Abstract
Wnt/β-catenin signaling is central to bone development and homeostasis in adulthood and its deregulation is associated with bone pathologies. Dickkopf-1 (DKK1), a soluble inhibitor of Wnt/β-catenin signaling required for embryonic head development, regulates Wnt signaling by binding to the Wnt coreceptor lipoprotein-related protein-5 (LRP5)/Arrow. LRP5 mutations causing high bone mass syndromes disrupt DKK1-mediated regulation of LRP5. Forced overexpression of Dkk1 in osteoblasts causes osteopenia, disruption of the hematopoietic stem cell (HSC) niche, and defects in HSC function. Dkk1 also inhibits fracture repair. Studies suggest that DKK1 activation in osteoblasts is the underlying cause of glucocorticoid- and estrogen deficiency–mediated osteoporosis, and at least partially underlies the teratogenic effects of thalidomide on limb development. DKK1 induces proliferation of mesenchymal stem cells (MSC) in vitro and may play a role in the development of high-grade undifferentiated pleomorphic sarcomas derived from MSC and osteosarcomas. DKK1 has been implicated in causing erosive arthritis, the osteolytic phenotypes of multiple myeloma and metastatic breast cancer, and osteoblastic metastases of prostate cancer. Preclinical studies have shown that neutralizing DKK1/Dkk1 and/or enhancing Wnt/β-catenin signaling may prove effective in treating bone pathologies. Here, we review the rapidly growing body of literature defining a pivotal role for DKK1 in bone health and disease.
Overview of Wnt signaling
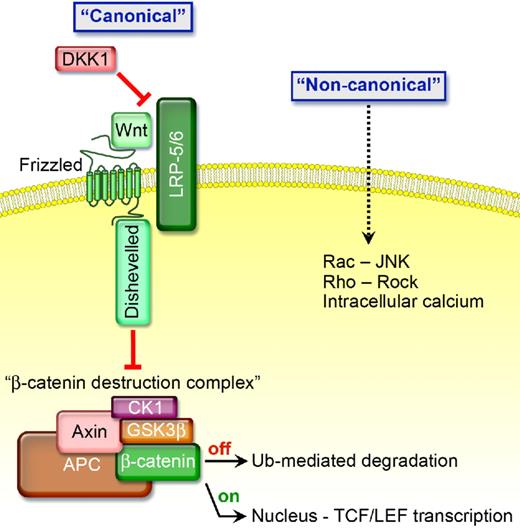
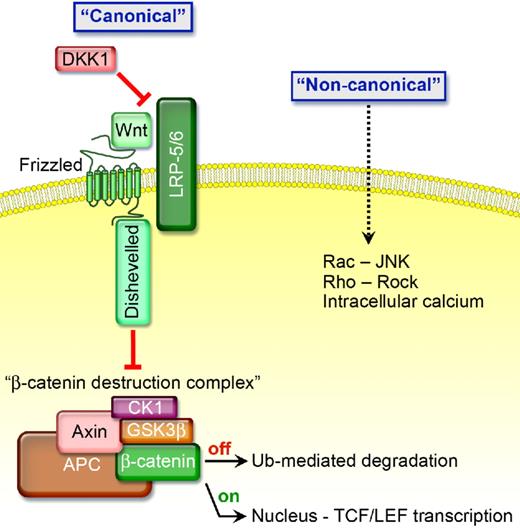
The Wnt family of glycoproteins signals through a “canonical” or “noncanonical” pathway (Figure 1). In the absence of Wnt's, glycogen synthase kinase-β (GSK3β), Axin, adenomatous polyposis coli (APC), and casein kinase Iα (CKIα) form a β-catenin destruction complex.1 CKIα phosphorylates β-catenin at Ser45, and then GSK3β phosphorylates β-catenin at Ser33/Ser37/Thr41. Phosphorylated β-catenin is recognized by β-transducin repeat protein, ubiquitinated, and degraded by proteasomes. In contrast, activation of canonical Wnt signaling occurs when a Wnt protein binds to one of the 10 members of the frizzled (FZD) receptor family; Wnt-FZD then binds low-density lipoprotein-related protein-5 (LRP5) or LRP6. The resultant complex activates Dishevelled (Dvl), a protein that attracts Axin away from the destruction complex and antagonizes the ability of GSK3β to phosphorylate β-catenin, thereby preventing destruction complex formation. If β-catenin is not degraded, it translocates to the nucleus where it binds to the transcription factor T-cell factor-4 (TCF4) and enhances target gene expression.1
The presence of certain Wnt proteins activates “canonical signaling” by binding to FZD and low-density LRP5 and LRP6 coreceptors causing Dvl to attract proteins away from the β-catenin destruction complex thereby preventing β-catenin from being degraded. If not degraded, β-catenin is stabilized and translocates to the nucleus where it binds to the transcription factor TCF4 and enhances target gene expression, including cyclin D1, c-myc, c-jun, vascular endothelial growth factor (VEGF), and several others that are associated with enhanced cell growth. In the presence of DKK1, which competitively binds to LRP5/6 causing it to bind to Kremen and become inactive, GSK3β, Axin, APC, and CKIα form a β-catenin destruction complex. CKIα and GSK3β phosphorylate β-catenin causing it to be recognized by β-transducin repeat protein, ubiquitinated, and degraded by proteasomes, thereby inhibiting “canonical signaling.”
The presence of certain Wnt proteins activates “canonical signaling” by binding to FZD and low-density LRP5 and LRP6 coreceptors causing Dvl to attract proteins away from the β-catenin destruction complex thereby preventing β-catenin from being degraded. If not degraded, β-catenin is stabilized and translocates to the nucleus where it binds to the transcription factor TCF4 and enhances target gene expression, including cyclin D1, c-myc, c-jun, vascular endothelial growth factor (VEGF), and several others that are associated with enhanced cell growth. In the presence of DKK1, which competitively binds to LRP5/6 causing it to bind to Kremen and become inactive, GSK3β, Axin, APC, and CKIα form a β-catenin destruction complex. CKIα and GSK3β phosphorylate β-catenin causing it to be recognized by β-transducin repeat protein, ubiquitinated, and degraded by proteasomes, thereby inhibiting “canonical signaling.”
Canonical Wnt's promote caveolin-dependent LRP internalization and facilitate its interaction with Axin.2 In contrast, DKK1 binds to LRP5/6 causing the receptor to attract a Kremen; this interaction promotes clathrin-mediated internalization, thereby inactivating LRP, though some data suggest that Kremen may not be essential for DKK1-mediated Wnt inhibition.3,4 Moreover, R-Spondins amplify the activity of canonical Wnt's while antagonizing DKK1-mediated interaction with LRP and Kremen.5 Wise and SOST are also secreted Wnt inhibitors that bind to and inactivate LRP.6,7 Wnt inhibitory factor (WIF) proteins, which are structurally similar to the extracellular portion of the Derailed/Ryk class of transmembrane Wnt receptors, and secreted forms of frizzled proteins (sFRP, sizzled, and FrzB) act by directly binding Wnt molecules and can function as Wnt inhibitors, but may also stabilize Wnt's and facilitate Wnt signaling.8,9
DKK1 regulates bone development and accrual and maintenance of bone mass
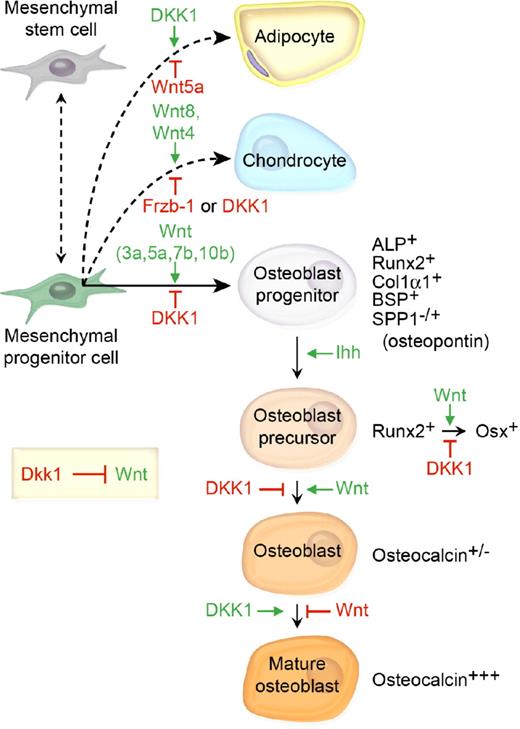
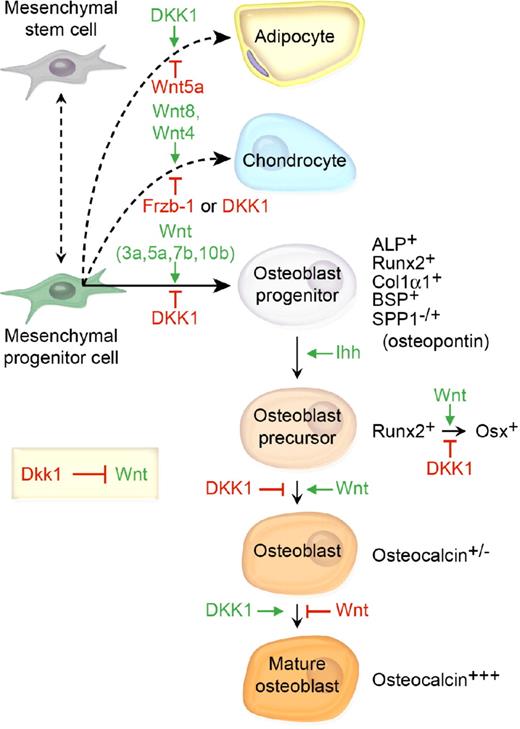
Bone marrow–derived mesenchymal stem cells (MSC) can differentiate into adipocytes, chondrocytes, or osteoblasts. Although the precise orchestration of Wnt signaling during bone development is dependent on complex microenvironmental cues, data from several groups suggest that Wnt3a, Wnt5a, Wnt7b, and Wnt10b are central to osteoblast differentiation (Figure 2).10-14 Increased β-catenin is found in cells committed to the osteoblast lineage, and loss of β-catenin in osteoblast precursor cells results in reduced bone deposition.15 In addition to promoting osteoblast commitment, canonical Wnt signaling inhibits adipocyte differentiation, primarily through accrual of stabilized β-catenin and subsequent transactivation of TCF-responsive genes.16,17 However, noncanonical Wnt signaling through Wnt5a inactivates peroxisome proliferator-activated receptor-γ (PPARγ), a key adipogenic transcription factor and activates Runx2.18
The prevailing model of the role of DKK1 and the mesenchymal cell lineage in bone development and bone mass homeostasis. Wnt 3a, 5a, and 7b and Ihh cooperatively promote osteoblast differentiation, whereas DKK1 inhibits osteoblastogenesis and shifts the developmental program toward adipogenesis. β-catenin/TCF1 promotes early osteoblast lineage commitment through induction of the master bone development protein Runx2.97 Runx2 then promotes transcriptional activation of a second master bone development transcription factor Osterix (Osx), which uses DKK1 to repress Runx2 expression to further promote osteoblast progenitor maturation.98 Finally, phenotypic lineage-specific markers for osteoblast maturation are illustrated.99,100
The prevailing model of the role of DKK1 and the mesenchymal cell lineage in bone development and bone mass homeostasis. Wnt 3a, 5a, and 7b and Ihh cooperatively promote osteoblast differentiation, whereas DKK1 inhibits osteoblastogenesis and shifts the developmental program toward adipogenesis. β-catenin/TCF1 promotes early osteoblast lineage commitment through induction of the master bone development protein Runx2.97 Runx2 then promotes transcriptional activation of a second master bone development transcription factor Osterix (Osx), which uses DKK1 to repress Runx2 expression to further promote osteoblast progenitor maturation.98 Finally, phenotypic lineage-specific markers for osteoblast maturation are illustrated.99,100
DKK1 counteracts the Wnt-mediated effects on bone differentiation and adipogenesis. A transient increase in DKK1 mRNA and protein expression occurred in preadipocytes within 6 hours of initiation of adipogenesis, and was associated with down-regulation of cytoplasmic and nuclear β-catenin.19 Furthermore, constitutive expression of DKK1 in 3T3-L1 preadipocytes promotes adipogenic differentiation.19 Msx2 is a homeodomain transcription factor first identified in osteoblasts. Recently, transgenic overexpression of Msx2 in mice was found to inhibit adipogenesis while enhancing osteogenic differentiation, increasing bone formation and bone volume, and resulting in increased Wnt7a and Wnt7b and decreased DKK1 expression.20 In vitro examination of C3H10T1/2 osteoprogenitor cells revealed that Msx2 inhibited Dkk1 promoter activity and also reduced RNA polymerase association with Dkk1 chromatin.20 These data suggest that DKK1 inhibits osteoblastogenesis and induces adipogenesis, effectively switching the MSC differentiation pathway (Figure 2). Finally, separate Wnt family members, namely Wnt8 and to a lesser degree Wnt4, promote chondrocyte differentiation. Not surprisingly, the frizzled-related protein Frzb/sFRP3, which antagonizes Wnt1 and Wnt8 signaling, has been shown to inhibit chondrocyte differentiation in vivo (Figure 2).21,22 Interestingly, recombinant bone morphogenic protein-2 (Bmp2), implanted into the muscle of mice, up-regulated Wnt ligands and receptors, activated β-catenin–mediated TCF-dependent transcriptional activity, and facilitated endochondral bone formation.23 In that model, Dkk1 inhibited β-catenin signaling as well as chondrogenesis and osteogenesis, thereby preventing endochondral bone formation.23
Dkk1 is known to promote programmed cell death (PCD) in the developing limb and Dkk1 expression is induced by Bmp4 and Bmp5.24-26 Using a chicken development model, we recently reported that thalidomide induces oxidative stress in the developing limb mesenchyme and up-regulates BMP signaling and DKK1 expression; this leads to an inhibition of canonical Wnt signaling and increased PCD.27 Importantly, both thalidomide-induced PCD and limb deformities could be partially inhibited by blocking Dkk1 or activating Wnt signaling downstream of the ligand-receptor interaction.27
Evidence from animal models and human studies supports an anabolic role for Wnt signaling in accrual and maintenance of bone mass, mediated by enhanced osteoblast differentiation/activity with concomitant suppression of osteoclast differentiation/activity.23,28-31 Osteoblasts produce osteoprotegrin (OPG) and receptor activator of NF-κB (RANK) ligand (RANKL). RANKL binds to RANK and enhances osteoclast differentiation/activity. OPG, a soluble decoy receptor for RANKL, competitively inhibits RANKL-RANK interaction; therefore, it is the OPG:RANKL ratio that determines the net effect on osteoclasts.31,32
Dkk1−/− mice die shortly after birth and display severe developmental phenotypes including head defects and limb dysmorphogenesis.25 The “doubleridge mouse” (Dkk1d/d) harbors a transgene inserted 150 kb from the DKK1 gene, which acts cis to reduce Dkk1 expression (ie, is hypomorphic for Dkk1).33 Genetic crosses of Dkk1d/d by Dkk−/+ mice result in incremental decreases in Dkk1 expression (eg, [Dkk1] = Dkk1+/+ > Dkk1+/d > Dkk1d/d > Dkk1d/−) leading to concomitant incremental increases in bone mass.34 Interestingly, Dkk1d/d mice demonstrate postaxial polysyndactyly, and that phenotype is partially or completely rescued by reduced expression of Lrp5 or Lrp6.33 In agreement with DKK1 genetic studies, mice lacking Lrp5 (ie, Lrp5−/−) have lower bone mass compared with wild-type littermates and, in contrast, mice that express a mutant form of Lrp5, which does not bind to Dkk1, display increased bone mass.35,36 Finally, mice that lack expression of both Lrp5 and Lrp6 have more severe bone loss than Lrp5−/−, suggesting that both Wnt coreceptors contribute to bone development and homeostasis.32
Binding of DKK1 to LRP5 is currently the best-characterized mechanism of modulation of Wnt signaling in bone in humans.28 LRP5 gain-of-function mutations which alter the first epidermal growth factor (EGF)–like domain (ie, LRP5 β-propeller 1 region) prevent DKK1-LRP5 interaction and are the cause of high bone mass (HBM) and mandibular, bucca, and lingual exostoses.37,38 LRP5 protein from patients with HBM mutations is not as sensitive to DKK1-mediated inhibition as the wild-type LRP5 protein.38 However, this finding was unexpected because when DKK1 was first identified as an inhibitor of Wnt signaling, it was found to interact strongly with the third and fourth EGF-like domains, not the first and second.39 Wnt ligands interact with the first and second EGF-like domains of LRP6 and by extension LRP5.39 Ai et al recently found that all HBM mutant proteins were indeed less inhibited by DKK1 than wild-type LRP5.40 LRP6 is a close homolog of LRP5 and interacts with DKK1. Engineering LRP6 to harbor a mutation in an equivalent amino acid as a HBM mutation (G158V in LRP5) rendered it unable to interact with DKK1.40 SOST, the gene mutated in the bone dysplasia syndrome, sclerotosis, binds to LRP5 and inhibits its ability to transduce a Wnt signal.6,41 The SOST protein binds to the first propeller domain of LRP5 and HBM mutations inhibit SOST binding to LRP5.42 Investigators recently confirmed that neither DKK1 nor SOST efficiently inhibited HBM-LRP5.43 This study also showed that coexpression of DKK1 and SOST did not inhibit HBM-LRP5 mutants better than either inhibitor by itself, that DKK1 and SOST do not simultaneously bind to wild-type LRP5, and that DKK1 is able to displace SOST from previously formed sclerostin-LRP5 complexes.43 A separate and distinct LRP5 loss-of-function mutation leads to reduced Wnt signaling, and is the genetic basis of the familial syndrome osteoporosis-pseudoglioma (OPPG), a disease characterized by low bone mass resulting in premature osteoporosis, fractures, and bone deformities.28,32 These mutations do not influence DKK1 function. Mice harboring null mutations in either Kremen 1 (Krm1−/−) or Kremen 2 (Krm2−/−) have normal bone formation and bone mass, while double mutants Krm1−/−; Krm2−/− show enhanced Wnt signaling, ectopic postaxial forelimb digits, expanded apical ectodermal ridges, and increased bone formation and volume.44 Triple mutants Krm1−/−; Krm2−/−; Dkk1+/− showed enhanced growth of ectopic digits, suggesting that Dkk1 has at least some Kremen-independent function during limb development.44
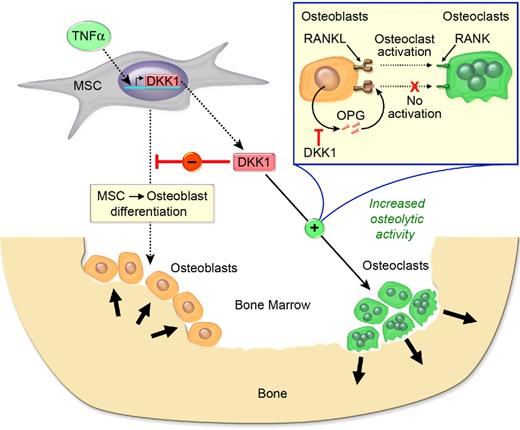
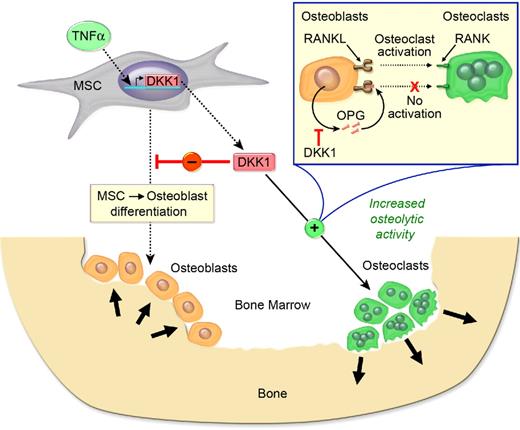
Genetic data regarding the effects of DKK1 on bone mass are supported by experiments where ovariectomized or sham-operated rats were given 20 μg/kg per day Dkk1 sense or antisense oligonucleotide or vehicle for 28 days, and it was noted that Dkk1 antisense oligonucleotide significantly abrogated the suppressive effect of ovariectomy (ie, estrogen deficiency) on femur weight, mineral content, mineral density, and peak load.45 In contrast, dexamethasone, an agent known to induce osteoporosis, up-regulates Dkk1 expression in primary human osteoblasts and may provide a molecular explanation for osteoporosis caused by long-term glucocorticoid use.46 Moreover, knockdown of Dkk1 expression by antisense oligonucleotide (Dkk1-AS) abrogated dexamethasone suppression of alkaline phosphatase activity and osteocalcin expression in MC3T3-E1 preosteoblasts, and mitigated dexamethasone-mediated suppression of mineral density, trabecular bone volume, osteoblast surface, and bone formation rate in bone tissue and ex vivo osteogenesis of primary bone marrow mesenchymal cells.47 The mechanism appears to be that Dkk1-AS abrogates dexamethasone-induced expression of nuclear β-catenin and phosphorylated Akt-Ser473.47 Dkk1 inhibited β-catenin signaling as well as healing of tibia fractures.48 Evidence indicates that DKK1 is also a major determinant of bone and joint pathology in inflammatory arthritis.49 DKK1 protein expression is enhanced in synovial fibroblasts and is increased in the serum of patients with rheumatoid arthritis (RA) compared with healthy controls.49 An anti–tumor necrosis factor-α (TNF-α) antibody reduced serum DKK1 levels in patients with RA. In contrast to RA, where bone erosion occurs, serum DKK1 concentrations in patients with ankylosing spondylitis, a disease characterized by osteophyte formation, were decreased compared with healthy controls.49 Dkk1 was induced in a TNF-α–transgenic mouse model of inflammatory arthritis; anti-Dkk1 antibody prevented bone erosion and promoted osteophyte formation, but did not affect inflammation.49 Finally, the same group showed that the effects of Dkk1 on bone were mediated by inhibition of Wnt signaling, which directly impaired new bone formation and limited OPG expression, thereby shifting the OPG:RANKL ratio to favor bone resorption. These data support the concept that DKK1 inhibits bone formation while promoting bone resorption (Figure 3).
DKK1 is both anabolic and anticatabolic in bone. TNF-α enhances DKK1 secretion which inhibits MSC-derived osteoblastogenesis and lowers OPG levels, resulting in reduced bone accretion. In addition, DKK1 enhances RANKL levels, and the increased RANKL:OPG ratio activates osteoclast activity, leading to bone resorption.
DKK1 is both anabolic and anticatabolic in bone. TNF-α enhances DKK1 secretion which inhibits MSC-derived osteoblastogenesis and lowers OPG levels, resulting in reduced bone accretion. In addition, DKK1 enhances RANKL levels, and the increased RANKL:OPG ratio activates osteoclast activity, leading to bone resorption.
DKK1 in cancer and bone metastases
DKK1 expression in normal tissues and cancer
DKK1 mRNA is expressed at low levels in most normal human tissues with the exception of the placenta.50,51 A wide range of DKK1 gene expression levels has been reported at various phases of tumorigenesis in multiple cancer phenotypes including prostate, breast, colorectal, esophageal, lung, and multiple myeloma (MM).50,51 Prostate cancers usually express lower DKK1 levels compared with normal prostate tissue, and bone metastases of prostate cancer typically have excess new bone formation (osteoblastic metastases).52 Interestingly, the osteolytic human prostate cancer cell line, PC-3, expresses significantly higher levels of DKK1 than its derivative cell line PC-3M.52 However, DKK1 expression was undetectable in the mixed osteoblastic/osteolytic prostate cancer cell lines LNCaP, C4-2, C4-2B, and ACE-1.52,53 DKK1 was found to be highly expressed by MDA-MB-231 (osteolytic model) and MCF7 (mixed osteolytic and osteoblastic model) human breast cancer cell lines, whereas ZR-75-1 and T47D cells (osteoblastic model) had no detectable levels.50,54 Bone marrow aspirates taken from hindlimbs of mice inoculated with human breast cancer cells that induce bone metastases (MDA-BO2, a subclone of MDA-MB-231 cells) had higher DKK1 levels compared with noninoculated control mice.55 In addition, serum DKK1 concentrations from patients with breast cancer and bone metastases were significantly higher compared with women with breast cancer in complete remission, patients with breast cancer metastases at nonbone sites, and healthy women.55 Human primary lung and esophageal cancers, lung cancer cell lines, and esophageal cancer cell lines express DKK1 mRNA.56 In colon cancer and melanoma, DKK1 expression was down-regulated compared with the respective normal tissues.57,58 Qian et al reported that DKK1 protein expression was detected in most MM cell lines and clinical MM samples.51
DKK1 in osteolytic and osteoblastic metastases
As described earlier, activation of canonical Wnt signaling induces osteoblast differentiation and increases survival of pluripotent mesenchymal progenitor cells. Bone is a frequent site of metastases in some cancers, and evidence suggests that Wnt signaling and DKK1 are involved in bone metastases. MM and neuroblastoma metastasize to bone resulting in osteolytic lesions. Prostate cancer bone metastases demonstrate both bone formation and bone resorption, but formation of new woven bone typically predominates. Bone metastases in breast cancer are typically osteolytic, but occasionally osteoblastic lesions occur.
Taken together, seemingly disparate data from several tumor phenotypes suggest that DKK1 promotes osteolytic metastases, and may facilitate the conversion of osteoblastic metastases to an osteolytic phenotype and vice versa. Prostate cancer cells induce osteoblastic metastases through activation of Wnt signaling.52,59 DKK1 was found to block prostate cancer-associated osteoblastic metastases without affecting tumor growth, while inhibition of DKK1 in osteolytic prostate cancer cells altered the nature of bone metastases from osteolytic to osteoblastic.59 Consistent with this notion, DKK1 was found to increase early in prostate cancer and decrease as tumors progress from primary tumor to metastasis.60 The same group demonstrated that breast cancer cell-derived DKK1 blocked Wnt3a-induced OPG expression and differentiation of osteoblast precursor C2C12 cells, and that these effects could be neutralized by anti-DKK1 antibody.61 Endothelin-1 (ET-1) binds to ETA receptors and promotes new bone formation by increasing osteoblast proliferation and maturation. ET-1 levels may be up-regulated in prostate cancer patients with osteoblastic metastases, and ET-1 overexpression in ZR-75-1 breast cancer cells resulted in increased new bone formation at metastatic sites in nude mice.62 Although ET-1 had no effect on the expression of Wnt's in osteoblasts, it suppressed Dkk1 levels, which was associated with an increase in osteoblast number and new bone formation.63 Most recently, neuroblastoma cell lines were also found to secrete DKK1.64 Proliferation of human (h) MSCs collected from pediatric-aged donors increased when cultured in conditioned media from neuroblastoma cells, and these effects were reversed by anti–DKK1-neutralizing antibody.64
However, the most extensive data suggesting that DKK1 promotes osteolytic metastases come from studies of MM-associated bone disease. MM cells secrete several Wnt-signaling inhibitors, such as sFRP-2, sFRP-3, and DKK1, which have been shown to increase bone loss by decreasing osteoblast function and increasing osteoclast maturation.65 We found that DKK1 plays an important role in MM-induced osteolysis by inhibiting osteoblast differentiation.66 In addition, high serum and bone marrow DKK1 concentrations correlate with osteolytic bone lesions, and serum from MM patients can block BMP-2-induced osteoblast differentiation in vitro in a DKK1-dependent manner.66 Surprisingly, increased DKK1 expression was not detected in patients with advanced stages of MM, suggesting a role during early stages of the malignancy when tumor-microenvironment interactions are essential for survival and resistance to chemotherapy. We subsequently analyzed the functional role of activation and DKK1-mediated inhibition of canonical Wnt signaling on BMP-2–induced osteoblast differentiation. We found that recombinant DKK1 (rDKK1) and plasma from MM patients with high levels of DKK1 blocked Wnt3a-induced β-catenin accumulation.67 In addition, DKK1 abrogated BMP-2–mediated osteoblast differentiation.67 Based on these data, we concluded that autocrine Wnt signaling in osteoblasts is necessary to promote BMP-2–mediated differentiation of preosteoblasts, while Wnt signaling alone is not capable of inducing such differentiation. DKK1 inhibits this process and may be a key factor regulating preosteoblast differentiation and MM bone disease.67 These findings led us to examine the effects of DKK1 on Wnt3a-regulated OPG and RANKL expression in osteoblasts. We confirmed that pretreatment with rDKK1 completely abolished Wnt3a-induced OPG mRNA and protein expression by mouse and human osteoblasts, and that coculture of osteoblasts with either a DKK1-expressing MM cell line or primary MM cells diminished OPG expression in the osteoblasts.68 Moreover, bone marrow sera from MM patients suppressed Wnt3a-induced OPG expression and enhanced RANKL expression in osteoblasts in a DKK1-dependent manner.68 Giuliani et al showed that DKK1 and sFRP3 were produced by MM cells, and that MM cells inhibited osteoblast formation and differentiation.69,70 However, though they demonstrated Dkk1-dependent inhibition of canonical Wnt signaling in murine preosteoblasts, they found that DKK1 did not alter Wnt signaling in human preosteoblasts or hMSC, and ascribed the mechanism of osteoblast inhibition to MM-induced blockade of Runt-related transcription factor-2/core-binding factor Runt domain-α subunit-1 (RUNX2/CBFA1) in human bone marrow osteoblast progenitors.69,70
The plasma cell dyscrasia monoclonal gammopathy of undetermined significance (MGUS) is present in 3% of the population older than 50 years of age, and is associated with a 1% per year risk of converting to overt MM.71,72 A key differentiating feature distinguishing MGUS from MM is the presence of focal osteolytic bone lesions in MM. We showed that DKK1 expression by MM tumor cells is significantly linked to the presence or absence of bone lesions in MM, whereas cells from patients with MGUS expressed low levels of DKK1.66 Kaiser et al recently conducted a larger follow-up study where they correlated serum levels of DKK1 with skeletal X-rays in 184 newly diagnosed MM patients and 33 cases of MGUS. These investigators showed that serum DKK1 was elevated in MM as compared with MGUS, MM without lytic lesions had significantly lower DKK1 levels than patients with lytic bone disease, and DKK1 levels also correlated with the number of bone lesions in MM.73
Proposed model of DKK1-mediated promotion of bone metastases
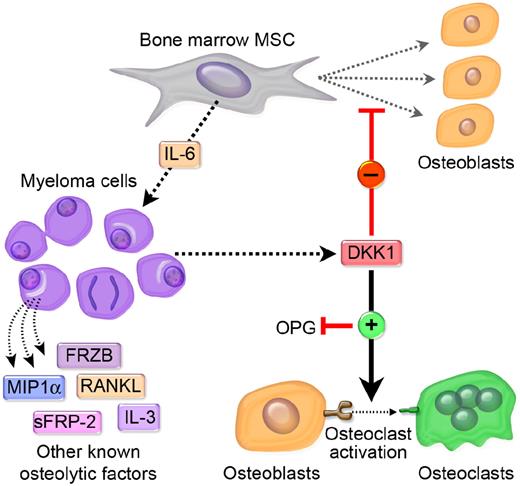
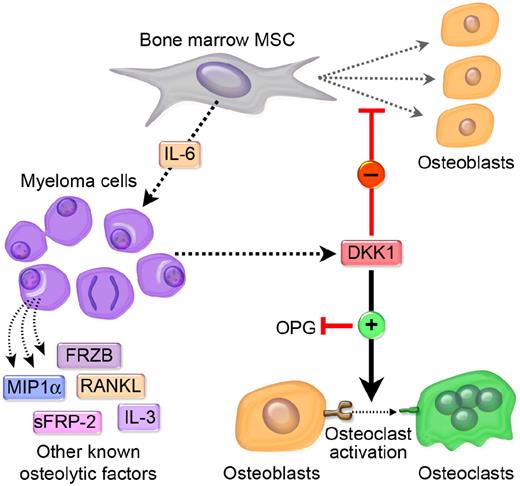
A complex dynamic model is emerging that suggests that DKK1 has differential, context-specific effects on various tumor cell phenotypes as well as on bone cells, often resulting in osteolytic bone metastases. Various factors can regulate DKK1 secretion from cancer cells. Some cancer cells secrete large amounts of DKK1, which may or may not affect tumor cell growth. However, the bulk of the evidence suggests that tumor-associated DKK1 secretion can inhibit Wnt-mediated osteoblast differentiation and OPG secretion, and increase RANKL production. A decrease in the OPG:RANKL ratio serves to enhance osteoclastogenesis and promotes osteoclastic bone resorption. We postulate that osteolysis serves to help create a tumor microenvironment that physically permits tumors to expand, and that undifferentiated osteoblast precursors and/or activated osteoclasts produce factors that promote interaction of tumor cells with the surrounding bone microenvironment, which might enhance proliferation and increase chemotherapy resistance of tumor cells within the lytic lesion. Indeed, in MM DKK1-mediated suppression of MSC differentiation leads to increased production of IL-6 and perhaps other MM growth factors. This would create a vicious cycle of bone destruction and tumor growth. In addition to DKK1, other tumor cell–derived factors such as RANKL, interleukin 3 (IL-3), sFRP-2, macrophage-inflammatory protein 1a (MIP1a), IL-1, IL-6, IL-8, IL-11, TNF-α, prostaglandin E (PGE), and parathyroid hormone-related protein (PTHrP) may also promote an osteolytic environment.74,75 We have outlined a diagrammatic representation of our proposed model as it relates to MM in Figure 4.
Metastatic cancer cells that secrete elevated levels of DKK1 disrupt the balance of osteoblastogenesis and osteoclastogenesis in favor of an osteolytic microenvironment that is conducive to tumor growth. MM has a unique and absolute requirement for the bone marrow microenvironment for its growth and survival and MM plasma cells may cultivate this “soil” by synthesizing and secreting DKK1. The primary effect of DKK1 appears to be the disruption of the differention of MSC in the osteoblasts (OB). RANK signaling regulates osteoclast development and Wnt signaling in MSC/OB differentially regulates RANKL and OPG, a RANKL decoy, which together modulate osteoclast development. DKK1 suppression of Wnt in MSC/OB leads to increased production of RANKL and IL-6 and decreased production of OPG. The shift in RANKL:OPG ratios leads to increased osteoclastogenesis and IL-6 is a potent survival factor of MM cells. The subsequent loss of osteoblasts and increase in osteoclasts causes lytic bone destruction, hypercalcemia, and loss of normal bone marrow function. A vicious cycle of bone destruction and tumor growth ensues. The absence of DKK1 in a subset of MM suggests that other soluble factors produced by MM cells may contribute to this process. These include MIP1a, sFRP-2, IL-3, RANKL, and possibly sFRP-3 (see “Proposed model of DKK1-mediated promotion of bone metastases” for more details).
Metastatic cancer cells that secrete elevated levels of DKK1 disrupt the balance of osteoblastogenesis and osteoclastogenesis in favor of an osteolytic microenvironment that is conducive to tumor growth. MM has a unique and absolute requirement for the bone marrow microenvironment for its growth and survival and MM plasma cells may cultivate this “soil” by synthesizing and secreting DKK1. The primary effect of DKK1 appears to be the disruption of the differention of MSC in the osteoblasts (OB). RANK signaling regulates osteoclast development and Wnt signaling in MSC/OB differentially regulates RANKL and OPG, a RANKL decoy, which together modulate osteoclast development. DKK1 suppression of Wnt in MSC/OB leads to increased production of RANKL and IL-6 and decreased production of OPG. The shift in RANKL:OPG ratios leads to increased osteoclastogenesis and IL-6 is a potent survival factor of MM cells. The subsequent loss of osteoblasts and increase in osteoclasts causes lytic bone destruction, hypercalcemia, and loss of normal bone marrow function. A vicious cycle of bone destruction and tumor growth ensues. The absence of DKK1 in a subset of MM suggests that other soluble factors produced by MM cells may contribute to this process. These include MIP1a, sFRP-2, IL-3, RANKL, and possibly sFRP-3 (see “Proposed model of DKK1-mediated promotion of bone metastases” for more details).
Dkk1 in the regulation of the hematopoietic stem cell (HSC) Niche and HSC function
Osteoblasts form the bone marrow hematopoietic stem cell niche.76 We originally postulated that increased expression of DKK1 in MM bone marrow, and subsequent defects in osteoblast differentiation, might lead to the immunosuppression and anemia that frequently accompany this disease, via defects in HSCs. In support of a role for Dkk1 in causing functional defects in HSC, Fleming and colleagues recently explored the impact of Wnt signaling within the context of the HSC niche.77 Using an osteoblast-specific promoter to drive the expression of DKK1, they noted changes in trabecular bone and in HSC function in transgenic mice. Wnt signaling was inhibited in HSCs, and the cells exhibited reduced p21Cip1 expression, increased cell cycling, reduced numbers of quiescent cells, and a decline in regenerative function after transplantation. This effect was microenvironment-determined and, importantly, was irreversible if the cells were transferred to a normal host. The authors suggest that Wnt-pathway activation in the HSC niche is required to preserve the reconstituting function of endogenous HSC.77 In agreement with these biological observations, MSCs secrete DKK1 that serves to promote their proliferation and block their differentiation in an autocrine manner.78 As mentioned above, high-grade undifferentiated pleomorphic sarcoma was shown to be derived from MSCs, and DKK1 was shown to play a role in this process.79 Finally, DKK1 serum levels in pediatric patients with osteosarcoma (OS) were significantly elevated, and DKK1 was maximally expressed by the OS cells at the tumor periphery, suggesting that DKK1 contributes to tumor expansion by inhibiting repair of surrounding bone.80 In vitro, DKK1 and RANKL are coexpressed by rapidly proliferating human OS cells.80 Both DKK1 and conditioned media from OS cells reduced osteogenesis by human MSCs, and immunodepletion of DKK1 or adding a GSK3β inhibitor reduced the DKK1 effects.80
Given that autologous HSC transplantation plays such a pivotal role in the management of MM, it is possible that the irreversible loss of reconstitution capabilities of HSC exposed to high levels of DKK1 may account for the poor engraftment and reconstitution of hematopoiesis that can occur. Given that HSCs isolated from DKK1-transgenic mice had fewer quiescent HSCs compared with normal controls, it will be interesting to investigate whether HSC mobilized from patients with MM also exhibit shifts in the ratios of p21Cip1-expressing and quiescent HSC that is correlated with DKK1 levels. Along these lines, it is noteworthy that several groups have reported that use of the immunomodulatory drugs thalidomide and lenalidomide, but not bortezomib, during induction chemotherapy is associated with a significant reduction in cell yield during hematopoietic stem cell mobilization in patients with MM.81 We have previously shown that while bortezomib does not activate DKK1, thalidomide, and to an even greater extent, lenalidomide, potently up-regulates DKK1 synthesis by MM plasma cells after short-term therapeutic doses.82,83 Collectively, these data suggest that the negative effects of thalidomide and lenalidomide on HSC mobilization might be traced to supraphysiological levels of DKK1. If this hypothesis is confirmed experimentally, new therapeutic strategies to neutralize DKK1 might prevent the negative effects of these drugs on hematopoietic stem cell biology.
Potential therapeutic advances that modulate DKK1 effects
Amelioration of MM in an animal model using neutralizing antibodies against DKK1 represents a key advance in the area of modulating DKK1 for therapeutic benefit. We engrafted patient-derived MM cells expressing varying levels of DKK1 into rabbit bones which were implanted into severe combined immunodeficiency (SCID) mice (ie, SCID-rab mice); the mice were then treated with control or DKK1-neutralizing antibodies for 4 to 6 weeks.84 The MM-containing implanted bones in mice treated with DKK1-neutralizing antibodies demonstrated a higher bone mass, increased numbers of osteocalcin-expressing osteoblasts, and a reduced number of multinucleated tartrate-resistant acid phosphatase (TRAP)–expressing osteoclasts, and the anabolic effects on bone were associated with reduced MM burden.84 Importantly, it was observed that bone mineral density (BMD) also rose in noninvolved implanted bones as well as the femurs of the mice treated with the antibody.84 Recently, we have been able to show that delivery of Wnt3a to bone in the SCID-hu model of human MM can ameliorate bone destruction and inhibit tumor growth.85 Similar results were also observed in the 5TGM mouse model of MM in mice treated with LiCl, a suppressor of GSK3β and activator of Wnt signaling.86 These antitumor effects of Wnt activation are noteworthy given the potential oncogenic effects of Wnt3a and hyperactivation of canonical Wnt signaling. It is also noteworthy that although capable of inducing stabilization of β-catenin in MM cells in vitro, Wnt3a had no obvious proliferative or growth-inhibitory effects in vitro or in vivo when grown subcutaneously. Together these data suggest that Wnt activation in the bone can have bone anabolic effects, which in turn have an antitumor effect in MM.86,87
Several studies have recently reported that canonical Wnt signaling regulates OPG and RANKL expression in osteoblasts, suggesting that Wnt signaling in osteoblasts regulates osteoclastogenesis and, therefore, the coupling of bone accretion to bone resorption (ie, bone turnover).30,88,89 We recently reported that MM cell–derived DKK1 interrupts Wnt3a-regulated OPG and RANKL expression in osteoblasts.68 We found that pretreatment of these cells with rDKK1 completely abolished Wnt3a-induced OPG mRNA and protein in mouse and human osteoblasts, and Wnt-3a-induced OPG expression is diminished in osteoblasts cocultured with a DKK1-expressing MM cell line or primary MM cells.68 Importantly, we showed that bone marrow serum from MM patients significantly suppressed Wnt3a-induced OPG and enhanced RANKL expression in osteoblasts, in a DKK1-dependent manner.68
Gunn and colleagues have shown that conditioned media from MSC cultures can induce MM cell lines to produce DKK1, suggesting that an MSC-derived soluble factor induces DKK1 in MM cells.90 IL-6 is a growth and survival factor for MM cells.91 MSCs that produce high levels of DKK1 also produce high levels of IL-6, and growth of IL-6–dependent MM cell lines in MSC-conditioned media is inhibited when a neutralizing antibody to IL-6 is added to the culture.90 Fulciniti et al recently evaluated DKK1 as a therapeutic target in MM using a DKK1-neutralizing antibody and found that it increased differentiation of MSC to osteoblasts and reduced IL-6 levels.92 Osteoblast differentiation of MSCs cultured in the presence of the DKK1-producing and IL-6–dependent cell line INA-6 was suppressed, and the anti-DKK1 antibody was able to restore osteoblast activity in this coculture system in a dose-dependent manner. These investigators observed no direct effect of the anti-DKK1 antibody on growth or survival of human MM cell lines; however, the antibody induced growth inhibition of MM cells cultured with bone marrow stromal cells and this was associated with down-regulation of IL-6 produced by MSCs. Growth of INA-6 MM cells in the SCID-hu murine model revealed direct correlation between the level of soluble human DKK1 in murine blood and tumor growth. Anti-DKK1 treatment was associated with increased trabecular bone, number of osteoblasts and osteocalcin within 1 mo, and the anti-DKK1 antibody suppressed MM growth after 4 weeks. Taken together, these results suggest that DKK1 may be a master regulator of MM-associated osteolytic bone disease and disease progression by directly interrupting Wnt-regulated differentiation of osteoblasts, which in turn leads to increased production of IL-6, which then leads to increased osteoclastogenesis by increasing RANKL to OPG ratios.
Finally, the proteasome inhibitor bortezomib (Velcade) was recently approved for the treatment of relapsed refractory MM.93 Although the mechanism of the anti-MM effect of this drug is not known, recent studies suggest that it may act, at least in part, by inducing osteoblast differentiation. Garrett et al were the first to show that proteasome inhibition induces osteoblast differentiation.94 Zangari and colleagues subsequently reported that response to bortezomib could be linked to increases in bone alkaline-phosphatase expression in patients with MM.95 Consistent with these studies, Oyajobi et al showed that bortezomib stimulates new bone formation and that was related to DKK1 down-regulation.96
Conclusions
DKK1 is central to embryonic and adult bone development and bone health and disease, and has emerged as a central molecular player among various factors that promote bone metastases. DKK1 ensures proper skeletal development and homeostasis, LRP5-mediated DKK1 signaling is essential to maintain bone mass, and DKK1 has been implicated in genetic bone metabolism syndromes that result from LRP5 mutations. Preclinical data suggest that DKK1 is involved in glucocorticoid- and estrogen deficiency–mediated osteoporosis, and at least partially underlies the teratogenic effects of thalidomide. In addition, DKK1 may contribute to erosive arthritis. The timing of DKK1 expression and its role in tumorigenesis and metastasis appears to be dependent on the specific tumor phenotype and the type of bone metastasis. Evidence suggests that DKK1 promotes osteolytic metastases, such as in MM, and likely has a modulatory role in the development of osteoblastic metastases as well. Finally, animal models have been helpful in preclinical evaluation of neutralizing DKK1 antibodies as potential therapy for MM-associated bone disease. Given the central role of autologous stem cell transplantation in the clinical management of MM and other diseases, we also eagerly await further studies addressing the effects of DKK1 on HSC biology. Over the next few years, additional studies on DKK1 will elucidate more knowledge on bone biology, bone health and disease, and the pathogenesis of bone metastases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by The National Cancer Institute (CA101875 [J.J.P.] and CA55819 [J.D.S.]), The Lebow Fund to Cure Myeloma (J.D.S.), The Nancy and Stephen Grand Philanthropic Fund (J.D.S.), and by a Senior and Translational Research Award from the Multiple Myeloma Research Foundation (Y.-W.Q.).
National Institutes of Health
Authorship
Contribution: J.J.P., B.M.H., N.K.T., T.J.R., M.V., Y.-W.Q., and J.D.S. reviewed literature and each wrote portions of the paper; B.M.H. had overall editorial responsibility for the figures; and J.J.P., T.J.R., and J.D.S had overall editorial responsibility for the manuscript.
Conflict-of-interest disclosure: J.D.S. and the University of Arkansas Medical Center have a patent and patents pending on the use of DKK1 as a therapeutic target and diagnostic. All other authors declare no competing financial interests.
Correspondence: John D. Shaughnessy Jr, Myeloma Institute for Research and Therapy, University of Arkansas Medical Center, 4301 West Markham Street, Little Rock, AR 72205; e-mail: shaughnessyjohn@uams.edu; or Joseph J. Pinzone, The Ohio State University Medical Center, 1581 Dodd Drive, Columbus, OH; e-mail: joseph.pinzone@osumc.edu.