In this issue of Blood, Zhao and colleagues describe a feed back loop between the transcription factor Myb and micro RNA 15a. Myb induces expression of miR-15a which, in turn, fine-tunes the abundance of Myb in human hematopoietic cells.
More than 30 years ago, Myb was first described as an oncogene carried by avian retroviruses (v-Myb). Shortly afterward, inappropriate activation of the cellular proto-oncogene counterpart Myb (= v-Myb myeloblastosis viral oncogene-homolog) by retroviral insertion was demonstrated as a cause for leukemia in mice and chickens.1,2 Since then, it has become more and more evident that aberrant expression of Myb could be implicated in a multitude of human tumors, from colon carcinoma to breast cancer to leukemia.3-5 Deregulation of Myb can result from multiple mechanisms, including genomic rearrangements, gene amplification, inappropriate or overexpression as well as structural changes.1
The physiologic functions of Myb in its genuine role as transcription factor have received a lot of attention. The protein is involved in multiple processes, such as stem cell self-renewal and lineage decisions, and needs to be down-regulated to permit cellular differentiation. Dozens of Myb target genes have been identified, many of which are involved in cell-cycle progression (c-Myc, Cyclins A, B, E) or survival (eg Bcl2). Obviously, Myb is an extremely well-studied protein. No more surprises to be expected? Even an old dog can learn new tricks!
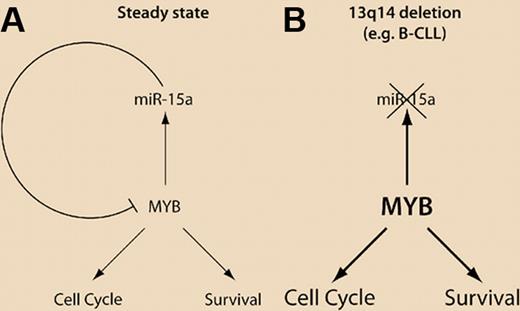
(A) Steady state of the Myb/miR-15a regulatory loop, resulting in feedback inhibition of MYB, preventing excessive cell proliferation and survival. (B) Deletion of 13q14 (comprising the miR-15a gene locus) is frequent in B-CLL. This could result in an increased abundance of Myb, inducing increased cell proliferation and survival.
(A) Steady state of the Myb/miR-15a regulatory loop, resulting in feedback inhibition of MYB, preventing excessive cell proliferation and survival. (B) Deletion of 13q14 (comprising the miR-15a gene locus) is frequent in B-CLL. This could result in an increased abundance of Myb, inducing increased cell proliferation and survival.
In this issue of Blood, Zhao et al demonstrate that Myb is not only a regulator in its own right but is subject itself to a control mechanism receiving lots of attention these days: it is regulated by a micro RNA, specifically miR-15a. This finding is not unexpected; however, Myb and miR-15a are more closely, interconnected than other couples because they feed back on each other. Myb is the very transcription factor that enables expression of its own inhibitor, the miR-15a precursor gene, which, after maturation, interferes with Myb mRNA translation.
The authors identified 2 consensus Myb-binding sites (t/cAACt/gG), conserved between mouse and man, and demonstrated experimentally by chromatin immunoprecipitation that Myb actually binds to these sites in vivo. In an important control experiment, knockdown of Myb by RNA interference resulted in the expected reduction of miR-15a levels. Since these experiments were all done in K562 erythroleukemic cells, representing a rather artificial model system, Zhao et al extended their analysis to primary human cells. First, they determined Myb versus miR-15a levels in cord blood–derived CD34+ hematopoietic progenitors undergoing gradual erythroid maturation in ex vivo cultures. There was a clear inverse correlation in the abundance of the 2 molecules at all time points. To substantiate this link in another experimental setting, either whole human bone marrow mononuclear cells or CD34+ progenitors were transfected with a miR-15a mimic (partially double-stranded RNA). The ensuing colony assays for CFU-GM, BFU-E, and CFU-E suggested that indeed miR-15a interferes with lineage-progression as well as proliferation-promoting functions of Myb.
This novel connection coupling of Myb and miR-15a reported by Zhao et al might have even more interesting pathophysiologic consequences because miR-15a has previously been reported to down-modulate expression of the antiapoptotic protein BCL2,6 which is prominently expressed in many hematopoietic cell types. Myb up-regulates BCL2, again arguing for a balancing act of the Myb/miR-15a couple, in this case affecting a down-stream target.
These considerations can be carried even further. In 68% of all B-cell chronic lymphocytic leukemia (B-CLL) cases, a 30kb chromosomal deletion can be detected (13q14), which encompasses the locus for miR-15a (and miR-16, another modulator of BCL2 expression).7 Thus, even in the absence of any genetic alterations in the Myb gene, loss of the “tumor suppressor” miR-15a may be sufficient to induce leukemia by enabling increased Myb expression and, in turn, compromised lineage progression, enhanced survival as well as increased proliferation. It might become rewarding to assess these and other possibilities in the future.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■