Abstract
Dexamethasone (DM) is a synthetic member of the glucocorticoid (GC) class of hormones that possesses anti-inflammatory and immunosuppressant activity and is commonly used to treat chronic inflammatory disorders, severe allergies, and other disease states. Although GCs are known to mediate well-defined transcriptional effects via GC receptors (GCR), there is increasing evidence that GCs also initiate rapid nongenomic signaling events in a variety of cell types. Here, we report that DM induces the phosphorylation of Lck and the activation of other downstream mediators, including p59Fyn, Zap70, Rac1, and Vav in resting but not activated human T cells. DM treatment also augments CXCL12-mediated signaling in resting T cells through its cell surface receptor, CXCR4 resulting in the enhanced actin polymerization, Rac activation, and cell migration on ligand exposure. Lck was found to be a critical intermediate in these DM-induced signaling activities. Moreover, DM-mediated Lck phosphorylation in T cells was dependent on the presence of both the GCR and the CD45 molecule. Overall, these results elucidate additional nongenomic effects of DM and the GCR on resting human T cells, inducing Lck and downstream kinase activation and augmenting chemokine signaling and function.
Introduction
Glucocorticoids (GCs) are used to treat diseases with an inflammatory or immune-mediated component, including autoimmune diseases, graft rejection, and leukemia. GCs act via the T-cell intracellular GC receptor (GCR) and may negatively regulate the expression of numerous genes associated with proinflammatory cytokine signaling.1,2 This inhibition of gene transcription appears to result from the ability of the GC/GCR complex to interfere with the activity of numerous transcription factors, either by binding to negative regulatory elements in the promoter region or through protein/protein interactions, impeding the ability of these factors to positively direct gene transcription.3 In addition to these genomic effects, several studies have also described nongenomic, rapid effects of GCs on immune cells.4–7 Dexamethasone (DM) can attenuate the early events of the T-cell receptor (TCR)–induced signaling cascade, including the activation of Src kinases via the GCR. GCR-deficient Jurkat cells and human T cells treated with the GCR blocker, Ru486, during cell activation failed to demonstrate any inhibition in kinase activation in response to DM. Interestingly, many of the inhibitory effects of GC have been observed in activated human or rodent T cells and immune cell subpopulations; however, the effects of DM on resting T cells are unclear.
CXCR4, a chemokine receptor specific for the chemokine ligand, CXCL12, is expressed on leukocytes and is involved in the recirculation of naive lymphocytes into lymphoid tissue.8 This receptor also plays a role in the retention of stem cells, differentiating B cells and neutrophils within bone marrow9 and controls B-cell positioning within lymph nodes, where its expression is regulated by interleukin-4.10 CXCR4 has been found to play a critical role in thymocyte chemotaxis and apoptosis11 as well as thymic development.12 CXCL12 was found to counteract the effects of DM on the apoptosis of CD4+CD8+ T cells. Interestingly, several reports have also demonstrated that exposure of T-cell lines to GC can up-regulate cell surface CXCR4 expression.13,14 Signals delivered through CXCR4-CXCL12 interactions result in potent chemotactic and pro-adhesive signals facilitating T- and B-lymphocyte migration.15–17 Several reports have suggested that the activation of the Src kinase, Lck, on treatment of T cells with CXCL12 may be involved in orchestrating the downstream signals necessary for chemotaxis.18,19 CXCR4 physically associates with the TCR on CXCL12 ligation and uses the ZAP70-binding immunoreceptor tyrosine-activation motif (ITAM) domains of the TCR for signal transduction.20 This association may actually account for several activities attributed to CXCR4 activation, including ERK activation, intracellular calcium mobilization, enhanced activator protein-1 activity, and cytokine production. These findings suggest a signaling linkage between the TCR and CXCR4 signaling pathways.
Here, we demonstrate that the treatment of resting T cells with the GCR agonist, DM, significantly enhances CXCR4-mediated signaling and function, possibly via the activation of Lck and several downstream kinases. The treatment of resting but not activated T cells with either CXCL12 or DM resulted in the activation of these kinases in a synergistic manner. Interestingly, the DM effects were found to actually require the presence of Lck, CD45, and the GCR. The relevance of these data to TCR and CXCR4 signaling will be discussed.
Methods
Reagents and supplies
CXCL12 was purchased from PeproTech (Rocky Hill, NJ). Polyclonal and monoclonal antibodies against Lck and Protein A/G PLUS agarose beads, monoclonal antiphosphotyrosine antibody (4G20) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody for antihuman CD3 and CD28 was purchased from BD Biosciences PharMingen (San Jose, CA). The antibodies for Fyn, Zap70, CD48, CD45, CXCR4, Csk, CD4, Vav1, and Vav2 were purchased from Upstate Biotechnology (Charlottesville, VA) or Abcam (Cambridge, United Kingdom). The activated Rac1 kits were purchased from Chemicon International (Temecula, CA). Monoclonal anti-CXCR4 antibody for fluorescence-activated cell sorter (FACS) was purchased from BD Biosciences PharMingen. DM (98% pure) and the blockers damnacanthal, lavendustin-A, genistein, staurosporine, RU486, and wortmannin were purchased from Sigma-Aldrich (St Louis, MO). The biochemical assay kit for CD45 was purchased from BIOMOL Research Laboratories (Plymouth Meeting, PA). A non–cell-permeable CD45-specific inhibitor, RK-682, was purchased from Calbiochem (San Diego, CA). Cholera toxin and phalloidin labeled with Alexa Fluor 488 were purchased from Invitrogen (Carlsbad, CA). Custom-made gel and other chemicals for Western blot were also purchased from Invitrogen.
T-cell lines and cultures
The human T-cell line, Jurkat cells (JE6.1) and the Jurkat cell lines deficient for Lck (JCaM 1.6), CD45 (J45.01), and the TCR-β chain (JRT 3.1) were obtained from ATCC (Manassas, VA). These cell lines were maintained according to ATCC instructions. Cells were harvested at 90% confluent stage with more than or equal to 99% viability. For primary T cells, leukapheresis packs were acquired from healthy human volunteers who provided informed consent in accordance with the Declaration of Helsinki and with approval from the National Institute on Aging Pheresis Unit (Institutional Review Board–approved protocol MRI2003-054). PBMCs and primary T cells (typically > 95%) were subsequently isolated as previously described.21–23 The culture conditions are described in each figure legend.
Flow cytometric analysis
FACS analysis was used to analyze the status of CXCR4 or CD4 or actin polymerization in different cell lines and primary T cells as previously described.22,23 For assessment of actin polymerization, phalloidin labeled with Alexa Fluor 488 was used. In parallel, the cells were treated with isotype immunoglobulin as a control. The samples were examined using a flow cytometer (FACScan; BD Biosciences, San Jose, CA).
Chemotaxis and intracellular calcium assays
Measurement of chemotaxis and calcium mobilization in response to DM or CXCL12 stimulation were also performed as previously described22,23 and discussed in the figure legends. In the chemotaxis studies, the data are expressed as the percent migration index.22,23 For intracellular calcium measurement, the data are presented as the relative ratio of fluorescence excited at 340 nm and 380 nm.
Statistical analysis
All the data were always repeated at least 3 times using duplicate or triplicate determination throughout the work. The Student t test was used for statistical analysis, and P below the .05 level was considered as significant wherever applicable.
Results
DM induces Lck phosphorylation in resting but not activated human T cells in a GCR-dependent manner
Previous studies have demonstrated that DM can inhibit the phosphorylation status of Lck and other Src kinases during TCR ligation, which influences the downstream events of T-cell activation.7 Similarly, here, we have found that the addition of DM to human T cells (Figure 1A top panel) and Jurkat T-cell cultures (Figure 1A bottom panel) during anti-CD3 and anti-CD28 mAb activation resulted in a significant reduction of Lck phosphorylation (range, 0.5- to 3-fold). In contrast, we found that DM treatment of human resting T cells (Figure 1A top panel) and Jurkat T cells (Figure 1A bottom panel) resulted in a significant induction (>2.5-fold) in the level of phosphorylated Lck. This result was surprising considering the established role of DM as an anti-inflammatory mediator and inhibitor of T-cell activation. Interestingly, treatment of resting T cells with the chemokine CXCL12 also demonstrated a dramatic increase in Lck phosphorylation (>2.5-fold), whereas no significant change in the Lck phosphorylation status was observed in the activated T-cell cultures. Moreover, a prominent additive effect on the Lck phosphorylation was observed in the resting T-cell populations on addition of both DM and CXCL12, whereas addition of CXCL12 with DM to the activated cell cultures failed to demonstrate any significantly greater inhibition in Lck phosphorylation compared with DM treatment alone (Figure 1A). These results suggest that DM exerts a differential effect on Lck phosphorylation depending on the activation state of the T cell and may provide an activation signal to resting T cells. Kinetic analysis of DM-mediated Lck phosphorylation (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) demonstrated that exposure of these cells to DM between 5 and 15 minutes resulted in the optimal phosphorylation of Lck followed by a significant decline in the magnitude with prolonged exposure.
DM induces Lck phosphorylation in resting T cells but not activated T cells in a GCR-dependent fashion. (A) Resting human T cells (top panel) and Jurkat cells [E6.1] (bottom panel) were treated with CXCL12 (100 ng/mL), DM (1 μM), or the combination for 5 minutes. In the activated T-cell cultures, primary human T cells were incubated with 5 μg/mL antihuman CD3 mAb in combination with 5 μg/mL antihuman CD28 antibody for 1 minute at a cell concentration of 5 × 106 cells/mL followed by treatment with CXCL12 and DM alone or with combination for 5 minutes. Treated cells were subsequently washed with ice-cold phosphate-buffered saline and then lysed with ice-cold radioimmunoassay (RIPA) buffer. Total Lck was immunoprecipitated from the lysates and phospho- and total Lck levels were assessed by immunoblotting using the phosphotyrosine and total Lck antibodies. In many of the gels, densitometry was performed on scanned gel images and assessed by UN-SCAN-IT gel digitizing software (Silk Scientific, Orem, UT). The data were then normalized and presented as fold change over control bands. Here, phospho-Lck was normalized against the corresponding band of total Lck. The final data are presented as the fold change over the control (P/T). (B) Primary human T cells were incubated in the presence or absence of 5 μM mifepristone (RU486) for 1 hour. The cells were then treated with CXCL12 (100 ng/mL) or DM (1 μM) or together for 5 minutes. Lysis of the cell and rest of the procedure for Lck immunoblot was followed as described in Figure 1A.
DM induces Lck phosphorylation in resting T cells but not activated T cells in a GCR-dependent fashion. (A) Resting human T cells (top panel) and Jurkat cells [E6.1] (bottom panel) were treated with CXCL12 (100 ng/mL), DM (1 μM), or the combination for 5 minutes. In the activated T-cell cultures, primary human T cells were incubated with 5 μg/mL antihuman CD3 mAb in combination with 5 μg/mL antihuman CD28 antibody for 1 minute at a cell concentration of 5 × 106 cells/mL followed by treatment with CXCL12 and DM alone or with combination for 5 minutes. Treated cells were subsequently washed with ice-cold phosphate-buffered saline and then lysed with ice-cold radioimmunoassay (RIPA) buffer. Total Lck was immunoprecipitated from the lysates and phospho- and total Lck levels were assessed by immunoblotting using the phosphotyrosine and total Lck antibodies. In many of the gels, densitometry was performed on scanned gel images and assessed by UN-SCAN-IT gel digitizing software (Silk Scientific, Orem, UT). The data were then normalized and presented as fold change over control bands. Here, phospho-Lck was normalized against the corresponding band of total Lck. The final data are presented as the fold change over the control (P/T). (B) Primary human T cells were incubated in the presence or absence of 5 μM mifepristone (RU486) for 1 hour. The cells were then treated with CXCL12 (100 ng/mL) or DM (1 μM) or together for 5 minutes. Lysis of the cell and rest of the procedure for Lck immunoblot was followed as described in Figure 1A.
Lck is an Src-family tyrosine kinase expressed in T lymphocytes, where it participates in the cellular immune response. Lck associates through its distinctive amino-terminal segment with the cytoplasmic tails of the CD4 and CD8 molecules. Activated Lck phosphorylates TCR-ϵ chains, which then recruit the ZAP70 kinase to promote T-cell activation. Lck is activated by autophosphorylation at Tyr394, and it is inactive when Tyr505 near the carboxy terminus is phosphorylated. Here, we have observed that, in resting T cells, tyrosine phosphorylation of Lck at Y394 is increased in the CXCL12 and/or DM-treated samples, with much lower levels of phospho-Y505 being observed in the same treated groups (Figure S1B). However, in the activated cultures the opposite results were observed with a dramatic reduction in Y394 phosphorylation in the DM, CXCL12, or combined groups compared with vehicle-treated control cells. Moreover, there was a greater induction of Y505 phosphorylation in the activated T cells treated with DM. In addition, Lck also undergoes phosphorylation on amino-terminal serine residues in response to T-cell stimulation. The phosphorylation on serine residues is accompanied by a decrease in the electrophoretic mobility of Lck on sodium dodecyl sulfate–polyacrylamide gels. The phosphorylation of serine 59 in Lck has been reported to inhibit Lck activity. In Figure S1B, we observed a decrease in serine phosphorylation of Lck in DM-treated resting but not activated T cells, which supports the Tyr394 Lck activation data.
In further support of CXCL12 and DM activation of the Lck signaling pathway, resting T cells treated with CXCL12 and/or DM demonstrated that a significant induction of Lck kinase activity was observed in response to both DM and CXCL12 with an even more dramatic induction (∼5-fold) when these treatments were combined (Figure S2A). These data are in agreement with the increased dissociation of Lck from immunoprecipitated CD4 molecules in resting human T cells and Jurkat T cells on treatment with DM and/or CXCL12 (Figure S2B). These data support our hypothesis that DM and CXCL12 provide an Lck activation signal in resting but not activated T cells, facilitating its kinase activity and downstream functions.
To determine whether DM-mediated Lck phosphorylation is dependent on the presence of GCR, resting human T cells (Figure 1B) were preincubated with mifepristone, a specific blocker of the GCR, followed by the treatment with DM or CXCL12 alone or in combination. The data revealed that the mifepristone-treated T cells demonstrated a clear repression in phosphorylation status of Lck in response to DM and/or CXCL12 compared with vehicle control-treated T cells. A partial inhibition in Lck phosphorylation was observed for the CXCL12-treated cell. These data clearly support a role for the GCR in the observed DM-mediated effects on resting T cells, including Lck phosphorylation.
DM potentiates the T-cell migration in response to CXCL12 in dose- and time-dependent manner
As the requirement of Lck phosphorylation has been shown to be necessary for CXCL12-mediated chemotaxis in T cell19 and DM appears to augment the CXCL12-induced Lck phosphorylation in resting T cells (Figure 1A), we have further examined its effects here on the CXCL12-mediated chemotaxis. DM-treated resting human T cells and Jurkat T cells demonstrated a significant increase in their ability to migrate in response to CXCL12 compared with their vehicle control–treated counterparts (Figure 2A). This DM-mediated enhancement of migration was found to be dose-dependent with gradually higher concentrations of DM resulting in the significant enhancement (P < .05) of CXCL12-mediated migration compared with vehicle control–treated cells (Figure 2B). Optimal DM effects were observed at 1 μM. Kinetic analysis using 1 μM DM on CXCL12-induced migration in Jurkat T cells revealed that an optimal DM-enhanced migratory response was obtained after 120 minutes of preincubation (Figure 2C).
DM augments CXCL12-induced migration of resting human T cells. (A) Primary human T cells and Jurkat cell were labeled with calcein-AM and then treated with 1 μM DM for 2 hours, after which the cells were placed in the upper wells of transwell migration chambers. In the lower well, media alone or CXCL12 (100 ng/mL) was added and the chambers were incubated for 2 hours in a CO2 incubator at 37°C. Triplicate well determinations were performed for each treatment group assayed. The level of fluorescence of cell migrating across the chamber was assessed using a microfluorimeter. (B) Cells were labeled with calcein-AM and treated with 9 different consecutive doses of DM including 0.1, 0.2, 0.4, 0.8, 1, 2, 4, 10, and 20 μM for 2 hours after which the cells were washed and assessed for migratory potential in response to CXCL12 (100 ng/mL). (C) Jurkat T cells were labeled with calcein-AM and treated with 1 μM DM for different time periods including 5, 15, 30, 60, 120, and 180 minutes. In all panels, the data were presented as the average of 3 individual experiments plus or minus SE. *Value was significant at P < .05 level. Cell migration is expressed in terms of percent migration index using the formulation: Percent migration index = [(migrated cells − background)/fluorescence of total input cell] × 100. Background T-cell migration was determined using the average number of cells migrating into the lower chamber in the absence of chemokine.
DM augments CXCL12-induced migration of resting human T cells. (A) Primary human T cells and Jurkat cell were labeled with calcein-AM and then treated with 1 μM DM for 2 hours, after which the cells were placed in the upper wells of transwell migration chambers. In the lower well, media alone or CXCL12 (100 ng/mL) was added and the chambers were incubated for 2 hours in a CO2 incubator at 37°C. Triplicate well determinations were performed for each treatment group assayed. The level of fluorescence of cell migrating across the chamber was assessed using a microfluorimeter. (B) Cells were labeled with calcein-AM and treated with 9 different consecutive doses of DM including 0.1, 0.2, 0.4, 0.8, 1, 2, 4, 10, and 20 μM for 2 hours after which the cells were washed and assessed for migratory potential in response to CXCL12 (100 ng/mL). (C) Jurkat T cells were labeled with calcein-AM and treated with 1 μM DM for different time periods including 5, 15, 30, 60, 120, and 180 minutes. In all panels, the data were presented as the average of 3 individual experiments plus or minus SE. *Value was significant at P < .05 level. Cell migration is expressed in terms of percent migration index using the formulation: Percent migration index = [(migrated cells − background)/fluorescence of total input cell] × 100. Background T-cell migration was determined using the average number of cells migrating into the lower chamber in the absence of chemokine.
DM-enhanced CXCL12-induced migration is a GCR- and Lck-dependent phenomenon
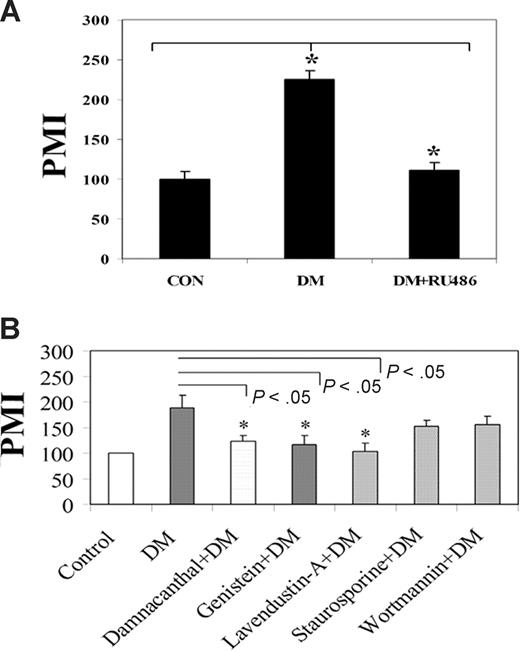
We have also used mifepristone to assess its ability to influence DM-mediated enhancement of CXCL12-induced T-cell migration. Resting human T cells (Figure 3A) were pretreated with the GCR antagonist followed by the treatment with DM, after which cell migration in response to CXCL12 was assessed. Here, DM-treated T cells demonstrated a significantly higher (P < .05) degree of CXCL12-mediated migration compared with control vehicle-treated cells, whereas, on mifepristone treatment, DM-treated T cells resulted in a significant attenuation (P < .05) in CXCL12-mediated migration compared with vehicle controls. Mifepristone alone did not demonstrate any alteration in migration alone in culture or any significant effects on cell viability (data not shown).
DM enhancement of T-cell migration is a GCR- and Lck-dependent event. (A) Primary human T cells were labeled with calcein-AM and incubated in the presence or absence of 5 μM mifepristone (RU486) for 1 hour. The cells were then treated with DM (1 μM) for 2 hours after which the cells were examined for their ability to migrate in response to CXCL12 (100 ng/mL) for 2 hours. (B) Primary T cells were treated with damnacanthal (500 nM for 1 hour), a Lck inhibitor, genistein (50 μM for 1 hour), a tyrosine kinase inhibitor, lavendustin-A (10 μM for 10 minutes), a Src kinase inhibitor, staurosporine (200 nM for 30 minutes), a protein kinase C inhibitor, or wortmannin (20 nM for 30 minutes), a phosphatidylinositol 3 kinase inhibitor. The dose and timing of each inhibitor treatment were previously optimized. The cells were then treated with 1 μM DM for 2 hours followed by a migration assay. The vehicle for all the treatment was maintained in parallel. No significant alterations in cell viability were detected for any of these inhibitors or vehicle as assessed by Trypan blue exclusion (data not shown). In all panels, the fluorescent signal of migrated cell was measured using a microfluorimeter. Triplicate well determinations were performed for each treatment group. The data were presented represent the average of 3 individual experiments plus or minus SE. *Significant value (P < .05). PMI indicates the percent migration index.
DM enhancement of T-cell migration is a GCR- and Lck-dependent event. (A) Primary human T cells were labeled with calcein-AM and incubated in the presence or absence of 5 μM mifepristone (RU486) for 1 hour. The cells were then treated with DM (1 μM) for 2 hours after which the cells were examined for their ability to migrate in response to CXCL12 (100 ng/mL) for 2 hours. (B) Primary T cells were treated with damnacanthal (500 nM for 1 hour), a Lck inhibitor, genistein (50 μM for 1 hour), a tyrosine kinase inhibitor, lavendustin-A (10 μM for 10 minutes), a Src kinase inhibitor, staurosporine (200 nM for 30 minutes), a protein kinase C inhibitor, or wortmannin (20 nM for 30 minutes), a phosphatidylinositol 3 kinase inhibitor. The dose and timing of each inhibitor treatment were previously optimized. The cells were then treated with 1 μM DM for 2 hours followed by a migration assay. The vehicle for all the treatment was maintained in parallel. No significant alterations in cell viability were detected for any of these inhibitors or vehicle as assessed by Trypan blue exclusion (data not shown). In all panels, the fluorescent signal of migrated cell was measured using a microfluorimeter. Triplicate well determinations were performed for each treatment group. The data were presented represent the average of 3 individual experiments plus or minus SE. *Significant value (P < .05). PMI indicates the percent migration index.
To assess whether the DM-mediated effects on CXCL12-mediated migration were also dependent on Lck activation, we used pharmacologic inhibitors specific to tyrosine kinase, Src kinase, and Lck within our assay system. All of these inhibitors were dissolved in vehicles and solvents recommended by the manufacturer, and individual vehicle controls were used and examined for each inhibitor examined. Optimal incubation time and inhibitor doses were determined for all of these experiments (data not shown). The results in Figure 3B revealed a significant abrogation of DM-induced increase in CXCL12-mediated migration in resting human T cells treated with the Lck-specific blocker damnacanthal, the tyrosine kinase blockers, genistein, and Src kinase blocker lavendustin-A. No significant effects were observed using the protein kinase C blocker staurosporine or the PI3 kinase blocker wortmannin (Figure 3B). In addition, no significant change in CXCL12-mediated migration was observed in response to any of the vehicle controls examined, including dimethyl sulfoxide, lavendustin-B, or chloroform-methanol (data not shown).
DM augments CXCL12-mediated actin polymerization and the activation of Rac1 and Vav
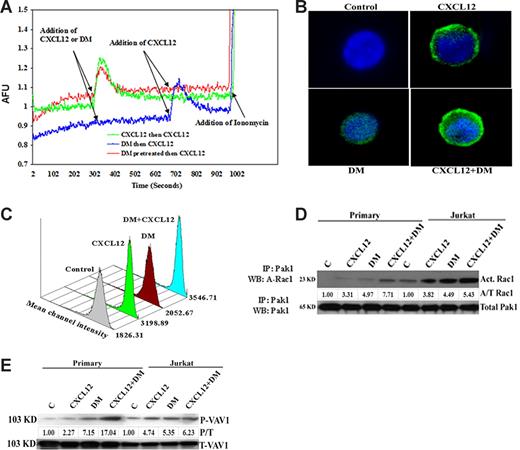
To determine whether DM potentiates CXCL12 function via augmentation of CXCR4 signaling, we examined the effects of DM on CXCR4-mediated activity in T cells. The results shown in Figure 4A reveal that CXCL12 induces a strong intracellular calcium mobilization signal on exposure to Fura 5–labeled T cells with the expected reduction in calcium mobilization on pretreatment of these same cells with CXCL12 (homologous desensitization). DM treatment of T cells alone failed to influence the intracellular calcium levels and also failed to influence the magnitude of CXCL12-mediated calcium response (Figure 4A). Moreover, preincubation of T cells with DM (in red) for 5 minutes at room temperature also failed to change the amplitude of the CXCL12-induced calcium spike compared with control CXCL12-treated cells and had no effect on CXCL12-mediated desensitization. Thus, it appears that DM is not influencing intracellular calcium levels or chemokine-induced calcium mobilization in resting T cells.
DM does not induce intracellular Ca++ but directly induces and augments CXCL12-induced actin polymerization and Rac1 and Vav activation. (A) Jurkat cells were loaded with 2 μM Fura-2 after which the levels of intracellular calcium were assessed using a spectrofluorimeter. Fluorescence was monitored at λex1 = 340 nm, λex2 = 380 nm, and λem = 510 nm. At all times, the baseline was maintained for 300 seconds followed by addition of CXCL12 (100 ng/mL) or DM (1 μM) in the cuvette. A second addition of CXCL12 was performed at 700 seconds to assess receptor desensitization. Ionomycin was added at 1000 seconds of all samples to assess Fura load and cellular viability and responsiveness. In the DM-treated groups, the cells were pretreated with 1 μM DM for 5 minutes to examine its direct effects on cellular calcium levels and on CXCL12-induced intracellular calcium mobilization. (B) Primary T cells were treated with CXCL12 (100 ng/mL) and DM (1 μM) or the combination for 5 minutes and were fixed followed by staining for polymerized actin using phalloidin labeled with Alexa Fluor 488. The cells were subsequently cytospinned on a slide coated with poly-L-lysine and then mounted with vectashield mounting media and examined using a Zeiss Axiovert S100 microscope (Carl Zeiss, Thornwood, NY) using a Spot Enhanced EN-1400 CCD camera and analyzed by Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI). The fluorescence image was acquired using the green filter for Alexa Fluor 488; 1, 2, 3, and 4 in the panel indicated the control, CXCL12, DM, and combined treatment, respectively. (C) Primary human T cells were treated with CXCL12 (100 ng/mL), DM (1 μM), or the combination for 5 minutes. The cells were subsequently fixed with 2% paraformaldehyde solution for 1 hour followed by incubation with phalloidin-Alexa Fluor 488. Data acquisition was performed by forward scatter versus side scatter. MFI indicates the mean fluorescence intensity. (D) Primary human T cells were treated with CXCL12 (100 ng/mL), DM (1 μM), or the combination for 5 minutes and then lysed with RIPA buffer. Rac1 was immunoprecipitated by Pak1 using the kit supplied by Chemicon International followed by separation in sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then immunoblotted. The densitometry of each band was performed and each value was first normalized against the total Pak1 and then presented as fold change over control of activated Rac1 over Pak1. (E) Similar to Rac1, the same procedure was used for Vav1 and Vav2 except that the immunoprecipitation was performed by assessing total Vav1 and Vav2, and the densitometric value of each phospho-Vav1 and Vav2 band was initially normalized against the total Vav1 or Vav2 (P/T) and then presented as fold change over control.
DM does not induce intracellular Ca++ but directly induces and augments CXCL12-induced actin polymerization and Rac1 and Vav activation. (A) Jurkat cells were loaded with 2 μM Fura-2 after which the levels of intracellular calcium were assessed using a spectrofluorimeter. Fluorescence was monitored at λex1 = 340 nm, λex2 = 380 nm, and λem = 510 nm. At all times, the baseline was maintained for 300 seconds followed by addition of CXCL12 (100 ng/mL) or DM (1 μM) in the cuvette. A second addition of CXCL12 was performed at 700 seconds to assess receptor desensitization. Ionomycin was added at 1000 seconds of all samples to assess Fura load and cellular viability and responsiveness. In the DM-treated groups, the cells were pretreated with 1 μM DM for 5 minutes to examine its direct effects on cellular calcium levels and on CXCL12-induced intracellular calcium mobilization. (B) Primary T cells were treated with CXCL12 (100 ng/mL) and DM (1 μM) or the combination for 5 minutes and were fixed followed by staining for polymerized actin using phalloidin labeled with Alexa Fluor 488. The cells were subsequently cytospinned on a slide coated with poly-L-lysine and then mounted with vectashield mounting media and examined using a Zeiss Axiovert S100 microscope (Carl Zeiss, Thornwood, NY) using a Spot Enhanced EN-1400 CCD camera and analyzed by Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI). The fluorescence image was acquired using the green filter for Alexa Fluor 488; 1, 2, 3, and 4 in the panel indicated the control, CXCL12, DM, and combined treatment, respectively. (C) Primary human T cells were treated with CXCL12 (100 ng/mL), DM (1 μM), or the combination for 5 minutes. The cells were subsequently fixed with 2% paraformaldehyde solution for 1 hour followed by incubation with phalloidin-Alexa Fluor 488. Data acquisition was performed by forward scatter versus side scatter. MFI indicates the mean fluorescence intensity. (D) Primary human T cells were treated with CXCL12 (100 ng/mL), DM (1 μM), or the combination for 5 minutes and then lysed with RIPA buffer. Rac1 was immunoprecipitated by Pak1 using the kit supplied by Chemicon International followed by separation in sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then immunoblotted. The densitometry of each band was performed and each value was first normalized against the total Pak1 and then presented as fold change over control of activated Rac1 over Pak1. (E) Similar to Rac1, the same procedure was used for Vav1 and Vav2 except that the immunoprecipitation was performed by assessing total Vav1 and Vav2, and the densitometric value of each phospho-Vav1 and Vav2 band was initially normalized against the total Vav1 or Vav2 (P/T) and then presented as fold change over control.
Ligation of G-protein coupled receptors is often accompanied by a dramatic remodeling of the actin cytoskeleton.24 The data in Figure 4B demonstrated that CXCL12 and DM induce actin polymerization, although the CXCL12-induced polymerization was more prominent than DM treatment. The combined treatment revealed even greater polymerization. No actin polymerization was observed in vehicle control–treated T cells. This actin polymerization was also assessed by flow cytometric analysis using a natural ligand of actin known as phalloidin, which was labeled with Alexa Fluor 488. Our results (Figure 4C) demonstrate a similar pattern of actin polymerization as shown in Figure 4B, where combined treatment demonstrated the highest level of actin polymerization compared with DM or CXCL12 treatment alone and the phosphate-buffered saline vehicle alone.
This DM-mediated enhancement of actin polymerization prompted us to examine the status of several important small guanosine triphosphate (GTP) binding proteins involved in the cascade for actin polymerization, namely, Rac1 and Vav. Here, activated Rac1 was immunoprecipitated using p21-activated kinase 1. Activated Rac1 increased dramatically in response to CXCL12 or DM in resting human T cells and Jurkat cells, compared with vehicle control–treated cells (Figure 4D). Moreover, the combination of CXCL12 and DM resulted in an additive effect on these important signaling proteins responsible for actin polymerization. Similar results were also observed in the phosphorylation of Vav1 (Figure 4E). These data demonstrated an increase in the phosphorylation of Vav1 in response to DM treatment in human resting T cells and Jurkat cells. Similar to Rac1 activation, an additive effect on phosphorylated Vav1 was also observed when CXCL12 and DM were combined in the treatment of these cells. Similar effects were observed on Vav2 phosphorylation (data not shown).
Fyn59 and Zap70 are activated by DM in resting T cells
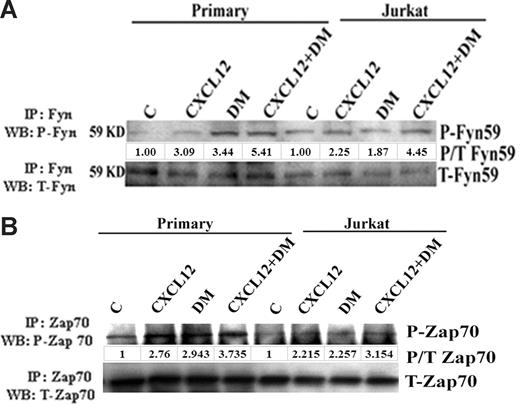
These results support the possibility that several of the downstream mediators of Lck may also be activated by DM and CXCL12 alone or in combination. Fyn59 and Zap70 are important members of Src kinase family and have been previously reported to be phosphorylated by Lck on TCR activation.18,25 Here, resting human T cells and Jurkat were treated with CXCL12 and/or DM followed by an immunoprecipitation of Fyn59 or Zap70. Similar to our other studies, an increase in the phosphorylation of p59Fyn (Figure 5A) and Zap70 (Figure 5B) was observed in response to DM and CXCL12 treatment of resting human T cells or Jurkat T cells with an even greater increase using combined treatments.
DM induces the phosphorylation of p59Fyn and Zap70. Primary human T cells and Jurkat cells were treated with CXCL12 (100 ng/mL) and DM (1 μM) or the combination for 5 minutes and then lysed with RIPA buffer as mentioned in Figure 1. Total Fyn59 (A) and Zap70 (B) were immunoprecipitated using the total Fyn59 and Zap70 antibodies, and then phosphorylation status was assessed by immunoblotting using the antiphosphotyrosine antibody. The membrane was then reprobed using the total antibody of Fyn59 or Zap70. The densitometric value of each phospho-band was first normalized against the total (P/T) of each molecule and then presented as fold change over control.
DM induces the phosphorylation of p59Fyn and Zap70. Primary human T cells and Jurkat cells were treated with CXCL12 (100 ng/mL) and DM (1 μM) or the combination for 5 minutes and then lysed with RIPA buffer as mentioned in Figure 1. Total Fyn59 (A) and Zap70 (B) were immunoprecipitated using the total Fyn59 and Zap70 antibodies, and then phosphorylation status was assessed by immunoblotting using the antiphosphotyrosine antibody. The membrane was then reprobed using the total antibody of Fyn59 or Zap70. The densitometric value of each phospho-band was first normalized against the total (P/T) of each molecule and then presented as fold change over control.
DM-induced phosphorylation of Lck is a CD45-dependent phenomenon
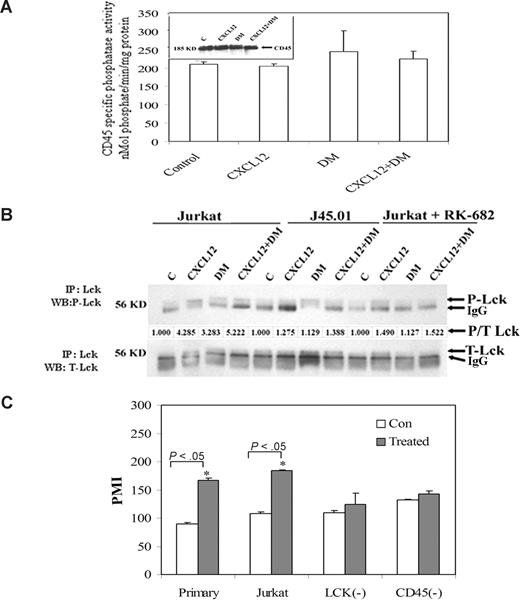
CD45 is a membrane-bound phosphatase that plays a critical role as a positive regulator of Lck.26 Thus, a possible role for CD45 in the DM-induced phosphorylation of Lck was also examined. Here, we have analyzed the effects of DM and CXCL12 alone or in combination on CD45-specific and total phosphatase activity in Jurkat T cells. The data failed to demonstrate any significant change in CD45 specific (Figure 6A) or total phosphatase activity (data not shown) in response to DM or CXCL12 treatment. However, blocking of CD45 activity using RK-682 demonstrated a significant attenuation in DM-induced Lck phosphorylation (Figure 6B) compared with vehicle control–treated Jurkat T cells. Moreover, only a partial blocking of CXCL12-induced Lck phosphorylation was observed using this CD45-specific phosphatase inhibitor. These studies suggest that active CD45 is required for the observed DM- and CXCL12-mediated effects on Lck phosphorylation although not directly inducing any phosphatase activity alone or in combination. The Lck and CD45 requirements are further supported by the use of a CD45-deficient Jurkat cell line, J45.01, which failed to elicit any Lck phosphorylation in response to DM or CXCL12 alone or in combination compared with wild-type Jurkat cells (Figure 6B). CD45-deficient line as well as an Lck-deficient line, Jcam 1.6, failed to demonstrate any DM-mediated effects on CXCL12-induced migration (Figure 6C). Moreover, these 2 deficient cell lines also demonstrated poor migration in response to CXCL12 at any dose tested compared with migration observed in the wild-type Jurkat cells and primary human T cells (Figure 6C). The poor migratory effects of the J45.01 and Jcam 1.6 cell lines do not appear to be the result of significant differences in the cell surface levels of CXCR4 compared with wild-type Jurkat cells regardless of any treatment (data not shown).
DM-induced phosphorylation of Lck is CD45-dependent event. (A) CD45 specific phosphatase activity was assayed from the control, CXCL12-treated, and DM-treated Jurkat cells using a kit manufactured by BIOMOL International. Jurkat cells were treated with CXCL12 (100 ng/mL) and DM (1 μM) alone or in combination for 5 minutes and after which the cells were lysed with a phosphate-free lysis buffer supplied by the manufacturer. CD45 was immunoprecipitated using monoclonal antibody after which the phosphatase activity was assayed. A phosphoprotein was used as a substrate with total reaction volume 100 μL over a 30-minute incubation period in which 50 μg of experimental sample was added. The activity was calculated from an inorganic phosphate standard curve. Finally, the specific phosphatase activity was expressed in nmol phosphate/minutes per mg of protein. In addition, the presence of CD45 in the lysate was confirmed by immunoblot and is shown as an insert in panel A. (B) Wild-type Jurkat cells and the CD45-deficient Jurkat cell line (J45.01) were treated with CXCL12 (100 ng/mL) and/or DM (1 μM) for 5 minutes or after pretreatment with CD45 blocker, RK-682 (25 μM) for 30 minutes followed by treatment with CXCL12 and/or DM for 5 minutes. CD45-deficient cell line was used as negative control for the experiment. Immunoprecipitation and immunoblotting of phospho-Lck and total Lck were performed, and the data are presented as described in Figure 1A. It should be noted that the ability of DM to inhibit the serine phosphorylation of Lck (shown in Figure S1B) suggests that the lower band noted as IgG in these gels may also be a different gel migrating form of phosphorylated Lck. (C) Primary human T cells, wild-type Jurkat T cells (JE6.1), and Jurkat cells deficient for Lck (JCaM1.6) and CD45 (J45.01) cells were labeled with calcein and treated with 1 μM DM or vehicle for 2 hours, after which CXCL12 migration assays. Data represented the average value of 3 individual experiments plus or minus SE. PMI indicates the percent migration index.
DM-induced phosphorylation of Lck is CD45-dependent event. (A) CD45 specific phosphatase activity was assayed from the control, CXCL12-treated, and DM-treated Jurkat cells using a kit manufactured by BIOMOL International. Jurkat cells were treated with CXCL12 (100 ng/mL) and DM (1 μM) alone or in combination for 5 minutes and after which the cells were lysed with a phosphate-free lysis buffer supplied by the manufacturer. CD45 was immunoprecipitated using monoclonal antibody after which the phosphatase activity was assayed. A phosphoprotein was used as a substrate with total reaction volume 100 μL over a 30-minute incubation period in which 50 μg of experimental sample was added. The activity was calculated from an inorganic phosphate standard curve. Finally, the specific phosphatase activity was expressed in nmol phosphate/minutes per mg of protein. In addition, the presence of CD45 in the lysate was confirmed by immunoblot and is shown as an insert in panel A. (B) Wild-type Jurkat cells and the CD45-deficient Jurkat cell line (J45.01) were treated with CXCL12 (100 ng/mL) and/or DM (1 μM) for 5 minutes or after pretreatment with CD45 blocker, RK-682 (25 μM) for 30 minutes followed by treatment with CXCL12 and/or DM for 5 minutes. CD45-deficient cell line was used as negative control for the experiment. Immunoprecipitation and immunoblotting of phospho-Lck and total Lck were performed, and the data are presented as described in Figure 1A. It should be noted that the ability of DM to inhibit the serine phosphorylation of Lck (shown in Figure S1B) suggests that the lower band noted as IgG in these gels may also be a different gel migrating form of phosphorylated Lck. (C) Primary human T cells, wild-type Jurkat T cells (JE6.1), and Jurkat cells deficient for Lck (JCaM1.6) and CD45 (J45.01) cells were labeled with calcein and treated with 1 μM DM or vehicle for 2 hours, after which CXCL12 migration assays. Data represented the average value of 3 individual experiments plus or minus SE. PMI indicates the percent migration index.
Interactions among GCR, CD45, and Lck on DM treatment in human T cells
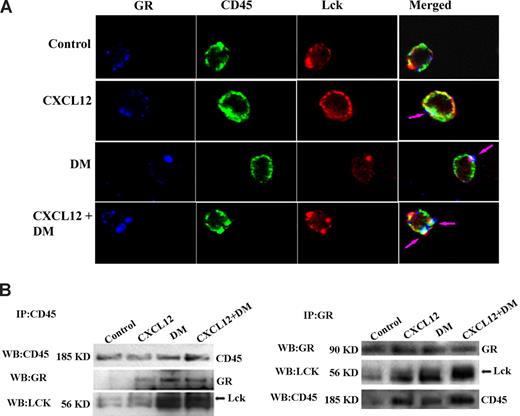
These data suggest the involvement of GCR, Lck and CD45 in the enhancement of CXCL12-mediated migration by DM and in the ability of DM to mediate Lck activation in resting T cells. It seems possible that there are direct interactions and colocalization of these molecules after DM surpasses the cell membrane. To examine this possibility, we examined the molecular interaction among these 3 molecules at a single-cell level using confocal microscopy. Resting human T cells were treated with DM or CXCL12 alone or in combination, and the colocalization of CD45, Lck, and GCR was examined using fluorochrome-labeled antibodies. Our results revealed that there was a clear colocalization among CD45, Lck, and GCR on the cell membrane on treatment of resting T cells with either CXCL12 or DM, but the magnitude of colocalization among these 3 molecules was highest in combined treatment compared with DM or CXCL12 treatment alone. No colocalization was observed in vehicle control–treated cells (Figures 7A, S3). Quantitation of the degree of colocalization was found to be significant for DM and CXCL12 alone and even greater in combined cultures (Figure S3).
DM-induced effects on CXCL12 migration appear to be Lck-, GCR-, and CD45-dependent. (A) Resting T cells were treated with CXCL12 (100 ng/mL), DM (1 μM), or the combination for 5 minutes and were fixed with 2% paraformaldehyde for 1 hour; 10% donkey serum was used for 1 hour at 37°C to block the slides followed by staining with anti-CD45 antibody for 1 hour. The cells were then permeabilized with 90% methanol for 30 minutes at −20°C. Anti-Lck and GC receptor antibodies were added together in blocking buffer, and the slides were incubated overnight at 4°C. Secondary antibody corresponding for each primary antibody labeled with Alexa Fluor 555, Alexa Fluor 647, and Alexa Fluor 488 for the Lck, GR, and CD45, respectively, was added to the slides and incubated for 1 hour at room temperature. The slides were then mounted with Prolong Gold (Invitrogen) mounting media, and image acquisition was done by a confocal microscope (Carl Zeiss) as previously described.50,51 Isotype controls for each primary antibody were also used in parallel as negative controls. Blue, green, and red indicated the presence of GCR, CD45, and Lck, respectively, and most right panel indicated by the merge of all 3 colors (white). Development of white patches on the merge-image indicated the colocalization of 3 molecules. Single-plane images were examined. (B) Primary human T cells were treated with CXCL12 (100 ng/mL) or DM (1 μM) or a combination for 5 minutes after which the cells were lysed with RIPA buffer. CD45, Lck, and GCR were immunoprecipitated using antibodies to total CD45, Lck, and GCR, respectively. Equivalent amounts of protein were used for immunoprecipitation, and antigen was pulled down from the lysate with antibody-coupled agarose beads after which immunoblotting for each of molecules was performed. Arrows are shown for some of the blots to focus on the bands of interest. See Figure S3 for further quantitation of colocalization after treatment.
DM-induced effects on CXCL12 migration appear to be Lck-, GCR-, and CD45-dependent. (A) Resting T cells were treated with CXCL12 (100 ng/mL), DM (1 μM), or the combination for 5 minutes and were fixed with 2% paraformaldehyde for 1 hour; 10% donkey serum was used for 1 hour at 37°C to block the slides followed by staining with anti-CD45 antibody for 1 hour. The cells were then permeabilized with 90% methanol for 30 minutes at −20°C. Anti-Lck and GC receptor antibodies were added together in blocking buffer, and the slides were incubated overnight at 4°C. Secondary antibody corresponding for each primary antibody labeled with Alexa Fluor 555, Alexa Fluor 647, and Alexa Fluor 488 for the Lck, GR, and CD45, respectively, was added to the slides and incubated for 1 hour at room temperature. The slides were then mounted with Prolong Gold (Invitrogen) mounting media, and image acquisition was done by a confocal microscope (Carl Zeiss) as previously described.50,51 Isotype controls for each primary antibody were also used in parallel as negative controls. Blue, green, and red indicated the presence of GCR, CD45, and Lck, respectively, and most right panel indicated by the merge of all 3 colors (white). Development of white patches on the merge-image indicated the colocalization of 3 molecules. Single-plane images were examined. (B) Primary human T cells were treated with CXCL12 (100 ng/mL) or DM (1 μM) or a combination for 5 minutes after which the cells were lysed with RIPA buffer. CD45, Lck, and GCR were immunoprecipitated using antibodies to total CD45, Lck, and GCR, respectively. Equivalent amounts of protein were used for immunoprecipitation, and antigen was pulled down from the lysate with antibody-coupled agarose beads after which immunoblotting for each of molecules was performed. Arrows are shown for some of the blots to focus on the bands of interest. See Figure S3 for further quantitation of colocalization after treatment.
Our hypothesis of interactions between these 3 molecules was further reinforced using reciprocal immunoprecipitation of treated human T cells. CD45 and GCR were immunoprecipitated from DM- or CXCL12-treated resting human T cells, and interactions between these molecules were examined using Western blot analysis of CD45, GCR, or Lck. These data revealed an interaction between all of these molecules on treatment with CXCL12 and DM alone or in combination (Figure 7B). Similar results were revealed examining total Lck immunoprecipitation (data not shown). Vehicle control–treated cells demonstrate little to no basal interaction between these molecules, suggesting that DM and/or CXCL12 facilitate an association between Lck, CD45, and GCR. Moreover, we also examined possible associations between CXCR4 and Lck on DM and CXCL12 treatment. In reciprocal immunoprecipitation, we failed to observe any interactions between CXCR4 and Lck after DM and/or CXCL12 treatment (Figure S4A).
Discussion
GCs are thought to modulate the expression of many proinflammatory genes at the transcriptional level but may also affect a membrane proximal step of the signaling cascade initiated by antigen and cytokine receptors.27 Inhibition of TCR signaling requires long-term exposure to GC/GCR binding and de novo protein synthesis, suggesting that this inhibitory property of GC is a consequence of novel gene transcription initiated by the hormone-receptor complex and not merely a consequence of GC insertion into the plasma membrane. GCs can alter membrane localized signaling events in resting T-cell hybrids, before TCR stimulation.2,27,28 Here, we report the rapid nongenomic effect of the synthetic GC, DM, on the early signaling events typically initiated by TCR ligation in T cells. Previous studies have reported that DM or GC treatment can inhibit TCR-mediated signaling responses,28–32 including Lck, Fyn, and ZAP70 phosphorylation within activated T cells.7 We have observed similar effects on activated T cells where DM treatment resulted in decreased Lck phosphorylation in this manuscript. However, on examination of the effects of DM on resting human T cells, we observed a contrary effect with DM actually mediating the activation of Lck and several downstream mediators in a TCR-independent manner (Figure S4B). The bell-shaped dose-response curve to DM in both resting human T cells and Jurkat T cells suggests a receptor-mediated event, which we confirmed through the use of the pharmacologic GCR blocker, mifepristone. These studies support the ability of DM to interact with a membrane-bound or cytosolic form(s) of the GCR. This may play a role in potentiating DM effects on Lck phosphorylation and CXCL12-induced migration and signaling (Figure 3A,B).
A possible mechanism for this DM enhancement of CXCL12-mediated chemotaxis may be via its ability to modulate the cell surface expression of the CXCL12 receptor, CXCR4, as previously described.13,14,33 There have been several reports demonstrating that GCs and DM can directly up-regulate CXCR4 expression in monocytes and T lymphocytes, resulting in an increased capacity of these cells to migrate in response to CXCL12.13,14,33 However, in those published studies, GC treatment required more extensive incubation times (up to 16 hours) before any effects on CXCR4 expression could be observed. In contrast, our DM effects on cell migration were observed as early as 5 minutes after DM treatment, with optimal effects on migration being noted at the 2-hour time interval, which failed to demonstrate any change in CXCR4 cell surface expression on primary human T cells or Jurkat cells (Figure S5A). The effects of DM on Lck phosphorylation were observed as early as 2 to 5 minutes after treatment, suggesting a transcription-independent mechanism controlling CXCR4-mediated migration and signaling (Figure S1A). Moreover, pretreatment of T cells with the CXCR4 ligand, CXCL12, in the presence or absence of DM results in a significant and expected down-regulation in the cell surface expression of CXCR4, most probably because of the receptor internalization, which is a classic characteristic of chemokine on its receptor34 (data not shown).
The induction of cell migration and actin polymerization in T cells in response to the CXCL12 ligand has been reported by numerous laboratories17,22,23,35,36 ; however, no data concerning the influence of DM on these processes exist. We observed that DM alone induced actin polymerization (Figure 4B,C) and did so synergistically with CXCL12. DM treatment of T cells and Jurkat cells also promoted the activation of the small GTP-binding protein Rac1 (Figure 4D) and the oncoproteins Vav1 or Vav2 (Figure 4E and data not shown). These molecules are important signaling components that play an important role in organizing the actin cytoskeleton in T cells or tumor cells.37–39 Our data also support the involvement of Lck and Src kinases in DM-mediated enhancement of CXCL12-induced migration and signaling (Figures 1A, 3). Similar to other reported kinetics for the activation of Lck,40,41 we also observed that maximal Lck phosphorylation occurs between 5 and 15 minutes after DM exposure (Figure S1A). Previous reports have demonstrated the activation of Lck by CXCL1219,42 ; however, to date, no studies have reported the direct effects of DM or GCs on chemokine-induced Lck phosphorylation or migration. Interestingly, DM-mediated enhancement of cell migration (Figure 3A) was found to be a GCR-dependent event, suggesting that direct interactions between DM and GCR are influencing CXCR4 signaling and activity.
In addition to Lck, Fyn59 and Zap70 are known to be 2 important downstream kinases activated by Lck that play a role in TCR signaling.43,44 Activated Lck phosphorylates TCR-ϵ chains, which then recruit the ZAP70 kinase to promote T-cell activation. Lck is activated by autophosphorylation at Tyr394 in the activation loop, and it is inactive when Tyr505 near the carboxy terminus is phosphorylated and interacts with its own SH2 domain. Similarly, in agreement with the increase in Tyr394 phosphorylation in response to DM and/or CXCL12, we have observed the phosphorylation of Fyn59 and Zap70 (Figure 5A,B) in response to these same stimuli. Interestingly, our CXCL12 results regarding the phosphorylation of Zap70 are in agreement with the work of Kremer et al where they demonstrate the induction of Zap70 phosphorylation in response to CXCL12.44 Together, these data suggest that DM may be “priming” the migratory signaling machinery in the T cell–promoting concurrent or subsequent responses to chemokine signals and providing an activation signal similar to that seen on TCR ligation.
Another possible means by which DM is facilitating TCR-independent Lck phosphorylation may be via the ability of DM to influence the activity of the CD45 molecule. CD45 is a membrane-bound phosphatase that plays a critical role as positive regulator of Lck by site-specific dephosphorylation of a tyrosine residue of the Lck regulator Csk, a cognate inhibitory motif for most of the Src kinase.45 We failed to observe any effects on the cellular CD45-specific or total phosphatase activity in response to DM or CXCL12 (Figure 6A), which is supported by the earlier work of Van Laethem and Leo.46 However, we did find that DM-induced Lck phosphorylation required the presence of the CD45 molecule as demonstrated by our inhibitor studies (Figure 6B). Furthermore, our confocal and immunoprecipitation experiments indicate that DM treatment facilitates a physical interaction between the GCR, CD45, and Lck molecules. The precise mechanisms mediating these interactions remain unclear.
Several additional studies have been performed in our attempt to determine how DM may facilitate the interactions between CD45 and Lck. One possibility included the potential ability of DM and/or CXCL12 to increased CD45 association with lipid rafts. CD45 is a membrane-bound phosphatase that has a positive regulatory role on the kinase domain of Lck through inhibition of negative modulator of Lck molecule Csk. CD45 is almost always localized in the nonraft regions of the cell membrane; however, during T-cell activation, it has been proposed that CD45 comes in close proximity to the raft by changing its transmembrane domain and assists in Lck phosphorylation by inhibiting Csk through physical interaction. However, DM failed to initiate any change in the fraction localization of CD45 or CD48 (or CD4 and CXCR4; data not shown) compared with vehicle control–treated cells, suggesting that increased localization of CD45 and CD4 is not necessary for DM-mediated Lck activation (Figure S5B). Moreover, DM and CXCL12 treatment failed to influence the phosphorylation of the regulators of Lck, namely Csk and Lck interacting transmembrane adaptor 1 (LIME1)47 in resting T cells (data not shown). On cellular activation, Csk is hypothesized to dephosphorylate the Lck through regulating the tyrosine phosphatase CD45,45 resulting in the release of Lck from the CD4 complex.
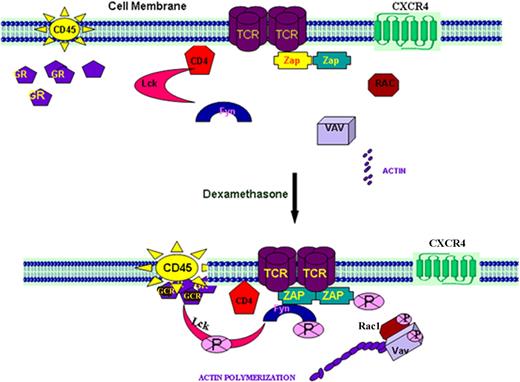
In our current model (described in Figure 8), Lck is associated with CD4 molecules through its cognate-binding domain. On TCR activation, Lck dissociates from CD4 molecule, presumably because of interactions between CD45 and Csk, resulting in its release into the cytosol where it is phosphorylated and is now capable of phosphorylating its various substrates.48,49 CD45 differentially regulates the negatively acting pTyr-505 and positively acting pTyr-394 p56lck tyrosine kinase phosphorylation sites.50–53 CD45 expression is necessary to dephosphorylate p56lck pTyr-394, facilitating T-cell activation. In our studies, DM and/or CXCL12 are also capable of mediating Lck activation in resting but not activated T cells. Moreover, a decrease in the phosphorylation of Lck Tyr505 (as well as in serine phosphorylation) and an increase in the phosphorylation of Tyr394 lead to an increase in Lck kinase activity on DM and/or CXCL12 treatment. Whether the CD4 release of Lck is the result of alterations in the lipid raft structure and/or an increase in the interactions or adjacencies of CD45 and CD4 remains to be defined. DM binding to GCR may facilitate an interaction with CD45 and/or the cell membrane influencing its ability to interact with the CD4 and/or Csk molecules, resulting in the release the CD4-bound Lck. Moreover, Lck activation by CXCL12 is thought to be involved in orchestrating the downstream signaling pathways necessary for chemotaxis.18,19 As CXCR4 has been shown to physically associate with the TCR on CXCL12 ligation and uses the ZAP70-binding ITAM domains of the TCR for signal transduction,20 this association may actually account for several activities attributed to CXCR4 activation, including kinase activation, intracellular calcium mobilization, and cytokine production. However, unlike the DM cultures, we also found that the CXCL12 effects on Tyr394 Lck phosphorylation required the presence of the TCR (Figure S4B) supporting a linkage between the TCR and CXCR4 signaling pathways. Thus, the combined signaling effects of DM and CXCL12 on Lck, Rac, and Vav activation and actin polymerization may account for the augmented CXCR4-mediated signaling and responses in the presence of DM. Moreover, these data also demonstrate an as-yet undescribed role for GCR in T-cell signaling and may have relevance to both chemokine and TCR signaling events in the resting T cell during the GC therapy for a variety of inflammatory diseases in humans.
Schematic presentation of the probable mechanism of induction of Lck through the GCR and CD45. Under resting T-cell conditions, Lck is attached to the CD4 molecules and Zap70 is associated with the TCR. CD45, the membrane-bound phosphatase molecule, is localized to the plasma membrane keeping its interactive domain toward the cytosol. Fyn and downstream small GTPase molecules, such as Rac1 and Vav, are also present in the cytosol. All of these molecules are not phosphorylated in their inactive resting state. On DM treatment, we think that the GCR, on ligation with DM in resting T cells interacts with CD45 molecule, which may in turn influence its ability to localize with and/or bind to the CD4-bound Lck possibly facilitating its release because of phosphorylation.50–54 CD45 differentially regulates the negatively acting pTyr-505 and positively acting pTyr-394 p56lck tyrosine kinase phosphorylation sites. We propose that DM facilitates CD45 dephosphorylation of p56lck pTyr-394. Our data have revealed that there appears to be a physical association between these 3 molecules in response to DM treatment. Although how and where these molecules actually interface remain to be defined, activated Lck stimulates Fyn59, which in turn phosphorylates Zap70. Activated Src kinases initiate the activation of small GTPases, Rac1 and Vav, which ultimately promote actin polymerization and cell migration. This phenomenon also appears to occur in the presence of CXCL12 and is further augmented when these mediators are combined. The activation of Lck on treatment of resting T cells with CXCL12 may be involved in orchestrating the downstream signaling pathways necessary for chemotaxis.18,19 CXCR4 has been shown to physically associate with the TCR on CXCL12 ligation and uses the ZAP70-binding ITAM domains of the TCR for signal transduction.20 This association may actually account for several activities attributed to CXCR4 activation, including MAP kinase activation, intracellular calcium mobilization, enhanced activator protein-1 activity, and cytokine production. Such an association may also account for the similar TCR-like signals observed in response to CXCL12 treatment. On treatment with DM, the combined DM-CXCL12-mediated effect on Lck, Rac, and Vav activation and actin polymerization may account for the augmented CXCR4-mediated signaling and responses in the presence of DM. Moreover, these data also demonstrate an as-yet undescribed role for GCR in T-cell signaling.
Schematic presentation of the probable mechanism of induction of Lck through the GCR and CD45. Under resting T-cell conditions, Lck is attached to the CD4 molecules and Zap70 is associated with the TCR. CD45, the membrane-bound phosphatase molecule, is localized to the plasma membrane keeping its interactive domain toward the cytosol. Fyn and downstream small GTPase molecules, such as Rac1 and Vav, are also present in the cytosol. All of these molecules are not phosphorylated in their inactive resting state. On DM treatment, we think that the GCR, on ligation with DM in resting T cells interacts with CD45 molecule, which may in turn influence its ability to localize with and/or bind to the CD4-bound Lck possibly facilitating its release because of phosphorylation.50–54 CD45 differentially regulates the negatively acting pTyr-505 and positively acting pTyr-394 p56lck tyrosine kinase phosphorylation sites. We propose that DM facilitates CD45 dephosphorylation of p56lck pTyr-394. Our data have revealed that there appears to be a physical association between these 3 molecules in response to DM treatment. Although how and where these molecules actually interface remain to be defined, activated Lck stimulates Fyn59, which in turn phosphorylates Zap70. Activated Src kinases initiate the activation of small GTPases, Rac1 and Vav, which ultimately promote actin polymerization and cell migration. This phenomenon also appears to occur in the presence of CXCL12 and is further augmented when these mediators are combined. The activation of Lck on treatment of resting T cells with CXCL12 may be involved in orchestrating the downstream signaling pathways necessary for chemotaxis.18,19 CXCR4 has been shown to physically associate with the TCR on CXCL12 ligation and uses the ZAP70-binding ITAM domains of the TCR for signal transduction.20 This association may actually account for several activities attributed to CXCR4 activation, including MAP kinase activation, intracellular calcium mobilization, enhanced activator protein-1 activity, and cytokine production. Such an association may also account for the similar TCR-like signals observed in response to CXCL12 treatment. On treatment with DM, the combined DM-CXCL12-mediated effect on Lck, Rac, and Vav activation and actin polymerization may account for the augmented CXCR4-mediated signaling and responses in the presence of DM. Moreover, these data also demonstrate an as-yet undescribed role for GCR in T-cell signaling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: M.C.G. and D.D.T. wrote the paper, performed research, and analyzed data; D.B., G.C., A.C., F.I., and A.B. performed research; and D.D.T. supervised and planned the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dennis D. Taub, Laboratory of Immunology, Clinical Immunology Section, National Institute of Aging Intramural Research Program, National Institutes of Health, Baltimore, MD 21224-6825; e-mail: taubd@grc.nia.nih.gov.

![Figure 1. DM induces Lck phosphorylation in resting T cells but not activated T cells in a GCR-dependent fashion. (A) Resting human T cells (top panel) and Jurkat cells [E6.1] (bottom panel) were treated with CXCL12 (100 ng/mL), DM (1 μM), or the combination for 5 minutes. In the activated T-cell cultures, primary human T cells were incubated with 5 μg/mL antihuman CD3 mAb in combination with 5 μg/mL antihuman CD28 antibody for 1 minute at a cell concentration of 5 × 106 cells/mL followed by treatment with CXCL12 and DM alone or with combination for 5 minutes. Treated cells were subsequently washed with ice-cold phosphate-buffered saline and then lysed with ice-cold radioimmunoassay (RIPA) buffer. Total Lck was immunoprecipitated from the lysates and phospho- and total Lck levels were assessed by immunoblotting using the phosphotyrosine and total Lck antibodies. In many of the gels, densitometry was performed on scanned gel images and assessed by UN-SCAN-IT gel digitizing software (Silk Scientific, Orem, UT). The data were then normalized and presented as fold change over control bands. Here, phospho-Lck was normalized against the corresponding band of total Lck. The final data are presented as the fold change over the control (P/T). (B) Primary human T cells were incubated in the presence or absence of 5 μM mifepristone (RU486) for 1 hour. The cells were then treated with CXCL12 (100 ng/mL) or DM (1 μM) or together for 5 minutes. Lysis of the cell and rest of the procedure for Lck immunoblot was followed as described in Figure 1A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/3/10.1182_blood-2008-04-151803/5/m_zh80240828500001.jpeg?Expires=1769382597&Signature=PtcoljmA6~lukMNwU1bMG8wkgeSvjn~pULnVHCHEYUvXRPcV-TRTCARQ3~BPFWmAgMx5uBHetFDyjoLK0ZWPHqe1KZg4p~oHDjPuL93lQw6UyiITJngpJQZDgYisT669zgf-I-PRimZuEeFsY0v2aHiz9K5wiqhI2rGY3eipnr20ctGw6dBs6AeTvGiphS6bHP~uIJ8RjCYC34FT7htLRtANTc-WLQ1Vyvbf7nbyqblwUC4pws420INV9T1L7u9~1BhN6YPBSPPlYRm1wLFd36Sp0mrEf0sMDhibYAiMj5Crz3WB70C3m58mVdTtvDWwATCnlV0Wn-So7TF~5PaghQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. DM augments CXCL12-induced migration of resting human T cells. (A) Primary human T cells and Jurkat cell were labeled with calcein-AM and then treated with 1 μM DM for 2 hours, after which the cells were placed in the upper wells of transwell migration chambers. In the lower well, media alone or CXCL12 (100 ng/mL) was added and the chambers were incubated for 2 hours in a CO2 incubator at 37°C. Triplicate well determinations were performed for each treatment group assayed. The level of fluorescence of cell migrating across the chamber was assessed using a microfluorimeter. (B) Cells were labeled with calcein-AM and treated with 9 different consecutive doses of DM including 0.1, 0.2, 0.4, 0.8, 1, 2, 4, 10, and 20 μM for 2 hours after which the cells were washed and assessed for migratory potential in response to CXCL12 (100 ng/mL). (C) Jurkat T cells were labeled with calcein-AM and treated with 1 μM DM for different time periods including 5, 15, 30, 60, 120, and 180 minutes. In all panels, the data were presented as the average of 3 individual experiments plus or minus SE. *Value was significant at P < .05 level. Cell migration is expressed in terms of percent migration index using the formulation: Percent migration index = [(migrated cells − background)/fluorescence of total input cell] × 100. Background T-cell migration was determined using the average number of cells migrating into the lower chamber in the absence of chemokine.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/3/10.1182_blood-2008-04-151803/5/m_zh80240828500002.jpeg?Expires=1769382597&Signature=3C2hNK-~wxfuDHE-9yGVN5QZIsrzUF-Ad9q87jnAhge5KccC60nN7KRBqxOO362U736yUvskGFGh-t17k8QOrONHFaeMKEUSecyxG5Bk3-dS7KA7w4Vh6r0Csb-a7cYtaW~7DLvQILX3fP0pE51EgRIOzsVBNLjoGEFLo3N6g3Y9sGIUhs1X3TgCLMfyyV7uszOEMpLcudYLgfurtau34wzGSmeROZDxUNx9YsnXHr8CKAYp6MXeKH1PWeCUppCLR6RymBt9Rx8RQHUrVNU9v7chrkSU-0nl9xkXNW2kDHSrQy2e4hsqobG9RFZbs1NMsyr44anpJF081rY4hxTmpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)