistic infections after HSCT42 has been linked to a defect in thymic function.4,6,22 So, our data showing that thymic function is impaired by aGVHD during the first months after transplantation may have consequences in terms of treatment. Decreasing incidence of aGVHD with aggressive GVHD prophylaxis and treatment, especially in young patients, could temper its effect on IR. Alternatively, experimental models, especially in mice, but also human transplantation data, have shown that it is possible to increase thymic function after HSCT.43 Some of them, such as stimulation of the growth hormone (GH) pathway44,45 or sex steroid blockade,46 are supposed to counterbalance the effect of aging on the thymic function. Others, such as the keratinocyte growth factor (KGF), have a protective effect on thymic epithelial cells that would preserve the thymus of conditioning treatment injuries.47 Finally, treatments with exogenous IL-748 or Flt3L49 have been shown to increase thymic output, and promote T-cell precursor survival and naive T-cell homeostasis. In humans, several clinical trials have been initiated, as follows: in HSCT, KGF has been given safely without, however, study of the thymic function.50 Administration of antiandrogen therapy in HSCT patients51 or GH in HIV patients52 has been shown to increase thymic function especially with the production of new naive CD4+ T cells and, finally, administration of recombinant human IL-7 in cancer patients53 increases the number of sjTREC+ circulating T cells, and above all induces naive T-cell proliferation. The clinical use of these therapeutic agents, alone or in combination, could so be a way to alleviate, in allogeneic HSCT, the effect of age and GVHD on the restoration of a potent thymic function.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank G. Henry and I. Fournier for excellent technical help.
This work was supported by research grants from the Sixth Framework Programme (FP6) ALLOSTEM (503319) London, United Kingdom; Agence de la Biomédecine, St Denis, France; and Cancéropôle Ile-de-France Paris, France.
Authorship
Contribution: E.C. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; M.B. collected data, performed statistical analysis, and analyzed and interpreted data; C.D. and J.B. performed research; C.R. and M.C. contributed analytical tools and collected data; R.P.L. and V.R. collected data; D.C. critically reviewed the manuscript; G.S. analyzed and interpreted data, and critically reviewed the manuscript; A.T. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: All the authors declare no competing financial interests.
Correspondence: Dr Emmanuel Clave, Laboratoire d'Immunologie et d'Histocompatibilité AP-HP, Inserm UMRS-940, Institut Universitaire d'Hématologie, Hôpital Saint-Louis, 1, Avenue Claude Vellefaux, F-75475 Paris, CEDEX 10, France; e-mail: emmanuel.clave@univ-paris-diderot.fr.