Abstract
In this issue of Blood, Clave and colleagues explore the impact of acute GVHD on thymic recovery following hematopoietic stem cell transplantation and begin to address the mechanism by which alloreactivity may impair thymic function in humans.
Although recovery of the innate immune system occurs rapidly following allogeneic hematopoietic stem cell transplantation (HSCT), full reconstitution of adaptive immunity is protracted, resulting in risk of infection and disease relapse. Reconstitution of T cells occurs via 2 major pathways.1 Mature T cells contained in the graft, remaining in the host following conditioning, or given as a separate infusion have tremendous potential to expand and can regenerate substantial numbers of progeny. However, this process results in an oligoclonal T-cell repertoire that is limited by the specificities contained in the starting population of T cells and further constricted during the expansion process. Full immunologic recovery, repertoire diversification, and immune competence coincides with recovery of thymic function but can be quite delayed.2 It is the delay in thymic recovery that is at the crux of the protracted course of full T-cell recovery following HSCT. Ultimately, a better understanding of the mechanisms limiting thymic function will be critical if we are to improve outcomes following HSCT.
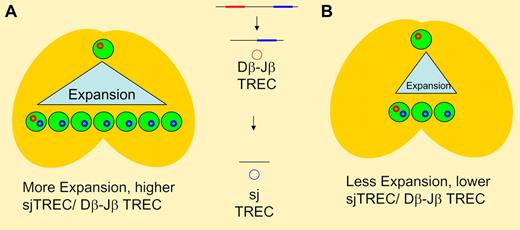
TCR rearrangement involves sequential deletions of intervening DNA sequences in germline DNA resulting in episomal DNA circles (TREC). Dβ-Jβ excision occurs earlier than sj TREC generation, with thymocyte proliferation occurring between the 2 steps. In panel A, greater intrathymic expansion results in higher thymic T-cell output and a higher ratio of sj/Dβ-Jβ TREC when compared to panel B where less intrathymic expansion results in lower thymic output and a lower ration of sj TREC to Dβ-Jβ TREC. With the same expansion seen in panel A (and the same TREC ratio), decreased thymic output would indicate changes either earlier (at the T-cell progenitor stage) or later (such as apoptosis of postrearrangement thymocytes) than the 2 rearrangement steps.
TCR rearrangement involves sequential deletions of intervening DNA sequences in germline DNA resulting in episomal DNA circles (TREC). Dβ-Jβ excision occurs earlier than sj TREC generation, with thymocyte proliferation occurring between the 2 steps. In panel A, greater intrathymic expansion results in higher thymic T-cell output and a higher ratio of sj/Dβ-Jβ TREC when compared to panel B where less intrathymic expansion results in lower thymic output and a lower ration of sj TREC to Dβ-Jβ TREC. With the same expansion seen in panel A (and the same TREC ratio), decreased thymic output would indicate changes either earlier (at the T-cell progenitor stage) or later (such as apoptosis of postrearrangement thymocytes) than the 2 rearrangement steps.
A number of factors contribute to delayed thymic recovery including age, regimen-related toxicity, and chronic GVHD. Less well-defined in humans is the impact of acute GVHD. Mouse models have demonstrated loss of thymic architecture, impaired thymic selection, and decreased numbers of double positive thymocytes with GVHD (reviewed in Krenger and Hollander3 ). Loss of thymic epithelial cells with diminished levels of proliferative factors, such as IL-7 and flt3L, and aberrant T-cell selection contribute to reduced thymic output and the generation of “auto”-reactive T cells. Importantly, these defects occur with the infusion of alloreactive T cells without conditioning, demonstrating that alloreactivity is sufficient to mediate GVHD effects on the thymus and indicate that the thymus is a target organ. Although useful, mouse models of GVHD are imperfect and not always predictive of the situation in humans, where the ability to measure what is truly happening in the thymus is difficult since most data comes from peripheral T cells. T cell–receptor excision circles (TRECs), which are generated during T cell–receptor rearrangement, have been instrumental in providing a picture of thymic function in humans and have confirmed the detrimental impact of age, regimen-related toxicity, and chronic GVHD.4 However, the data for acute GVHD have been limited.5
Clave et al contribute to our understanding of thymic function following HSCT by performing longitudinal analyses in a cohort patients of varying ages treated at a single institution.6 Their results confirm the profound impact of chronic GVHD and age on thymic recovery. In addition, they demonstrate that patients with acute GVHD also have decreased signal joint (sj) TREC levels and an oligoclonal T-cell repertoire. As long as the acute GVHD successfully resolved, recovery of TREC was seen. Interestingly, the authors indicate in the discussion that the decline in TREC was seen even in patients with grade 1 GVHD. However, their analysis goes one step further by measuring TRECs generated during β chain rearrangement in a subset of patients. Since this rearrangement occurs earlier than sj TREC, the ratio of β TREC to sj TREC has been proposed to indicate the amount of proliferation occurring between the 2 rearrangement steps in the thymus 7 (see figure). Thus, this provides additional indirect information on a critical event in T-cell development. Clave et al demonstrate that β TREC levels decline in proportion to sj TREC with a stable ratio, suggesting that the decreased thymic output with acute GVHD is not due to a decline in thymocyte proliferation.
What are the implications of this study? As has been seen in mice, the thymus must be considered a target organ in acute GVHD along with skin, gastrointestinal tract, and liver. This is perhaps not surprising, given that all contain epithelial tissues. However, in contrast to some of the murine data, diminished thymocyte proliferation may not be the root cause. The authors suggest an earlier defect, perhaps at the T-cell progenitor stage. An equally plausible explanation would be loss of thymocytes to apoptosis. Regardless of the cause, these data once again demonstrate the delicate balance between the detrimental and potentially beneficial effects of alloreactivity (at least for malignancy) and suggest that HSCT physicians may need to reconsider the notion that “a little GHVD” is a good thing.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal