Abstract
Fms-like tyrosine kinase 3 ligand (FLT3L) plays a major role in dendritic cell (DC) biology. Deficiency of FLT3L causes a dramatic decrease in DC numbers, whereas increasing its availability (by repetitive injections for 7-10 days) leads to a 10-fold increase in DC numbers. In this study, we show that FLT3L treatment indirectly leads to an expansion of peripheral naturally occurring T regulatory cells (NTregs). The FLT3L-induced increase in NTregs was still observed in thymectomized mice, ruling out the role of the thymus in this mechanism. Instead, the increased number of NTregs was due to proliferation of preexisting NTregs, most likely due to favored interactions with increased number of DCs. In vitro, we show that DCs induce regulatory T-cell (Treg) proliferation by direct cell contact and in an interleukin-2–dependent, T-cell receptor–independent manner. FLT3L could prevent death induced by acute graft-versus-host disease (GVHD). This study demonstrates unique aspects in the regulation of Treg homeostasis by DCs, which were unappreciated until now. It also reinforces the relevance of FLT3L treatment in GVHD by its ability to increase both the number of tolerizing DCs and NTregs.
Introduction
Fetal liver kinase 2/Fms-like tyrosine kinase 3 (FLK2/FLT3) is a receptor tyrosine kinase, which was initially cloned from fetal liver population enriched with hematopoietic stem cell activity.1 FLT3 has sequence and structural homology with c-kit and is expressed mostly by hematopoietic cell progenitors and dendritic cells (DCs).2-4 FLT3L is the only known ligand for FLT3.
Both FLT3 and FLT3L-deficient mice are viable and fertile, and hematopoiesis is maintained in the absence of FLT3 signaling.5,6 The most striking phenotypes of these mice are the decrease in lymphoid and myeloid bone marrow progenitors as well as a decrease in numbers of DC and natural killer (NK) cells. As expected from data obtained in FLT3L-deficient mice, administration of FLT3L by repetitive injections leads to an increase in the number of bone marrow progenitors as well as DCs and NK cells.7-10 Moreover, our previous work showed that increased FLT3L availability had an inhibitory effect on B-cell lymphopoiesis, suggesting that, when present in supraphysiologic levels, FLT3L could enhance myeloid over lymphoid differentiation.10
DCs play a dual role in the immune system. Depending on their maturation/activation state, DCs are responsible for either inducing an immune response or inducing tolerance to self or noninnocuous antigens.11,12 In an immature state, DCs express low levels of major histocompatibility complex (MHC) class II antigens and costimulatory molecules, and are thought to induce T-cell tolerance by several distinct mechanisms.12 Maturation of DCs is induced by pathogens or endogenous danger signals sensed through a variety of receptors, which upon ligand binding induce MHC class II and costimulatory molecule up-regulation as well as inflammatory cytokine secretion. Once in a mature state, DCs prime T cells and induce immune response.13
There is considerable literature suggesting that DCs influence regulatory T-cell biology. Regulatory T cells (Tregs) encompass T-cell population, which prevent harmful immune responses against self.14,15 Among Tregs, the so-called naturally occurring CD4 regulatory T cells (NTregs) are the best characterized. This lineage is defined by the expression of the transcription factor Foxp3.16-18 A natural mutation or conditional deletion of Foxp3 leads to lethal autoimmune disease due to absence of functional NTregs.19-22 NTregs develop in the thymus,15 but conversion from effector to regulatory cells can take place in the periphery, and in some experimental settings, DCs were demonstrated to be instrumental in this process.23-26
Little is known about the rules governing NTreg homeostasis in the periphery in normal conditions, but it is clear that interleukin (IL)-2 is indispensable for NTreg survival, whereas it is dispensable for their development in the thymus.27,28 In IL-2–deficient mice, NTreg development still takes place in the thymus, but cells die rapidly after thymic egress because of lack of IL-2–mediated survival signals. Consequently, IL-2–deficient mice suffer from autoimmune disease because of the lack of peripheral NTregs.29,30 In addition, CD28 signaling has been shown to be implicated in thymic NTreg development as well as peripheral survival.31,32 In a diabetes model, CD28 blockade or deficiency exacerbates autoimmune symptoms.32 Finally, NTregs are known to proliferate upon antigen encounter in vivo.33-36
In the present study, we investigated the influence of FLT3L-mediated increase of DC numbers on regulatory T cell homeostasis. We demonstrate that repetitive injections of FLT3L led to expansion of NTregs in the periphery. NTreg increase was due to proliferation of pre-existing NTregs, most likely due to favored interactions with DCs. Numbers of both DCs and NTregs go back to normal shortly after stopping FLT3L treatment. Experiments conducted in vitro showed that DC-induced NTreg proliferation was IL-2–dependent and T-cell receptor (TCR)–independent, and required an additional contact-dependent DC-mediated signal whose nature remains unknown. FLT3L treatment partially prevented death induced by acute graft-versus-host disease (GVHD) in irradiated hosts, whereas it completely rescued nonirradiated hosts. This represents a new aspect of NTreg homeostasis and might provide alternate ways of controlling NTreg biology in vivo.
Methods
Mice
C57BL/6, C57BL/6.Ly5.1 congenic mice, DBA/2, (C57BL/6 × DBA/2)F1, and FLT3L knockout (KO) mice were bred in our animal facility. MHC class II–deficient mice37 were obtained from Taconic Farms (Germantown, NY), and C57BL/6 Foxp3EGFP reporter mice38 were a kind gift from author B.M. All mice were used between 7 and 16 weeks of age and were maintained in specific pathogen-free conditions. All animal experiments were carried out within institutional guidelines, with the approval of the Cantonal Veterinary Office of Basel, Switzerland. Mice were humanely killed by CO2 inhalation, and organs were removed by standard procedures.
Thymectomies
At 4 to 6 weeks of age, mice were anesthetized and thymus was removed by suction through a small upper sternal incision. That thymectomy had been complete was verified in each animal by anatomical inspection at the time of sacrifice.
FLT3L treatment
Recombinant human FLT3L (rFLT3L) was a kind gift of Amgen (Thousand Oaks, CA). A stock solution containing 1 mg of rFLT3L/mL was prepared in phosphate-buffered saline (PBS), and aliquots were stored at −20°C until use. For FLT3L treatment, mice generally received 10 to 20 μg of rFLT3L (0.2 mL) by intraperitoneal injection daily or every second day for 10 days, a treatment schedule previously used to increase DC number. In titration experiments (see “Changes in T-lymphocyte subsets after FLT3L treatment”), mice were treated with graded doses of 2 or 10 μg/0.2-mL injections.
Bone marrow chimeras
Bone marrow cell suspensions from donor mice were prepared by flushing femurs and tibias with PBS using a 23-g needle. After red blood cell lysis, T cells were depleted by resuspending cells in a mixture of rat IgM anti-CD4 (RL172) and anti-CD8 (31 M) monoclonal antibody (mAb) hybridoma supernatants and incubated for 20 minutes at 4°C. After a washing step, antibody-coated cells were lysed by adding rabbit complement dissolved in serum-free Dulbecco modified Eagle medium (DMEM). After incubation for 45 minutes at 37°C, cells were washed and resuspended in DMEM before injection. Hosts were lethally γ-irradiated with a single dose of 950 cGy using a cobalt source (Gammacell 40; Atomic Energy of Canada, Mississauga, ON) before receiving 5 × 106 cells intravenously.
Cell preparation
Single-cell suspensions from thymus or spleen were prepared by pressing through a 100-μm nylon mesh into 2% fetal calf serum (FCS) Iscove modified Dulbecco medium (IMDM). For flow cytometry, cells were washed and resuspended in PBS containing 2% FCS and 0.1% sodium azide (FACSwash). Viable cells were stained with trypan blue and counted in a hemocytometer. Total lymphocyte numbers in each subpopulation were calculated from the frequency estimated by fluorescence-activated cell sorter (FACS) analysis, and the total number of living cells was recovered per organ.
Flow cytometry
The following mAbs were used: anti-B220 (RA3-6B2), anti-CD3 (145.2c11), anti-CD4 (RM4-5), anti-CD11c (HL3), anti-DX5 (DX5), and anti-Ki67 (B56) were purchased from BD Biosciences (San Jose, CA); anti-NK1.1 (PK136) and anti-FoxP3 (mAb FJK-16S) were purchased from eBioscience (San Diego, CA); anti-5-bromo-2′-deoxyuridine (BrdU; MoBU-1) was purchased from Biolegend (San Diego, CA); anti-CD4 (GK1.5), anti-CD8α (53-6-72), anti-CD19 (1D3), anti-CD45.2 (1D4-2.1), and anti–pan-MHC class II (M5) antibodies were purified from the hybridoma supernatant and labeled by standard methods. Cell surface staining was performed as previously described, and analyses were performed on a FACSCalibur interfaced to a Macintosh computer with CellQuest (BD Biosciences) or FlowJo (TreeStar, Ashland, OR) software. Dead cells were excluded from analysis by a combination of light scatter and/or absence of propidium iodide staining. Intracellular staining for FoxP3 and Ki67 was performed according to the manufacturer's instructions (eBioscience and BD Biosciences). Cell sorting was performed on a FACSAria (BD Biosciences).
In vitro assay
Bone marrow-derived DCs (BMDCs) were differentiated in vitro using standard protocol.39 Differentiated DCs were harvested for stimulation after 6 to 10 days; when indicated, DCs were matured by overnight incubation with 100 ng/mL lipopolysaccharide (LPS). Bulk lymph node cells or sorted cells from C57BL/6 Foxp3EGFP reporter mice were labeled with PKH26 (Sigma-Aldrich; PKH26GL) and incubated alone or together with sorted CD19+ B cells or BMDCs in 2% to 5% FCS IMDM. FLT3L or IL-2 supernatant or 10 μg/mL anti-IL-2 (S4B6) was added when indicated.
When indicated, cells were stimulated with plate-bound anti-CD3 (2C11), and antibody was diluted in PBS at a final concentration of 5 μg/mL and incubated 2 hours at 37°C. Excess was then washed away. Proliferation was monitored by measurement of PKH26 fluorescence in FL2 channel using a FACSCalibur.
GVHD experiment
GVHD was induced by intravenous injection of pooled cell suspension from lymph node and spleen cells from C57BL/6 into nonirradiated or sublethally irradiated (C57BL/6 × DBA/2)F1. Animals were followed on a daily basis and euthanized when necessary.
BrdU experiment
Mice were pulsed by intraperitoneal injection of 1 mg of BrdU. On following days, BrdU was added to the drinking water at 1 mg/mL. Anti-BrdU staining was performed as already described.40
Results
Changes in T-lymphocyte subsets after FLT3L treatment
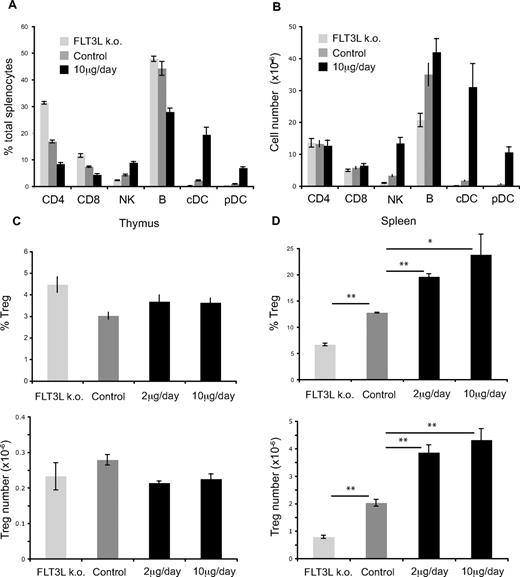
The effect of increased FLT3L availability on DC development in mice is well documented4-9,41 ; however, little is known about the direct or indirect impact of prolonged FLT3L treatment on T-cell populations. To study the influence of FLT3L levels on T-cell populations, wild-type (WT) mice were injected daily with 10μg/day of recombinant cytokine for 10 days and compared with WT control and FLT3L KO mice. Figure 1A,B shows the percentages and absolute numbers of the indicated splenic subpopulations in FLT3L KO, WT control, or WT-treated mice. Proportionally, changes in FLT3L level had the most striking effect on conventional DCs (FLT3L KO, 0.1 × 106; WT, 1.7 × 106; WT treated, 31 × 106) and plasmacytoid DCs (FLT3L KO, 0.02 × 106; WT, 0.7 × 106; WT treated, 10.6 × 106), as well as NK cell number (FLT3L KO, 1 × 106; WT, 3.3 × 106; WT treated, 13.4 × 106), as we reported previously.10 Expansion of the DC compartment was also observed in the bone marrow, thymus, and lymph nodes of treated mice (data not shown). Despite the differences of NK and DC numbers, the absolute numbers of CD4 or CD8 T cells were not drastically altered by changes in FLT3L levels (Figure 1B), and entire thymopoiesis was similar between control and treated mice (data not shown). We observed a decrease in number of B cells in FLT3L KO compared with WT mice, which is due to decreased number of B-cell precursors in the bone marrow (data not shown).
Influence of FLT3L treatment on lymphocyte subpopulations. C57BL/6 mice were injected intraperitoneally with either 2 or 10 μg, daily, or were left untreated. (A) Percentages of indicated splenic subpopulations in FLT3L KO, WT control, or WT-treated mice. (B) Absolute cell numbers of indicated splenic subpopulations in FLT3L KO, WT control, or WT-treated mice (C). Percentages of Foxp3+ cells among CD4SP (top) or absolute numbers of Foxp3+ cells (bottom) in thymus of FLT3L KO, WT control, or WT-treated mice. (D) Percentages of Foxp3+ cells among CD4 T cell or absolute numbers of Foxp3+ cells in spleen of FLT3L KO, WT control, or WT-treated mice. Each bar shows mean ± SEM. *P < .05; **P < .01. FLT3L KO,  ; WT control,
; WT control,  ; WT treated,
; WT treated,  and
and  .
.
Influence of FLT3L treatment on lymphocyte subpopulations. C57BL/6 mice were injected intraperitoneally with either 2 or 10 μg, daily, or were left untreated. (A) Percentages of indicated splenic subpopulations in FLT3L KO, WT control, or WT-treated mice. (B) Absolute cell numbers of indicated splenic subpopulations in FLT3L KO, WT control, or WT-treated mice (C). Percentages of Foxp3+ cells among CD4SP (top) or absolute numbers of Foxp3+ cells (bottom) in thymus of FLT3L KO, WT control, or WT-treated mice. (D) Percentages of Foxp3+ cells among CD4 T cell or absolute numbers of Foxp3+ cells in spleen of FLT3L KO, WT control, or WT-treated mice. Each bar shows mean ± SEM. *P < .05; **P < .01. FLT3L KO,  ; WT control,
; WT control,  ; WT treated,
; WT treated,  and
and  .
.
The role of DCs in extrathymic development of Tregs has been reported by many groups.23-26,42 Therefore, we wondered whether the influence of FLT3L level on DC numbers would have any influence on NTreg population. Variations of FLT3L concentration had no influence on the percentage or the absolute number of NTregs in the thymus (Figure 1C). However, there was a significant, dose-dependent increase in percentage and absolute number of Foxp3+ NTregs in the spleen (Figure 1D). Among CD4 T cells, FLT3L KO had 2-fold fewer Tregs than WT mice (6.7% vs 12.8%), and in the best case, FLT3L treatment increased Tregs by 2-fold (12.8% vs 24%). Both DC and NTreg numbers went back to normal values after stopping FLT3L treatment (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Because the total CD4 T-cell numbers in FLT3L KO, WT, or treated mice were similar (Figure 1B), we conclude that FLT3L level correlates with NTreg population size in the periphery, but not in the thymus.
Origin of Treg increase
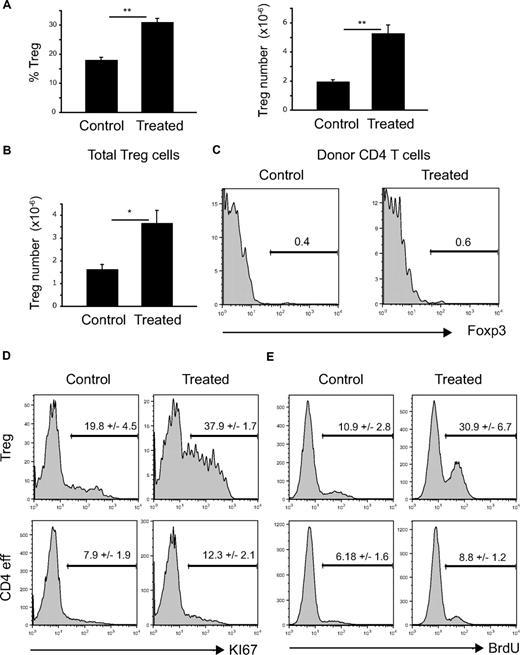
Several nonexclusive mechanisms could explain the increase in NTregs after repetitive FLT3L injections. First, we addressed the question as to whether the extra number of peripheral Tregs was due to enhanced thymic NTreg production. Although we could not observe an increase in CD4 single-positive (SP) Foxp3+ thymocytes (Figure 1C), FLT3L could potentially increase the rate of Treg development and output from the thymus. Therefore, mice were thymectomized before FLT3L treatment. We observed that even in the absence of thymic output, there was still a FLT3L-dependent increase in the percentage and absolute numbers of splenic Tregs (Figure 2A). This increase was comparable with that observed in nonthymectomized mice (Figure 1A), implying that the thymus does not contribute significantly to the increase in peripheral Ntregs.
Expansion of peripheral NTreg compartment due to increased proliferation of Foxp3+ CD4 T cells. (A) Mice were thymectomized before FLT3L treatment (10 μg intraperitoneal injection daily for 10 days). Histograms show percentages of Foxp3+ among CD4 T cells (left) and absolute numbers (right) in the spleen of control or treated mice. Each bar shows mean ± SEM. **P < .01. (B,C) CD45.1 WT mice were irradiated (100 rad) before intravenous injection of 2 × 106 sorted CD4+Foxp3− cells from CD45.2 Foxp3EGFP reporter mice. After 2 weeks, mice were treated with FLT3L (10 μg intraperitoneal injection daily for 10 days) or left as control. (B) Absolute numbers of CD4+Foxp3+ cells in the spleen of control or treated mice. Histogram shows mean ± SEM. *P < .05. (C) Histograms show percentage of Foxp3+ cells within CD45.2+D4+ donor cell population in control or treated animals. (D) Histograms show percentages of Ki67+ cells among Foxp3+ (top panels) or Foxp3− (bottom panels) CD4 T cells in control or treated animals. (E) Histograms show incorporation of BrdU in Foxp3+ (top panels) or Foxp3− (bottom panels) CD4 T cells in control or treated animals pulsed with BrdU.
Expansion of peripheral NTreg compartment due to increased proliferation of Foxp3+ CD4 T cells. (A) Mice were thymectomized before FLT3L treatment (10 μg intraperitoneal injection daily for 10 days). Histograms show percentages of Foxp3+ among CD4 T cells (left) and absolute numbers (right) in the spleen of control or treated mice. Each bar shows mean ± SEM. **P < .01. (B,C) CD45.1 WT mice were irradiated (100 rad) before intravenous injection of 2 × 106 sorted CD4+Foxp3− cells from CD45.2 Foxp3EGFP reporter mice. After 2 weeks, mice were treated with FLT3L (10 μg intraperitoneal injection daily for 10 days) or left as control. (B) Absolute numbers of CD4+Foxp3+ cells in the spleen of control or treated mice. Histogram shows mean ± SEM. *P < .05. (C) Histograms show percentage of Foxp3+ cells within CD45.2+D4+ donor cell population in control or treated animals. (D) Histograms show percentages of Ki67+ cells among Foxp3+ (top panels) or Foxp3− (bottom panels) CD4 T cells in control or treated animals. (E) Histograms show incorporation of BrdU in Foxp3+ (top panels) or Foxp3− (bottom panels) CD4 T cells in control or treated animals pulsed with BrdU.
Thus, if peripheral NTreg expansion was thymus independent, it could be due either to (1) the conversion of a CD4 T helper, non-NTreg to a NTreg phenotype and/or (2) the proliferation of pre-existing NTregs upon increased FLT3L availability. To address the first hypothesis, we investigated whether CD4+Foxp3− cells could become Foxp3+ upon FLT3L treatment. For this purpose, we injected 2 × 106 sorted CD4+Foxp3− CD45.2 cells from Foxp3EGFP reporter mice38 into lightly (100-rad) irradiated CD45.1 hosts and, 2 weeks later, treated mice with FLT3L for 10 days or left them as control. As shown in Figure 2B, treatment induced a significant increase in total NTregs but did not induce Foxp3 expression in transferred cells (Figure 2C). We conclude, therefore, that FLT3L-mediated NTreg increase was not due to conversion of effector to regulatory cells.
To address the second hypothesis, we determined CD4 T cells and NTreg proliferation upon FLT3L treatment using 2 different readouts, the expression of the proliferation-associated marker Ki67 (Figure 2D) and incorporation of BrdU (Figure 2E). As shown, FLT3L treatment significantly increased the percentages of Ki67+ and BrdU+ CD4+Foxp3+ cells, whereas it induced only a minor increase of Ki67+ or BrdU+ CD4+Foxp3−. This strongly suggests that FLT3L treatment induced a preferential increased proliferation of regulatory versus effector CD4 T cells.
Taken together, these results show that expansion of the peripheral NTreg compartment is due to proliferation of NTregs upon FLT3L treatment.
DCs support Treg proliferation
FLT3L-induced Treg proliferation could be due either to a direct signaling of the cytokine on the Treg population or to an indirect effect. Neither CD4 helper nor NTregs express detectable FLT3 at the protein level (data not shown), and sorted NTregs did not proliferate in vitro upon anti-CD3 stimulation in presence of FLT3L (Figure S2). Therefore, the effect of FLT3L on NTreg proliferation is very likely to be indirect.
FLT3L treatment increased both NK and DC numbers in the spleen (Figure 1A,B). FLT3L-induced expansion of NTregs took place even when mice were depleted of NK cells by injection of anti-NK1.1 mAb (data not shown), suggesting that NK cells were not instrumental in the expansion of NTregs.
Given their ability to stimulate T cells, DCs were the most plausible candidates responsible for the expansion of NTregs. We therefore addressed the capacity of DCs to induce NTreg proliferation. Because there is no simple, reliable, in vivo model available for long-term DC depletion, we addressed this hypothesis in vitro.
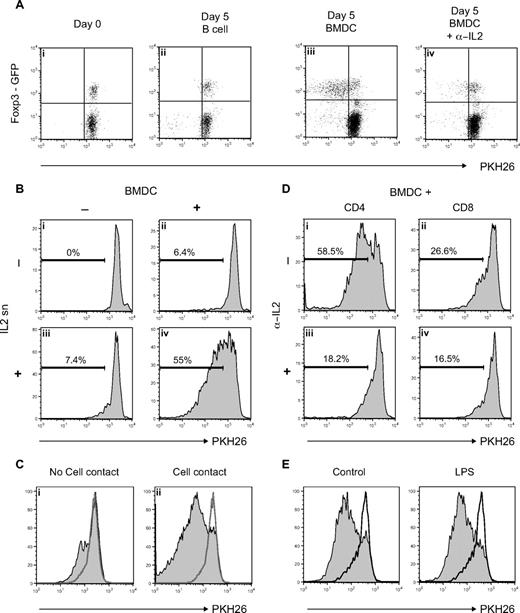
Figure 3A shows the proliferation of NTregs and CD4 helper cells as measured by PKH26 staining. Neither CD4 helper nor NTregs proliferated alone (data not shown) or in the presence of purified syngeneic B cells (Figure 3Aii). In contrast, coculture with syngeneic in vitro FLT3L-induced BMDCs39 led to a preferential proliferation of Foxp3+ cells (Fig 3Aiii). Cell division in these cultures was IL-2–dependent because it could be inhibited by addition of blocking anti–IL-2 antibody (Figure 3Aiv). Proliferation took place in the absence of any extra FLT3L and could not be blocked by addition of blocking anti-FLT3 antibody, again suggesting that there is no direct link between FLT3L and NTreg proliferation (data not shown). Sorted CD4+Foxp3− cells did not convert to a regulatory phenotype when incubated together with BMDCs (data not shown), ruling out any conversion mechanisms, as demonstrated in vivo (Figure 2B).
FLT3L-differentiated BMDCs induce proliferation of Tregs. (A) PKH26-labeled lymph node cell suspension from Foxp3EGFP reporter mice was incubated with sorted syngeneic B-cell or syngeneic in vitro FLT3L-differentiated BMDCs for 5 days with or without antagonistic anti-IL-2. Dot plots show PKH26 level on CD4 T cells. (B) PKH26-labeled CD4+Foxp3+ cells sorted from Foxp3EGFP reporter mice were incubated with or without extra IL-2 and with or without BMDCs. Histograms show PHK26 level on CD4+Foxp3+ cells after 5 days of incubation. (C) PKH26-labeled CD4+Foxp3+ cells sorted from Foxp3EGFP reporter mice were incubated with extra IL-2 only (empty histogram) or with BMDCs without (left panel) or with (right panel) cell contact. Histograms show PHK26 level on CD4+Foxp3+ cells after 5 days of incubation. (D) PKH26-labeled CD4+Foxp3+ cells sorted from Foxp3EGFP reporter mice were incubated with BMDCs and sorted CD4+Foxp3− (CD4:Tregs ratio, 4:1) or CD8+ (CD8:Tregs ratio, 2:1) T cells. Cocultures were done in presence or absence of antagonistic anti–IL-2. Histograms show PHK26 level on CD4+Foxp3+ cells after 5 days of incubation. (E) PKH26-labeled CD4+Foxp3+ cells sorted from Foxp3EGFP reporter mice were incubated with extra IL-2 only (empty histogram) or with control BMDCs (left panel) or LPS-activated BMDCs (right panel). Histograms show PHK26 level on CD4+Foxp3+ cells after 5 days of incubation.
FLT3L-differentiated BMDCs induce proliferation of Tregs. (A) PKH26-labeled lymph node cell suspension from Foxp3EGFP reporter mice was incubated with sorted syngeneic B-cell or syngeneic in vitro FLT3L-differentiated BMDCs for 5 days with or without antagonistic anti-IL-2. Dot plots show PKH26 level on CD4 T cells. (B) PKH26-labeled CD4+Foxp3+ cells sorted from Foxp3EGFP reporter mice were incubated with or without extra IL-2 and with or without BMDCs. Histograms show PHK26 level on CD4+Foxp3+ cells after 5 days of incubation. (C) PKH26-labeled CD4+Foxp3+ cells sorted from Foxp3EGFP reporter mice were incubated with extra IL-2 only (empty histogram) or with BMDCs without (left panel) or with (right panel) cell contact. Histograms show PHK26 level on CD4+Foxp3+ cells after 5 days of incubation. (D) PKH26-labeled CD4+Foxp3+ cells sorted from Foxp3EGFP reporter mice were incubated with BMDCs and sorted CD4+Foxp3− (CD4:Tregs ratio, 4:1) or CD8+ (CD8:Tregs ratio, 2:1) T cells. Cocultures were done in presence or absence of antagonistic anti–IL-2. Histograms show PHK26 level on CD4+Foxp3+ cells after 5 days of incubation. (E) PKH26-labeled CD4+Foxp3+ cells sorted from Foxp3EGFP reporter mice were incubated with extra IL-2 only (empty histogram) or with control BMDCs (left panel) or LPS-activated BMDCs (right panel). Histograms show PHK26 level on CD4+Foxp3+ cells after 5 days of incubation.
Sorted NTregs proliferated only when they were cultured in presence of extra IL-2 and BMDCs (Figure 3Biv), whereas IL-2 (Figure 3Biii) or BMDCs (Figure 3Bii) alone only induced a weak proliferation. These results argue first that neither BMDCs nor NTregs produce IL-2, and second, that NTregs need a DC-mediated additional signal to proliferate in the presence of IL-2. This additional signal required direct cell contact, because Tregs did not divide significantly when they were incubated separately from BMDCs in a transwell culture (Figure 3Ci) compared with cells incubated in direct contact with BMDCs (Figure 3Cii).
We next investigated whether CD4 helper and/or CD8 T cells were providing IL-2 to Tregs. As shown in Figure 3C, sorted Tregs proliferated in the presence of BMDCs plus CD4 helper or CD8 (Figure 3Di,ii), and this proliferation could be inhibited by addition of blocking anti–IL-2 antibody (Figure 3Diii,iv). This demonstrates that, unlike BMDCs (Figure 3B), both CD4 helper and CD8 T cells can provide IL-2 in these settings.
Finally, the ability of DCs to induce NTreg proliferation was independent of the maturation status. As shown in Figure 3E, LPS-activated DCs were as potent as immature DCs in inducing NTreg proliferation. Similarly, granulocyte-macrophage CSF (GM-CSF)–differentiated BMDCs had the same ability to induce NTreg proliferation (Figure S3).
We show in this study that unlike B cells, BMDCs induce NTreg proliferation. Proliferation was IL-2 dependent and required an additional cell contact–dependent signal supplied by DCs.
DC-mediated Treg expansion is TCR independent
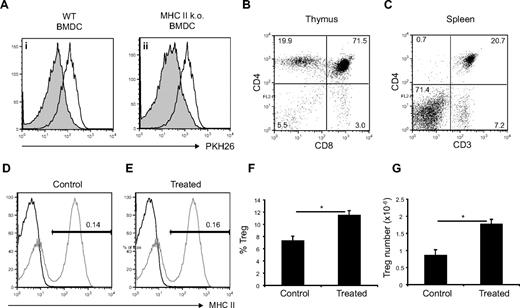
We concluded from the data shown in Figure 3B that IL-2 was necessary but not sufficient to induce sustained NTreg proliferation in vitro, and we postulated that BMDCs were providing a second signal, which was enhancing Treg proliferation. A strong TCR signal together with CD28 costimulation is known to induce NTreg proliferation in vitro in the presence of extra IL-2; hence, we looked to see whether DC-induced Treg proliferation was TCR dependent. As shown in Figure 4A, MHC class II KO BMDCs37 were as potent as WT BMDCs in inducing the proliferation of sorted NTregs when extra IL-2 was provided. This argues that DC-induced NTreg proliferation is independent of TCR-MHC class II interactions. Because sorted NTregs did not proliferate when stimulated with plate-bound anti-CD28 plus IL-2 (data not shown), we conclude that BMDCs were inducing NTreg proliferation by a TCR- and CD28-independent mechanism.
Mechanism of FLT3L-induced Treg proliferation is TCR independent. (A) PKH26-labeled CD4+Foxp3+ cells sorted from Foxp3EGFP reporter mice were incubated with WT BMDCs (filled histogram, left panel), MHC class II KO BMDCs (filled histogram, right panel), or alone (empty histogram) in presence of extra IL-2. Histograms show PHK26 level on CD4+Foxp3+ cells after 5 days of incubation. (B-G) WT mice were lethally irradiated and reconstituted with MHC class II KO bone marrow. (B) CD4 versus CD8 expression in thymus of MHC class II KO → WT chimera. (C) CD4 versus CD3 expression in spleen of MHC class II KO → WT chimera. (D,E) MHC II expression in the spleen of control or FLT3L-treated MHC class II KO → WT chimera (black line) versus WT control (gray line). (F,G) Percentages of Foxp3+ cells among CD4 T cell or absolute number of Foxp3+ cells in spleen of treated versus control MHC class II KO → WT chimera. Each histogram shows mean ± SEM. *P < .05.
Mechanism of FLT3L-induced Treg proliferation is TCR independent. (A) PKH26-labeled CD4+Foxp3+ cells sorted from Foxp3EGFP reporter mice were incubated with WT BMDCs (filled histogram, left panel), MHC class II KO BMDCs (filled histogram, right panel), or alone (empty histogram) in presence of extra IL-2. Histograms show PHK26 level on CD4+Foxp3+ cells after 5 days of incubation. (B-G) WT mice were lethally irradiated and reconstituted with MHC class II KO bone marrow. (B) CD4 versus CD8 expression in thymus of MHC class II KO → WT chimera. (C) CD4 versus CD3 expression in spleen of MHC class II KO → WT chimera. (D,E) MHC II expression in the spleen of control or FLT3L-treated MHC class II KO → WT chimera (black line) versus WT control (gray line). (F,G) Percentages of Foxp3+ cells among CD4 T cell or absolute number of Foxp3+ cells in spleen of treated versus control MHC class II KO → WT chimera. Each histogram shows mean ± SEM. *P < .05.
Next, we wondered whether FLT3L treatment would induce Treg expansion in the absence of TCR-MHC class II interactions in vivo. To test this hypothesis, we lethally irradiated WT mice and reconstituted them with bone marrow from MHC class II KO. In such chimeras, MHC class II molecules are still expressed on radioresistant cells of the thymus, allowing CD4 T cells to develop, but are completely absent on radiosensitive cells. As expected, in these chimeras, CD4SP and mature CD4 T cells could be found in the thymus (Figure 4B) and spleen (Figure 4C). In the spleen, MHC class II expression could not be detected either by FACS (Figure 4D) or by immunohistology (data not shown). As expected, FLT3L treatment led to an increase in the number of dendritic cells and had no influence on the expression of MHC class II in the spleen (Figure 4E). FLT3L induced an increase both in the percentage and absolute number of NTregs (Figure 4F,G).
Taken together, we conclude that MHC class II–TCR interactions are not necessary for DC-induced Treg proliferation both in vitro and in vivo. The nature of the second signal delivered by DCs, which is necessary for enhanced Treg proliferation, is still under investigation.
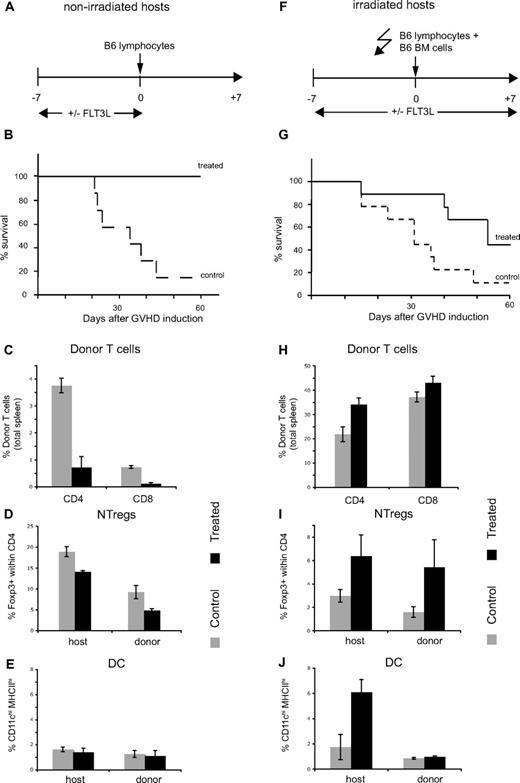
FLT3L treatment prevents mice from dying from acute GVHD
Given its ability to increase the number of DCs and NTregs, we challenged the potential of FLT3L treatment to rescue mice dying from acute GVHD. We investigated the effect of FLT3L pretreatment of nonirradiated and irradiated host (Figure 5A,F). As shown in Figure 5B, FLT3L pretreatment completely prevented death due to acute GVHD caused by injection of C57BL/6 lymphocytes into nonirradiated (C57BL/6 × DBA/2)F1. When treatment was applied after GVHD induction, it partially prevented death of the mice (Figure S4). In the pretreated group, 1 week after GVHD induction, the percentages of allogeneic CD4 and CD8 T cells in the spleen were almost 10 times lower compared with control group (Figure 5C). After treatment arrest, percentages of NTregs or DCs were not significantly increased in treated compared with control mice (Figure 5D,E).
FLT3L treatment prevents mice dying from by acute GVHD. (A-E) (C57BL/6 × DBA/2)F1 mice were treated with FLT3L (20 μg intraperitonal injection every second day from days −7 to 0) or left untreated. At day 0, mice were injected with 50 × 106 pooled lymph node/spleen from C57BL/6 mice. (F-J) (C57BL/6 × DBA/2)F1 mice were treated with FLT3L (20 μg intraperitoneal injection every second day from days −7 to 7) or left untreated. At day 0, mice were irradiated (600 rad) and injected with 50 × 106 pooled lymph node/spleen plus 8 × 106 bone marrow cells from C57BL/6. (B,G) Survival curves of control and treated mice. (C,H) Percentages of C57BL/6 CD4 and CD8 T cells in the spleen of control or treated mice at day 7. (D,I) Percentage of Foxp3+ cells within host or donor CD4 T cells in the spleen of control or treated mice at day 7. (E,J) Percentage of CD11chighMHCIIhigh cells within host or donor cells in the spleen of control or treated mice at day 7.
FLT3L treatment prevents mice dying from by acute GVHD. (A-E) (C57BL/6 × DBA/2)F1 mice were treated with FLT3L (20 μg intraperitonal injection every second day from days −7 to 0) or left untreated. At day 0, mice were injected with 50 × 106 pooled lymph node/spleen from C57BL/6 mice. (F-J) (C57BL/6 × DBA/2)F1 mice were treated with FLT3L (20 μg intraperitoneal injection every second day from days −7 to 7) or left untreated. At day 0, mice were irradiated (600 rad) and injected with 50 × 106 pooled lymph node/spleen plus 8 × 106 bone marrow cells from C57BL/6. (B,G) Survival curves of control and treated mice. (C,H) Percentages of C57BL/6 CD4 and CD8 T cells in the spleen of control or treated mice at day 7. (D,I) Percentage of Foxp3+ cells within host or donor CD4 T cells in the spleen of control or treated mice at day 7. (E,J) Percentage of CD11chighMHCIIhigh cells within host or donor cells in the spleen of control or treated mice at day 7.
We applied a similar protocol to irradiated host; however, we kept treatment for 1 week after conditioning (Figure 5F). We thought that a majority of host-tolerizing cells might undergo apoptosis after irradiation, and therefore, it would be beneficial to prolong FLT3L injections. FLT3L treatment only partially prevented death induced by injection of C57BL/6 lymphocytes into irradiated (C57BL/6 × DBA/2)F1 (Figure 5G) similarly to what has been shown by others.41 At day 7, the majority of T cells were of donor origin irrespective of whether hosts were treated or not. Within the host compartment, both NTregs and DCs were significantly increased in FLT3L-treated mice, whereas in the donor compartment, only NTregs were found in higher number compared with control mice (Figure 5I,J).
In conclusion, depending on whether hosts are irradiated or not, FLT3L treatment completely or partially prevents death due to acute GVHD. The beneficial effect of FLT3L correlates with an increased percentage of Tregs and DCs, but the exact cellular requirements of FLT3L-mediated protection need further investigation.
Discussion
Factors controlling NTreg homeostasis are not yet completely understood; however, it would seem that the size of the NTreg population is predetermined by numbers of survival and/or proliferation signals. Thus, the proportion of NTregs among total CD4 T cells is set early on during ontogeny and kept fairly constant until advanced adulthood.43 This is also true regarding the proportion of NTregs in different mouse strains; the ratio between regulatory and effector cells is varying significantly between strains, but is very constant within each strain.44 Altogether, it strongly suggests that a combination of factors keeps the NTreg compartment to a given size in homeostatic conditions. Moreover, it seems that even in lymphopenic situations like in pTα or IL-7–deficient mice, the size of the NTreg compartment is preserved.45
The unique role of IL-2 in NTreg biology is well described, and there is clearly a direct correlation between cytokine availability and NTreg numbers. In mice, increasing IL-2 availability by injection of highly potent IL-2/anti–IL-2 complex expands NTreg number in the periphery,46,47 whereas neutralizing IL-2 by injection of mAb induces a decrease in NTreg numbers and eventually leads to autoimmune disease due to absence of NTreg-mediated tolerance.48 Because IL-2 production by NTregs is minimal,49-51 they rely for their survival on IL-2 produced by conventional CD4 helper cells.52
The role of CD28 signaling in thymic NTreg development has been dissected31 ; however, its precise role in NTreg homeostasis in periphery is less clear. Although inhibition of CD28 by injection of blocking antibody leads to reduced numbers of peripheral NTregs,32 it is not completely understood to what extent this effect is NTreg intrinsic or due to a decrease in IL-2 production by CD4 effector cells caused by a lack of costimulatory signals. If injection of anti-CD28 superagonist antibody were reported to induce NTreg proliferation in vitro and in vivo,53 it does not mimic the physiologic situation in which CD80 and CD86, the natural ligands of CD28, also bind to the inhibitory molecules CTLA-4. NTregs are also known to proliferate upon antigen encounter in vivo33-36 and in vitro; NTregs can be stimulated with antigen-loaded antigen-presenting cells (APCs).35,54 However, in homeostatic conditions, one can wonder what proportion of Tregs receives such a strong TCR signal.
To date, therefore, the only reported ways of inducing NTreg proliferation have been by TCR stimulation, increased IL-2 availability, or CD28 costimulation. However, in this report, we show that FLT3L treatment also results in an increase in NTreg number, most likely by increasing DC number.
Thus, repetitive FLT3L injections expanded peripheral Tregs probably by favoring interactions between DCs and NTregs. This increase in NTregs was not due to phenotypic conversion from conventional to NTregs (Figure 2B). Numbers of both DCs and NTregs go back to normal shortly after stopping FLT3L treatment (Figure S1). We could show in vitro that DCs control NTreg proliferation by cell-contact mechanisms in a TCR-independent manner, allowing cells to divide in the presence of IL-2. We can postulate that the signals delivered by DCs are either positively influencing the IL-2 signaling pathway and/or giving an independent additional signal. Because neither FLT3L treatment nor NTreg stimulation by BMDCs had an effect on CD25 or IL-2Rβ expression by NTregs, and IL-2 was not a limiting factor in our in vitro experiments, we rather think that DCs provide an additional signal or signals to NTregs. This signal seems to determine very clearly whether or not cells will enter cell cycle in the presence of IL-2, and explains the absence of sustained proliferation of sorted NTregs in the presence of saturating IL-2. These conclusions imply that the expansion we observe in vivo and in vitro is very likely to be polyclonal. It might guarantee polyclonality of the NTreg repertoire in homeostatic conditions and reciprocally to avoid oligoclonality in a situation in which a considerable subpopulation of the NTregs is in constant contact with their cognate antigen.55
We suggest that DCs play a significant role in the control of the NTreg homeostasis in the periphery. This is strengthened by several facts. First, increasing DC numbers by, for example GM-CSF treatment, induces similar increases in NTreg numbers.56 Second, numbers of both DCs and NTregs go back to normal values shortly after DC numbers decrease after stopping FLT3L treatment (Figure S1). Third, in FLT3L KO mice, in which the most striking phenotype is the decrease in DC number (Figure 1B), there is a significant decrease in Treg number and percentage of peripheral CD4 T cells. Finally, the fact that Jh KO mice,57 which have no B cells, have comparable percentages of splenic NTregs compared with WT mice suggests that B cells, which represent the majority of splenic APCs in normal mice, do not play any role in controlling the size of the peripheral NTreg compartment (data not shown). This last point underlines the unique role of DCs in setting the size of peripheral NTreg population. Overall, this might be a mechanism for the immune system to ensure equilibrium between the number of professional APCs and the number of NTregs. Given their key role in T cell priming, it is probably necessary to keep a homeostatic link between DCs and Tregs, as it is the case for CD4 helper cells and NTregs.
The effect of FLT3L treatment on GVHD outcome in nonirradiated mice was dramatic. When FLT3L injections were performed before GVHD initiation, treatment could rescue 100% of mice (Figure 5B). The differences in CD4 and CD8 T-cell number in treated versus control animals 1 week after GVHD induction suggest that allogeneic T cells only expended in control mice (Figure 5C). Therefore, complete survival of treated mice might be due to the absence or significantly lowered alloimmune response against host. In this case, allogeneic response is probably controlled very rapidly in the initiation phase. In some situations of GVHD, recipient NK cells can reject donor cells, a mechanism called “hybrid resistance.” We wondered whether FLT3L treatment was enhancing rejection of allo-T by increasing the number of NK cells, which would explain the decreased number of alloreactive T cells. However, FLT3L treatment was still protective in mice depleted of NK cells, thereby excluding a role for NK cells (data not shown).
In contrast, FLT3L treatment of irradiated hosts only partially rescued mice from acute GVHD (Figure 5G). Because inflammation might be induced by irradiation, the alloimmune response is probably increased in lymphopenic hosts, and the majority of tolerizing cells, either DCs or NTregs, undergo apoptosis upon conditioning, FLT3L-mediated tolerance is certainly more difficult to achieve in irradiated compared with nonirradiated hosts. Moreover, survival of mice relies on a long-term, stable tolerance in contrast to unirradiated recipients.
FLT3L treatment correlates both with increased survival and increased percentages of DCs and NTregs. However, the mechanisms and cellular requirements by which FLT3L treatment improves the outcome of GVHD remain unknown. It was reported that FLT3L-mediated protection was due to increased numbers of immature DCs and that in vitro, compared with DCs from control animals, DCs from FLT3L-treated animals were far less potent in allo-T cell priming.41 Although we agree with these conclusions, we think in addition that it is not the only way by which FLT3L treatment protects from GVHD. Treg transfer can prevent death from GVHD after bone marrow transplantation,58,59 and others have demonstrated the importance of Tregs in allograft acceptance.60 Therefore, we think that FLT3L might prevent death due to GVHD partly by increasing the number of NTregs as well as immature DCs. The contribution of both cell populations in FLT3L-mediated protection needs to be further investigated in vivo.
We conclude that, by increasing both immature DC and Treg numbers, FLT3L treatment might be a valuable way of improving GVHD outcome.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
R.C. thanks Inserm for support. We thank Amgen (Thousand Oaks, CA) for a generous gift of rFLT3L.
This work was supported by grants from the Swiss National Science Foundation and European Community awarded from the EuroThymaide Consortium. A.R. is holder of the chair in immunology endowed by R. Hoffman-La Roche (Basel, Switzerland).
Authorship
Contribution: L.K.S. and N.B. performed experiments and analyzed data; L.K.S. and R.C. wrote the paper; L.K.S., N.B., R.C., and A.R. designed research; and B.M. contributed vital reagent.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Antonius Rolink, Departement Biomedizin Basel, Division of Molecular Immunology, University of Basel, Mattenstrasse 28, 4058 Basel, Switzerland; e-mail: antonius.rolink@unibas.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal