Abstract
Hepatitis C virus (HCV) primarily replicates within the liver, leading to hepatitis, fibrosis, and hepatocellular carcinoma. Infection is also associated with B-cell abnormalities, suggesting an association of the virus with B cells. The infectious JFH-1 strain of HCV can bind primary and immortalized B cells but fails to establish productive infection. However, B cell–associated virus readily infects hepatoma cells, showing an enhanced infectivity compared with extracellular virus. B cells express the viral receptors CD81, SR-BI, and the C-type lectins DC-SIGN and L-SIGN. Antibodies specific for SR-BI and DC-SIGN/L-SIGN reduced B-cell transinfection, supporting a role for these molecules in B-cell association with HCV. Stimulation of B cells with CD40 ligand and interleukin-4 promoted their ability to transinfect hepatoma cells. B cell–associated virus is resistant to trypsin proteolysis and HCV-specific neutralizing antibodies, consistent with particle internalization. HCV promoted the adhesion of primary B cells to Huh-7 hepatomas, providing a mechanism for B-cell retention in the infected liver. In summary, B cells may provide a vehicle for HCV to persist and transmit to the liver.
Introduction
Hepatitis C virus (HCV) is an enveloped positive-stranded RNA virus and the sole member of the hepacivirus genus, within the Flaviviridae. HCV establishes persistent infection in the majority of subjects leading to progressive liver disease, and it is the leading indication for liver transplantation in many parts of the world. HCV infection is also associated with B-cell lymphoproliferative disorders such as mixed cryoglobulinemia (MC) and non-Hodgkin lymphoma.1-3 MC is diagnosed in 50% of HCV-infected persons and may be asymptomatic or associated with systemic vasculitis and immune complex disease.4 Reports showing the clinical resolution of MC and lymphomas after successful interferon antiviral treatment suggest a pathogenic role for HCV in B-cell dysfunction.5,6
The liver is the primary target for HCV replication, and one of the characteristic features of chronic hepatitis C infection is the presence of lymphoid aggregates in the liver, appearing as well-formed germinal centers.7,8 B-cell localization and productive immunoglobulin (Ig) gene rearrangements are influenced by a variety of events, and the clonal expansion of B cells in the HCV-infected liver suggests an altered environment that favors their retention and proliferation. At present, the molecular basis underlying B-cell expansion and transformation in HCV infection is unknown.
The lack of culture systems supporting primary virus replication has made the interpretation of experiments assessing HCV replication in hematopoietic cells difficult9-11 (reviewed in Weng and Levy12 ). However, the development of HCV pseudoparticles (HCVpp)13,14 and a system supporting the generation of infectious HCV in cell culture (HCVcc) have enabled studies on viral entry.15-17 Current evidence suggests that at least 3 host-cell molecules are important for HCV infection of hepatocytes in vitro: scavenger receptor class B member I (SR-BI),18,19 the tetraspanin CD81,14,15,19,20 and the tight junction protein Claudin-1 (CLDN1).21 Other factors such as glycosaminoglycans, the C-type lectins dendritic cell–specific ICAM-3–grabbing nonintegrin (DC-SIGN), and liver/lymph node–specific ICAM-3–grabbing nonintegrin (L-SIGN)22 have been implicated in HCV binding; however, their role is less well established (reviewed in von Hahn and McKeating23 ).
We show that primary and immortalized B cells bind HCVcc strain JFH-1 but fail to support a productive infection. However, B cell–associated virus can readily infect hepatoma cells showing enhanced infectivity compared with extracellular virus. Antibodies specific for SR-BI and DC-SIGN/L-SIGN reduced transinfection, supporting a role for these molecules in B-cell association with HCV. B cell–associated virus is resistant to HCV-specific neutralizing antibodies. HCV promoted primary B-cell adhesion to Huh-7 hepatomas, providing a potential mechanism for B-cell retention in the chronically HCV-infected liver. Thus, B cells may provide a vehicle for HCV to evade humoral immune responses and transmit to the liver.
Methods
Cells, antibodies, and reagents
Huh-7.5 (provided by C. Rice, The Rockefeller University, New York, NY) were propagated in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% nonessential amino acids. Group I Burkitt lymphoma (BL) L3055–Bcl-2 and KEM cells were propagated in RPMI/10% FBS. Peripheral blood mononuclear cells (PBMCs) and liver-derived B cells were isolated from healthy and HCV-infected donors, as previously described.24,25 Briefly, B cells were purified from mononuclear cell populations by CD19-coated magnetic beads (Dynal Biotech, Oslo, Norway) to greater than 95% purity, and their phenotype was confirmed by anti-CD20 labeling and flow cytometry. Ethics approval for the study was given by the South Birmingham Local Research Ethics Committee (Queen Elizabeth Hospital, Birmingham, United Kingdom) and the University Hospital Birmingham Trust (Queen Elizabeth Hospital), and informed consent was obtained in accordance with the Declaration of Helsinki.
The primary antibodies used were anti-CLDN1 1C5-D9 (Novus Biologicals, Littleton, CO), anti-CD81 M38 (F. Berditchevski, University of Birmingham), anti-SRBI 25 (BD Biosciences, San Jose, CA), rabbit polyclonal anti–SR-BI (provided by T. Huby, Inserm, France), anti–DC-SIGN clone 507 (R&D Systems, Minneapolis, MN), anti–L-SIGN clone 604 (R&D Systems), anti-CD20 clone H147 (Invitrogen, Carlsbad, CA), and anti-NS5A 9E10 (C. Rice, Rockefeller University). IgG was purified from the sera of 5 healthy and 5 chronic HCV-infected patients by protein G–sepharose chromatography and pooled for neutralization studies (GE Healthcare, Little Chalfont, United Kingdom). Secondary-labeled antibodies were obtained from Invitrogen: Alexa Fluor 488 goat anti–mouse IgG and Alexa Fluor 488 goat anti–rabbit IgG. Bafilomycin and scavenger receptor inhibitors polyinosinic acid (poly-I), fucoidin, polycytidylic acid (poly-C), and chondroitin were obtained from Sigma-Aldrich (St Louis, MO).
HCVcc generation and infection
HCVcc was generated as previously described.15 Briefly, RNA was transcribed in vitro from full-length genomes using the Megascript T7 kit (Ambion, Austin, TX) and electroporated into Huh-7.5 cells. High-titer stocks were generated by 2 serial passages through naive Huh-7.5 cells.21 Supernatants were collected at 72 and 96 hours after infection, pooled, and stored at −80°C. Infected cells were detected by methanol-fixation and stained for NS5A using the anti-NS5A 9E10 antibody and an Alexa 488–conjugated anti–mouse IgG. Infection was quantified by enumerating NS5A+ cells and was defined as the number of focus-forming units (FFUs).26
Quantitative RT-PCR of HCV RNA
To measure the cell-associated and cell-free HCV RNA levels, RNA was extracted using the RNeasy mini kit (QIAGEN, Valencia, CA) and QIAamp MinElute Virus kit (QIAGEN), respectively, according to the manufacturer's instructions. HCV amplification was performed as previously described21 in a single tube reverse transcriptase–polymerase chain reaction (RT-PCR) in accordance with the manufacturer's guidelines (CellsDirect kit; Invitrogen) and fluorescence was monitored in a 7900 HT real-time PCR machine (ABI, Foster City, CA). In all reactions the housekeeping gene GAPDH was included as an internal endogenous control for amplification efficiency and RNA quantification (primer-limited endogenous control; ABI).
B-cell transinfection of Huh-7.5 cells
B cells were stimulated by incubating with a monolayer of L fibroblasts expressing CD40L in the presence of interleukin-4 (IL-4), as previously described.27 One million naive or stimulated B cells in RPMI/10% FBS were incubated with 3 to 5 × 106 FFU of JFH-1, J6/JFH, and H77/JFH in a final volume of 100 μL for 2 hours at 37°C. Unbound virus was removed by 5 sequential washes and low-speed centrifugation. As a control for the efficiency of washing, the final wash buffer was tested for infectious virus and was generally found to be negative. B cells were cocultured with Huh-7.5 cells seeded at 1.5 × 104 per well of a 48-well plate and incubated for 72 hours at 37°C. Infection was assessed by fixing the monolayer with prechilled methanol, and NS5A+ cells visualized by staining with monoclonal antibody (mAb) 9E10 and a goat anti–mouse Alexa 488–conjugated secondary antibody.
B cells were incubated with Bafilomycin A1, the scavenger receptor inhibitors poly-I, fucoidin, and controls poly-C and chondroitin at 100 μg/mL for 1 hour at 37°C, the reagents were removed by extensive washing, the cells were incubated with a defined infectious titer of JFH-1 for 2 hours at 37°C and transinfection was quantified. Virus was not directly exposed to inhibitors at this stage of the experiment, but independent experiments showed minimal effect(s) on virus infectivity.
Antibody inhibition of B-cell transinfection
Increasing concentrations of IgG from healthy (IgGcontrol) or HCV-infected patients (IgGHCV) were incubated with JFH-1 for 1 hour at 37°C, and the immune complexes were assessed for B-cell transinfection or directly incubated with Huh-7.5 cells to measure the neutralizing capacity of the IgG.
To define the receptors on B cells engaging virus, B cells were incubated with anti-CD81 M38, anti–DC-SIGN 507, anti–L-SIGN 604, rabbit anti–SR-BI, or control antibodies at 10 μg/mL for 1 hour on ice. This concentration of antibody was shown to saturate the cellular receptors by flow cytometry. Unbound antibodies were removed by washing, the cells were incubated with JFH-1 for 2 hours at 37°C, and transinfection was quantified.
Enzyme and neutralizing antibody protection assay
CD40L/IL-4–stimulated B cells were incubated with JFH-1 for 1 hour, 2 hours, or 3 hours, and unbound virus was removed by washing. Trypsin (2.5 μg/mL) was added for 5 minutes at 37°C, followed by enzyme inactivation with 10% FBS/RPMI. Alternatively, control or IgGHCV (200 μg/mL) was added to the virus-exposed B cells for 30 minutes at 37°C. Trypsin and IgG were removed by washing, the B cells cocultured with Huh-7.5 cells, and transinfection assessed.
Static adhesion assay
Huh-7.5 cells were seeded to confluence in 24-well plates. The following day, untreated and CD40L/IL-4–stimulated B cells were labeled with 5 CMFDA according to the manufacturer's instructions (Invitrogen), and 0.5 × 106 B cells were added per well of hepatoma cells for 4 hours at 37°C. Nonadhered cells were removed by washing, and the hepatoma-bound cells were fixed with prechilled methanol. Fixed cells were stained with DAPI to enumerate total cell nuclei. Adhered green lymphocytes were counted per field of view using a Nikon (Melville, NY) fluorescence microscope at ×20 magnification. The ratio of lymphocytes to total cell number was estimated for an average of 10 fields of view per condition. Huh-7.5 cell monolayers were confirmed as intact after the washes.
Flow cytometry
Cells were incubated in 1% BSA/2% normal goat serum/PBS (fluorescence-activated cell sorting [FACS] buffer) for 20 minutes on ice. Receptor-specific antibodies were incubated with B cells in FACS buffer for 30 minutes on ice, and unbound antibody was removed by washing. Secondary Alexa 488–conjugated goat antibodies were incubated for a further 30 minutes on ice; cells were washed and fixed in 1% paraformaldehyde. Cell staining was assessed by flow cytometry using a FACSCalibur (BD Biosciences), and data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Western blotting
Huh-7.5 and lymphocyte cell pellets were lysed in PBS, 1% Triton-X100, 0.1% sodium deoxycholate, 0.1% SDS containing protease (Complete; Roche, Indianapolis, IN) and phosphatase (PhoStop; Roche) inhibitors for 1 hour on ice. Lysates were clarified by centrifugation (14 000 rpm, 30 minutes), and the protein concentration was determined using Protein Assay Reagent (Pierce Chemical, Cramlington, Northumberland, United Kingdom) according to the manufacturer's instructions. Protein lysates (20 μg) were separated by 12% SDS–polyacrylamide gel electrophoresis (PAGE), transferred to PVDF membranes for incubation with anti–SR-BI (anti-Cla1; BD Biosciences) and anti-β actin (Sigma-Aldrich) antibodies. Secondary horseradish peroxidase–conjugated sheep anti–mouse IgG (Jackson Immunoresearch Laboratories, West Grove, PA) was detected by enhanced chemiluminescence (Geneflow, Alexandria, VA).
Statistical analysis
Error bars indicate standard deviations. Comparisons between samples were made using nonparametric tests (Mann-Whitney [unpaired samples] and Wilcoxon [paired samples]); multiple comparisons were assessed using Kruskall-Wallis ANOVA and Dunn test. All tests were performed using the Prism 4.0 software package (GraphPad Software, San Diego, CA).
Results
B cell–associated HCV can infect Huh-7.5 hepatoma cells
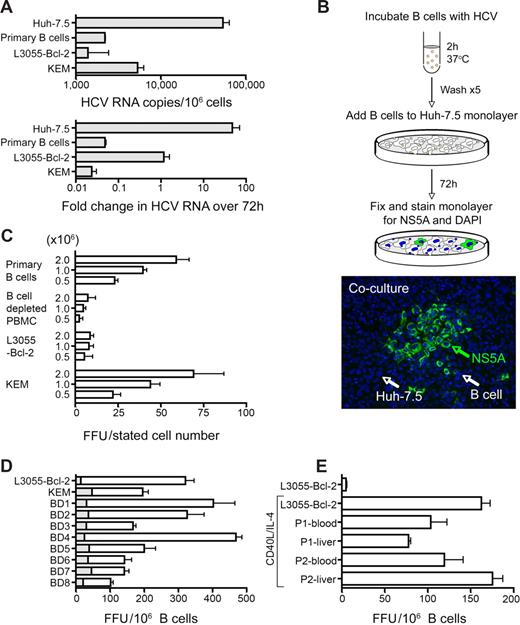
To investigate whether HCV can bind B cells, JFH-1 was incubated with PBMC-derived B cells, 2 BL cell lines (L3055–Bcl-2 and KEM), the permissive Huh-7.5 hepatoma cell line, and Chinese hamster ovary (CHO) cells for 2 hours on ice, unbound virus was removed by washing, and cell-associated HCV RNA was quantified. JFH-1 bound to B cells with a mean of between 2000 and 7000 copies of HCV RNA per million cells, compared with 76 000 genome copies bound to Huh-7.5 cells (Figure 1A). There was no detectable HCV RNA bound to CHO cells. To address whether HCV replicates in B cells the cultures were maintained, and viral RNA levels were quantified. HCV RNA increased 40-fold in Huh-7.5 cells over 3 days; however, there was no detectable increase in viral genomes in the B cells after 3 (Figure 1A) or 7 days (data not shown). The differential decay of HCV RNA in the different B cells did not associate with cell viability. Consistent with the nonreplicating nature of JFH-1 RNA in B lymphocytes, we failed to detect HCV antigen in virus-inoculated B cells cultured over time, whereas Huh-7.5 cells expressed core and NS5A antigens that were readily detectable by indirect immunofluorescence or flow cytometry (Figure 1B). These data show that B cells can bind HCV JFH-1; however, the genome failed to replicate, suggesting that B cells are not active sites for viral replication.
HCV can associate with B cells and transinfect hepatoma cells. (A) PBMC-derived primary B cells, Burkitt lymphoma B-cell lines L3055-Bcl-2 and KEM, and Huh-7.5 hepatoma cells were allowed to bind JFH-1 for 2 hours on ice. HCV RNA copy numbers were determined by quantitative RT-PCR, and the fold change in HCV RNA was defined after 72 hours. Error bars represent the SD from 3 or 4 replicates. (B) Cartoon depicting the protocol for measuring B-cell transinfection of Huh-7.5 cells. The image depicts a group of NS5A+ (green) infected Huh-7.5 cells, which are designated as FFUs. The Huh-7.5 cell monolayer and remaining B cells were visualized by DAPI, staining their large oval and small round nuclei, respectively. (C) Increasing numbers of PBMC-derived purified B cells and the corresponding B cell–depleted fraction, L3055–Bcl-2, or KEM were incubated with JFH-1 and cocultured with Huh-7.5 cells. Data represent the FFU in Huh-7.5 cells per stated number of cocultured lymphoid cells. (D) Effect of CD40L and IL-4 stimulation on PBMC-derived B cell (8 blood donors; BD1-8), L3055–Bcl-2 or KEM cell JFH-1 transinfection of Huh-7.5 cells. Resting ( ) and CD40L/IL-4–stimulated (□) cells are depicted. (E) CD40L/IL-4–stimulated PBMC and liver-derived B cells from 2 HCV-infected patients (P1 and P2) can transinfect Huh-7.5 cells with JFH-1. Error bars represent the SEM from 3 replicate infections.
) and CD40L/IL-4–stimulated (□) cells are depicted. (E) CD40L/IL-4–stimulated PBMC and liver-derived B cells from 2 HCV-infected patients (P1 and P2) can transinfect Huh-7.5 cells with JFH-1. Error bars represent the SEM from 3 replicate infections.
HCV can associate with B cells and transinfect hepatoma cells. (A) PBMC-derived primary B cells, Burkitt lymphoma B-cell lines L3055-Bcl-2 and KEM, and Huh-7.5 hepatoma cells were allowed to bind JFH-1 for 2 hours on ice. HCV RNA copy numbers were determined by quantitative RT-PCR, and the fold change in HCV RNA was defined after 72 hours. Error bars represent the SD from 3 or 4 replicates. (B) Cartoon depicting the protocol for measuring B-cell transinfection of Huh-7.5 cells. The image depicts a group of NS5A+ (green) infected Huh-7.5 cells, which are designated as FFUs. The Huh-7.5 cell monolayer and remaining B cells were visualized by DAPI, staining their large oval and small round nuclei, respectively. (C) Increasing numbers of PBMC-derived purified B cells and the corresponding B cell–depleted fraction, L3055–Bcl-2, or KEM were incubated with JFH-1 and cocultured with Huh-7.5 cells. Data represent the FFU in Huh-7.5 cells per stated number of cocultured lymphoid cells. (D) Effect of CD40L and IL-4 stimulation on PBMC-derived B cell (8 blood donors; BD1-8), L3055–Bcl-2 or KEM cell JFH-1 transinfection of Huh-7.5 cells. Resting ( ) and CD40L/IL-4–stimulated (□) cells are depicted. (E) CD40L/IL-4–stimulated PBMC and liver-derived B cells from 2 HCV-infected patients (P1 and P2) can transinfect Huh-7.5 cells with JFH-1. Error bars represent the SEM from 3 replicate infections.
) and CD40L/IL-4–stimulated (□) cells are depicted. (E) CD40L/IL-4–stimulated PBMC and liver-derived B cells from 2 HCV-infected patients (P1 and P2) can transinfect Huh-7.5 cells with JFH-1. Error bars represent the SEM from 3 replicate infections.
Recent reports show that B cells can transfer infectious HIV to T cells,28,29 and we hypothesized that B cell–associated HCV may be infectious for hepatoma cells. JFH-1 was incubated with PBMC-derived B cells, the B cell–depleted lymphocyte fraction from the same donor and L3055-Bcl-2 and KEM cells, unbound virus removed by washing, and the cells cocultured with Huh-7.5 cells. After 72 hours, the cocultured cells were fixed and stained for the nonstructural viral protein, NS5A. HCV infection of Huh-7.5 forms a focal area of NS5A+ cells that can be enumerated as FFUs (Figure 1B). Primary B cells and KEM transferred infectious virus to Huh-7.5 cells in a dose-dependent manner, whereas L3055–Bcl-2 and lymphocytes from B cell–depleted PBMCs failed to transfer virus (Figure 1C). Incubation of L3055–Bcl-2 with 5- and 10-fold greater levels of infectious JFH-1 resulted in a corresponding increase in FFUs in Huh-7.5, suggesting that the levels of virus used in these experiments are not saturating. B cells formed tight associations with the Huh-7.5 cell monolayer that persisted during the multiple wash steps of the fixation and staining protocol; however, NS5A+ B cells were not observed. B-cell transinfection varied between donors and was enhanced after their stimulation with CD40 ligand (CD40L) and IL-4 (Figure 1D). Neither treatment alone had an effect on transinfection. CD40L/IL-4 stimulated L3055–Bcl-2, and primary B cells transferred chimeric JFH viruses expressing the Core-NS2 region of H77 and J6 at comparable levels to that seen for JFH-1 (data not shown).
To ascertain whether B cells from chronically HCV-infected patients can transfer infectious JFH-1 to hepatoma cells, PBMCs and liver-derived B cells from 2 HCV-infected subjects were stimulated with CD40L and IL-4 and tested for their ability to transinfect Huh-7.5 cells. Peripheral and liver-derived cells from both patients transferred JFH-1 to Huh-7.5 at comparable levels to peripheral B cells from healthy donors (Figure 1E). To confirm that the virus replicating in Huh-7.5 cells was JFH-1, cocultures were performed with B cells not incubated with JFH-1, and transfer was not observed.
To study the mechanism(s) underlying the CD40L/IL-4–enhanced B-cell transinfection, we investigated the effect(s) of stimulation on L3055–Bcl-2 and KEM association with JFH-1. Stimulated L3055–Bcl-2 and KEM cells bound 4- and 2-fold greater levels of HCV RNA containing particles, respectively (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article), with a concomitant 32- and 5-fold increase in transinfection (Figure S1B), suggesting that additional parameters contribute to the increased frequency of B-cell transinfection.
Transfer of B cell–associated HCV to hepatoma cells is an active process
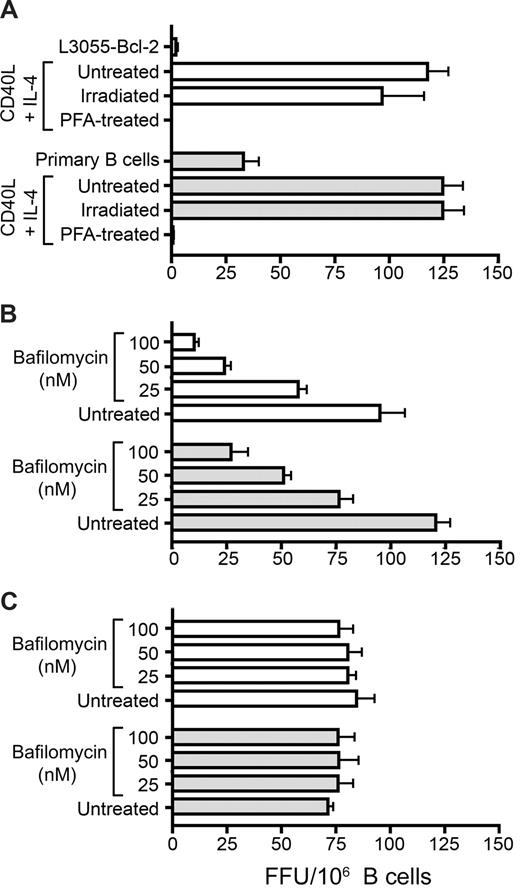
To address whether B cell–mediated transfer of virus is a passive or active process, we compared the effects of gamma irradiation and paraformaldehyde fixation on the transfer process. Irradiated or fixed cells were incubated with JFH-1 and cocultured with Huh-7.5 cells. Irradiated B cells transferred infection as efficiently as untreated cells. However, fixation abrogated this ability, indicating a need for active participation of the donor cell (Figure 2A). DC-SIGN–mediated transfer of HIV to T cells depends on the formation of a low pH endosomal compartment.30 We therefore tested the pH dependence of JFH-1 transinfection by incubating B cells with bafilomycin A1, a V-ATPase inhibitor that prevents acidification of endosomes before the addition of virus. Bafilomycin A1 inhibited transinfection in a dose-dependent manner (Figure 2B) but had no detectable effect on cell-free virus infectivity. B cells recovered their ability to transfer infection after 12 hours, showing the reversibility of inhibition (Figure 2C). These data suggest that B-cell transinfection is a pH-dependent active process requiring the trafficking of HCV particles within the cell.
B-cell transinfection is an active cellular process. (A) Untreated, gamma-irradiated, and paraformaldehyde-fixed CD40L/IL-4–stimulated L3055-Bcl-2 (□) and PBMC-derived ( ) B cells were incubated with JFH-1 and cocultured with Huh-7.5 cells, and transinfection was quantified. Stimulated L3055-Bcl-2 (□) or PBMC-derived (
) B cells were incubated with JFH-1 and cocultured with Huh-7.5 cells, and transinfection was quantified. Stimulated L3055-Bcl-2 (□) or PBMC-derived ( ) B cells were incubated with increasing concentrations of Bafilomycin A1, which prevents endosome acidification for 1 hour at 37°C and incubated with JFH-1 for 2 hours at 37°C immediately (B) or after a 12-hour recovery period (C). B cells were cocultured with Huh-7.5 cells and transinfection quantified. Error bars represent the SD of FFUs per 106 B cells from 3 replicate infections.
) B cells were incubated with increasing concentrations of Bafilomycin A1, which prevents endosome acidification for 1 hour at 37°C and incubated with JFH-1 for 2 hours at 37°C immediately (B) or after a 12-hour recovery period (C). B cells were cocultured with Huh-7.5 cells and transinfection quantified. Error bars represent the SD of FFUs per 106 B cells from 3 replicate infections.
B-cell transinfection is an active cellular process. (A) Untreated, gamma-irradiated, and paraformaldehyde-fixed CD40L/IL-4–stimulated L3055-Bcl-2 (□) and PBMC-derived ( ) B cells were incubated with JFH-1 and cocultured with Huh-7.5 cells, and transinfection was quantified. Stimulated L3055-Bcl-2 (□) or PBMC-derived (
) B cells were incubated with JFH-1 and cocultured with Huh-7.5 cells, and transinfection was quantified. Stimulated L3055-Bcl-2 (□) or PBMC-derived ( ) B cells were incubated with increasing concentrations of Bafilomycin A1, which prevents endosome acidification for 1 hour at 37°C and incubated with JFH-1 for 2 hours at 37°C immediately (B) or after a 12-hour recovery period (C). B cells were cocultured with Huh-7.5 cells and transinfection quantified. Error bars represent the SD of FFUs per 106 B cells from 3 replicate infections.
) B cells were incubated with increasing concentrations of Bafilomycin A1, which prevents endosome acidification for 1 hour at 37°C and incubated with JFH-1 for 2 hours at 37°C immediately (B) or after a 12-hour recovery period (C). B cells were cocultured with Huh-7.5 cells and transinfection quantified. Error bars represent the SD of FFUs per 106 B cells from 3 replicate infections.
To investigate whether B cell–associated virus is infectious for extended time periods, CD40L/IL-4–stimulated PBMC-derived B cells from 2 donors (BD1 and 2) and L3055–Bcl-2 were incubated with JFH-1 at 37°C for 2 hours and monitored for their ability to infect Huh-7.5 cells over an 8-hour period. L3055–Bcl-2 transinfection declined over time, showing a similar kinetics to cell-free particle infectivity (Figure S2). However, primary B cell–associated viral infectivity appeared more stable, showing a minimal decline after 4 hours (Figure S2).
CD40L/IL-4 and HCV promote B-lymphocyte association with Huh-7.5 hepatoma cells
We previously reported that CD40 ligation of L3055–Bcl-2 cells increased ICAM-1/CD54 expression,31 which may promote their adhesion to hepatoma cells. To investigate further, resting and CD40L/IL-4–stimulated L3055–Bcl-2 and PBMC-derived B cells from 7 healthy donors were labeled with CMFDA and assessed for their ability to adhere to Huh-7.5 cells. Stimulation increased L3055–Bcl-2 and primary B-cell adhesion to Huh-7.5 cells (Figure 3A), but in a manner that was not predictive of the high increase in transinfection observed after stimulation. Thus, it is likely that multiple parameters contribute to the increased efficiency of CD40L/IL-4–stimulated B cells to transinfect hepatoma cells. The ability of B cells to transmit infection to hepatocytes is likely to require close interactions between the 2 cell types. This led us to investigate whether HCV modulates the ability of B lymphocytes to bind hepatoma cells. Resting and CD40L/IL-4–stimulated B cells were incubated with JFH-1, and their adhesion to Huh-7.5 cells was quantified. Naive peripheral blood B cells from all 7 donors tested showed an increased ability to bind Huh-7.5 after exposure to HCV (Figure 3B).
Effect(s) of CD40/IL-4 and HCV on B-cell adhesion to Huh-7.5 hepatoma cells. Untreated and CD40L/IL-4–stimulated L3055-Bcl-2 (□) and PBMC-derived ( ) B cells were labeled with CMFDA and added to a confluent monolayer of Huh-7.5 cells for 15 minutes at 37°C. Nonbound cells were removed by gentle washing, and the adherent cells were enumerated (A). Alternatively, PBMC-derived B cells from 7 healthy donors were incubated with mock media or JFH-1 overnight, labeled with CMFDA, and quantified for their adhesion to Huh-7.5 cells (B). The data are presented as adhered lymphocytes per 10 000 Huh-7.5 cells. The first panel depicts the median values for mock (○) and HCV-exposed B cells (●), and the second panel indicates the response per donor and shows a significant increase in binding after HCV exposure (P = .0156, nonparametric paired t test [Wilcoxon]).
) B cells were labeled with CMFDA and added to a confluent monolayer of Huh-7.5 cells for 15 minutes at 37°C. Nonbound cells were removed by gentle washing, and the adherent cells were enumerated (A). Alternatively, PBMC-derived B cells from 7 healthy donors were incubated with mock media or JFH-1 overnight, labeled with CMFDA, and quantified for their adhesion to Huh-7.5 cells (B). The data are presented as adhered lymphocytes per 10 000 Huh-7.5 cells. The first panel depicts the median values for mock (○) and HCV-exposed B cells (●), and the second panel indicates the response per donor and shows a significant increase in binding after HCV exposure (P = .0156, nonparametric paired t test [Wilcoxon]).
Effect(s) of CD40/IL-4 and HCV on B-cell adhesion to Huh-7.5 hepatoma cells. Untreated and CD40L/IL-4–stimulated L3055-Bcl-2 (□) and PBMC-derived ( ) B cells were labeled with CMFDA and added to a confluent monolayer of Huh-7.5 cells for 15 minutes at 37°C. Nonbound cells were removed by gentle washing, and the adherent cells were enumerated (A). Alternatively, PBMC-derived B cells from 7 healthy donors were incubated with mock media or JFH-1 overnight, labeled with CMFDA, and quantified for their adhesion to Huh-7.5 cells (B). The data are presented as adhered lymphocytes per 10 000 Huh-7.5 cells. The first panel depicts the median values for mock (○) and HCV-exposed B cells (●), and the second panel indicates the response per donor and shows a significant increase in binding after HCV exposure (P = .0156, nonparametric paired t test [Wilcoxon]).
) B cells were labeled with CMFDA and added to a confluent monolayer of Huh-7.5 cells for 15 minutes at 37°C. Nonbound cells were removed by gentle washing, and the adherent cells were enumerated (A). Alternatively, PBMC-derived B cells from 7 healthy donors were incubated with mock media or JFH-1 overnight, labeled with CMFDA, and quantified for their adhesion to Huh-7.5 cells (B). The data are presented as adhered lymphocytes per 10 000 Huh-7.5 cells. The first panel depicts the median values for mock (○) and HCV-exposed B cells (●), and the second panel indicates the response per donor and shows a significant increase in binding after HCV exposure (P = .0156, nonparametric paired t test [Wilcoxon]).
HCV-immune complexes and B cells
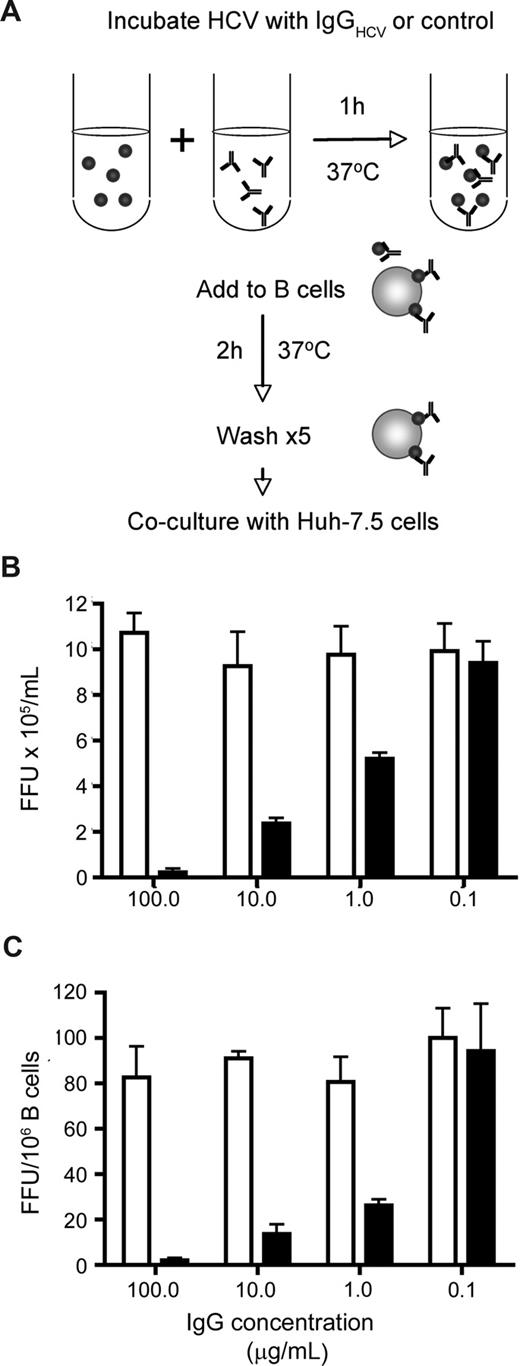
Antibodies specific for the glycoproteins of HIV and Dengue virus enhance infection of B cells and monocytes by Fc receptor uptake of immune complexes.32,33 In the case of Dengue virus, antibodies that saturate the particle neutralize infectivity, whereas subsaturating levels of antibody enhance viral infectivity.33 To investigate the effect(s) of HCV-specific antibodies on JFH-1 B-cell transinfection, virus was incubated with increasing concentrations of purified IgG from healthy and HCV-infected subjects (IgGHCV). The virus-IgG complexes were incubated with stimulated L3055–Bcl-2 cells for 2 hours, unbound virus was removed by washing, and the cells were cocultured with Huh-7.5. To assess the neutralizing activity of IgG for cell-free virus, immune complexes were tested for infection of Huh-7.5 cells. IgGHCV showed a dose-dependent inhibition of L3055–Bcl-2 associated and cell-free JFH-1 infectivity, with no detectable enhancement of transinfection at low concentrations (Figure 4).
HCV-immune complexes and B-cell association. HCV JFH-1 was incubated with increasing concentrations of IgG purified from healthy and HCV-infected subjects at 37°C for 1 hour. Virus-immune complexes were mixed with CD40L/IL-4–stimulated L3055–Bcl-2 cells at 37°C for 2 hours, unbound complexes were removed by washing, the cells were cocultured with Huh-7.5, and transinfection was quantified (depicted in A). The infectivity of cell-free JFH-1–immune complexes was tested by inoculating Huh-7.5 cells, and the data were expressed as FFU/mL (B). FFU per 106 B cells were calculated (C). Error bars represent the SD from 3 replicate infections.
HCV-immune complexes and B-cell association. HCV JFH-1 was incubated with increasing concentrations of IgG purified from healthy and HCV-infected subjects at 37°C for 1 hour. Virus-immune complexes were mixed with CD40L/IL-4–stimulated L3055–Bcl-2 cells at 37°C for 2 hours, unbound complexes were removed by washing, the cells were cocultured with Huh-7.5, and transinfection was quantified (depicted in A). The infectivity of cell-free JFH-1–immune complexes was tested by inoculating Huh-7.5 cells, and the data were expressed as FFU/mL (B). FFU per 106 B cells were calculated (C). Error bars represent the SD from 3 replicate infections.
B cells express HCV receptors: SR-BI and DC-SIGN mediate transinfection
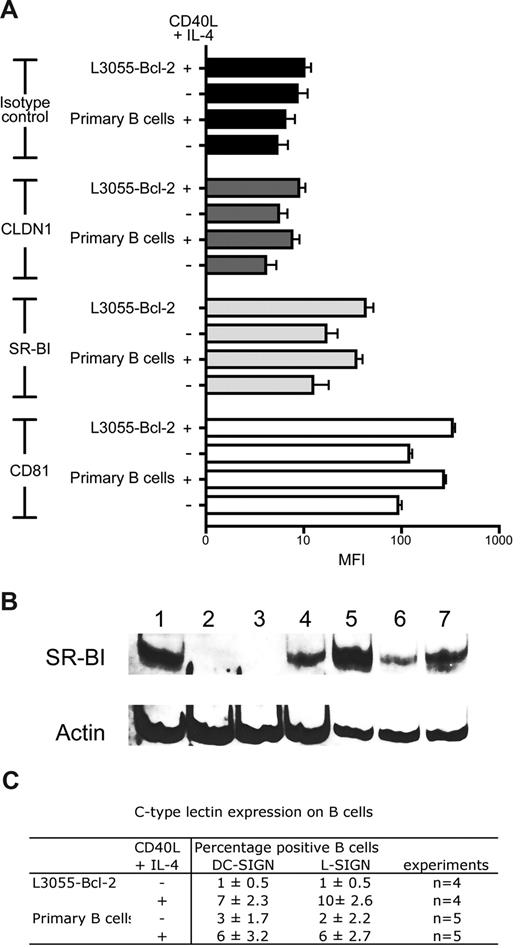
To investigate the mechanism(s) defining HCV interaction with B cells, we screened resting and CD40L/IL-4–stimulated cells for viral receptor expression. As previously described, B cells express CD81 that is up-regulated after stimulation. CLDN1 expression was undetectable on resting or stimulated primary or B-cell lines. In contrast to recently published data,34 L3055–Bcl-2 and primary B cells expressed SR-BI, and CD40L/IL-4 stimulation increased expression levels (Figure 5A). SR-BI expression was confirmed with an independent antibody that reacted with SDS-denatured protein lysates by Western blotting (Figure 5B). DC-SIGN/L-SIGN expression varied between donors as previously reported.28 Less than 3% of primary B cells expressed DC-SIGN and L-SIGN, and this increased to 3% to 9% after stimulation (Figure 5C). Unstimulated L3055–Bcl-2 cells did not express detectable levels of DC-SIGN/L-SIGN at the cell surface; however, CD40L/IL-4 stimulation increased expression, with 8% and 10% of cells staining positive for DC-SIGN and L-SIGN, respectively (Figure 5C).
B cells express CD81, SR-BI, and C-type lectins DC-SIGN and L-SIGN. (A) HCV viral receptor expression on untreated or CD40L/IL-4–stimulated PBMC-derived primary B cells, and L3055–Bcl-2 was characterized by flow cytometry. Median fluorescence intensity (MFI) values are plotted for isotype controls (■), CLDN1 ( ), SR-BI (
), SR-BI ( ), and CD81 (□) as an average from 3 tests, with error bars showing the SD (B) 20 μg total cellular protein from Huh-7.5 (lane 1); B cell–depleted fraction from BD1 (lane 2) and BD2 (lane 3); resting (lane 4) and CD40L/IL-4–stimulated PBMC-derived B cells (lane 5) from BD1; resting (lane 6) and CD40L/IL-4–stimulated PBMC-derived B cells (lane 7) from BD2 were separated by SDS-PAGE; the proteins were transferred to PVDF membranes and probed for SR-BI and actin expression. (C) Summary table of DC-SIGN/L-SIGN expression in untreated and CD40L/IL-4–stimulated PBMC-derived primary B cells and L3055–Bcl-2.
), and CD81 (□) as an average from 3 tests, with error bars showing the SD (B) 20 μg total cellular protein from Huh-7.5 (lane 1); B cell–depleted fraction from BD1 (lane 2) and BD2 (lane 3); resting (lane 4) and CD40L/IL-4–stimulated PBMC-derived B cells (lane 5) from BD1; resting (lane 6) and CD40L/IL-4–stimulated PBMC-derived B cells (lane 7) from BD2 were separated by SDS-PAGE; the proteins were transferred to PVDF membranes and probed for SR-BI and actin expression. (C) Summary table of DC-SIGN/L-SIGN expression in untreated and CD40L/IL-4–stimulated PBMC-derived primary B cells and L3055–Bcl-2.
B cells express CD81, SR-BI, and C-type lectins DC-SIGN and L-SIGN. (A) HCV viral receptor expression on untreated or CD40L/IL-4–stimulated PBMC-derived primary B cells, and L3055–Bcl-2 was characterized by flow cytometry. Median fluorescence intensity (MFI) values are plotted for isotype controls (■), CLDN1 ( ), SR-BI (
), SR-BI ( ), and CD81 (□) as an average from 3 tests, with error bars showing the SD (B) 20 μg total cellular protein from Huh-7.5 (lane 1); B cell–depleted fraction from BD1 (lane 2) and BD2 (lane 3); resting (lane 4) and CD40L/IL-4–stimulated PBMC-derived B cells (lane 5) from BD1; resting (lane 6) and CD40L/IL-4–stimulated PBMC-derived B cells (lane 7) from BD2 were separated by SDS-PAGE; the proteins were transferred to PVDF membranes and probed for SR-BI and actin expression. (C) Summary table of DC-SIGN/L-SIGN expression in untreated and CD40L/IL-4–stimulated PBMC-derived primary B cells and L3055–Bcl-2.
), and CD81 (□) as an average from 3 tests, with error bars showing the SD (B) 20 μg total cellular protein from Huh-7.5 (lane 1); B cell–depleted fraction from BD1 (lane 2) and BD2 (lane 3); resting (lane 4) and CD40L/IL-4–stimulated PBMC-derived B cells (lane 5) from BD1; resting (lane 6) and CD40L/IL-4–stimulated PBMC-derived B cells (lane 7) from BD2 were separated by SDS-PAGE; the proteins were transferred to PVDF membranes and probed for SR-BI and actin expression. (C) Summary table of DC-SIGN/L-SIGN expression in untreated and CD40L/IL-4–stimulated PBMC-derived primary B cells and L3055–Bcl-2.
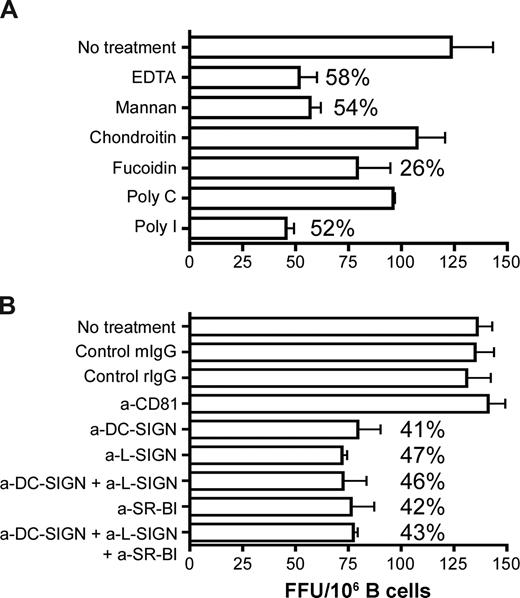
To study the role of C-type lectins, CD81 and SR-BI in HCV association and B-cell transinfection, we screened a range of inhibitors (Figure 6A) and blocking antibodies (Figure 6B) for their effect(s) on CD40L/IL-4–stimulated primary B-cell transinfection. B cells were treated with the inhibitors and receptor specific antibodies at concentrations shown to saturate the cell surface for 1 hour and then incubated with JFH-1 for 2 hours at 37°C. Unbound virus and antibodies were removed by extensive washing, and the cells were cocultured with Huh-7.5 cells for 72 hours before staining for NS5A+ cells. The broad range scavenger receptor inhibitors, fucoidin and poly-I (Figure 6A) as well as the specific anti–SR-BI antibody reduced transinfection by up to 50%.
The role of SR-BI and lectins in HCV association and B-cell transinfection. CD40L/IL-4–stimulated PBMC-derived primary B cells were incubated with (A) scavenger receptor inhibitors fucoidin and poly-I or the respective control molecules chondroitin or poly-C, mannan, or EDTA for 1 hour at 37°C and (B) anti-CD81, anti–SR-BI, anti–DC-SIGN/L-SIGN, and control antibodies (10 μg/mL) for 1 hour on ice. B cells were washed to remove inhibitors and unbound antibodies and incubated with JFH-1 for 2 hours at 37°C, the virus was removed by washing, the cells were cocultured with Huh-7.5, and transinfection was quantified. Data represent the SD FFUs per 106 B cells from 3 replicate infections. Percentage inhibition of transinfection compared with control molecules or isotype control antibodies are noted for each treatment.
The role of SR-BI and lectins in HCV association and B-cell transinfection. CD40L/IL-4–stimulated PBMC-derived primary B cells were incubated with (A) scavenger receptor inhibitors fucoidin and poly-I or the respective control molecules chondroitin or poly-C, mannan, or EDTA for 1 hour at 37°C and (B) anti-CD81, anti–SR-BI, anti–DC-SIGN/L-SIGN, and control antibodies (10 μg/mL) for 1 hour on ice. B cells were washed to remove inhibitors and unbound antibodies and incubated with JFH-1 for 2 hours at 37°C, the virus was removed by washing, the cells were cocultured with Huh-7.5, and transinfection was quantified. Data represent the SD FFUs per 106 B cells from 3 replicate infections. Percentage inhibition of transinfection compared with control molecules or isotype control antibodies are noted for each treatment.
DC-SIGN/L-SIGN interaction with viral glycoproteins depends on bivalent cations and can be competed with mannan. Pretreatment of B cells with the chelating agent EDTA or mannan reduced transinfection by 58% and 54%, respectively, and lectin-specific blocking antibodies reduced infection by comparable levels (Figure 6). Simultaneous incubation of B cells with both anti–SR-BI and anti–lectin antibodies did not increase the inhibitory activity above that observed when either antibody was tested alone. Anti–SR-BI and DC-SIGN/L-SIGN inhibition was dose dependent, showing negligible activity at subsaturating levels. Anti-CD81 and control antibodies had no effect on JFH-1 transinfection. Data for CD40L/IL-4–stimulated primary B cells are shown; similar levels of inhibition were observed with stimulated liver-derived and L3055–Bcl-2 B cells.
B cell–associated HCV acquires resistance to trypsin and neutralizing antibodies
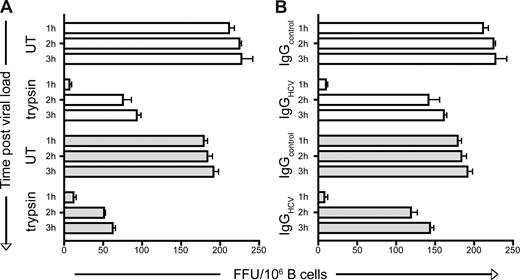
To address whether HCV enters B cells, we investigated the sensitivity of cell-associated virus to trypsin proteolysis and neutralizing antibodies (nAbs). CD40L/IL-4–stimulated L3055–Bcl-2 and primary B cells were incubated with JFH-1 for increasing periods of time at 37°C before treating with trypsin or purified IgG from healthy or HCV-infected subjects. Cells were extensively washed to remove neutralizing agents before coculturing with Huh-7.5 cells. After 1 hour, B cell–associated virus was sensitive to both trypsin and nAbs. However, by 2 hours resistance to both agents was noted, consistent with the internalization of infectious virus (Figure 7). Prior treatment of the B cells with trypsin had no effect on their ability to transfer JFH-1 infection or to adhere to Huh-7.5 cells, suggesting that the protection of cell-associated virus from trypsin at 37°C is due to particle internalization. Cell-free virus was sensitive to the effects of trypsin, showing a 90% decline in infectivity over 30 minutes (data not shown). The ability of B cell–associated HCV to resist nAbs and yet remain infectious for hepatoma cells provides a potential niche for the virus to evade immune surveillance.
B cell–associated JFH-1 acquires resistance to trypsin and nAbs. CD40L/IL-4–stimulated L3055–Bcl-2 (□) and PBMC-derived primary ( ) B cells bound JFH-1 were incubated for 1, 2, or 3 hours at 37°C and untreated (UT) or treated with trypsin (2.5 μg/mL) (A), control, or HCV+ IgG (200 μg/mL) (B) for 5 and 15 minutes, respectively. Enzyme and unbound IgG was removed by extensive washing, the B cells were cocultured with Huh-7.5 cells, and transinfection was quantified. Data represent the SEM FFUs per 106 B cells from 3 replicate infections.
) B cells bound JFH-1 were incubated for 1, 2, or 3 hours at 37°C and untreated (UT) or treated with trypsin (2.5 μg/mL) (A), control, or HCV+ IgG (200 μg/mL) (B) for 5 and 15 minutes, respectively. Enzyme and unbound IgG was removed by extensive washing, the B cells were cocultured with Huh-7.5 cells, and transinfection was quantified. Data represent the SEM FFUs per 106 B cells from 3 replicate infections.
B cell–associated JFH-1 acquires resistance to trypsin and nAbs. CD40L/IL-4–stimulated L3055–Bcl-2 (□) and PBMC-derived primary ( ) B cells bound JFH-1 were incubated for 1, 2, or 3 hours at 37°C and untreated (UT) or treated with trypsin (2.5 μg/mL) (A), control, or HCV+ IgG (200 μg/mL) (B) for 5 and 15 minutes, respectively. Enzyme and unbound IgG was removed by extensive washing, the B cells were cocultured with Huh-7.5 cells, and transinfection was quantified. Data represent the SEM FFUs per 106 B cells from 3 replicate infections.
) B cells bound JFH-1 were incubated for 1, 2, or 3 hours at 37°C and untreated (UT) or treated with trypsin (2.5 μg/mL) (A), control, or HCV+ IgG (200 μg/mL) (B) for 5 and 15 minutes, respectively. Enzyme and unbound IgG was removed by extensive washing, the B cells were cocultured with Huh-7.5 cells, and transinfection was quantified. Data represent the SEM FFUs per 106 B cells from 3 replicate infections.
Discussion
We show that JFH-1 and chimeric particles bearing J6 and H77 structural proteins can bind primary and immortalized B cells, consistent with reports detailing HCV RNA association with B cells in the peripheral blood.9 B cells bound 10- to 35-fold less JFH-1 RNA-containing particles than did the permissive Huh-7.5 cell line. All 3 viral strains failed to replicate and establish a productive infection in the B cells tested, showing the lack of association between viral RNA particle binding and replication. Boisvert et al35 reported the detection of positive-strand HCV RNA but not replicative intermediate negative-strand RNA with B cells from HCV-infected patients, supporting our data that HCV associates with B cells but fails to replicate in this cell type. B cell–associated JFH-1 is infectious for hepatoma cells, and stimulation of B cells with CD40L and IL-4 increased infectivity (Figure 1D). In these experiments the specific infectivity (ratio of infectious virus particles [assessed by counting FFUs] to genomic RNA [determined by quantitative RT-PCR]) of cell-free JFH-1 varied from 1/4500 to 1/12 000, whereas the specific infectivity of stimulated L3055–Bcl-2 virus was 1/250, suggesting that B cell–mediated delivery of HCV is an efficient route to infect hepatoma cells.
The rapid loss of cell-free virus infectivity was delayed by several hours by incubating JFH-1 with stimulated primary B cells (Figure S1), suggesting that viral sequestration by B cells in vivo may promote viral persistence. Similar reports show that B cell–associated Epstein-Barr virus27 and macrophage-associated HIV virus36 are more infectious for epithelial and T-cell targets, respectively, than cell-free virus.
CD40L/IL-4–stimulated B cells from the explanted liver and peripheral blood of 2 HCV-infected patients were able to capture JFH-1 and transinfect Huh-7.5 cells (Figure 1E) at comparable efficiencies to B cells from healthy donors. Coculture of B cells from HCV-infected patients did not promote replication of the patients' endogenous virus, which may reflect the reduced permissiveness of Huh-7.5 cells to clinical strains of HCV. Alternatively, given the unstable nature of in vitro–generated HCV particles (Figure S2), patient-derived B cell–associated virus may have degraded during the stimulation procedure.
The ability of primary and B-cell lines to transmit HCV increased after CD40L/IL-4 stimulation, concomitant with increases in CD81 and SR-BI expression (Figure 5A), DC-SIGN and L-SIGN expression (Figure 5C), HCV particle association (Figure S1A), and adhesion to Huh-7.5 cells (Figure 3). All of these processes are likely to be important in defining the efficiency of HCV transinfection.
We found that B cells expressed CD81 but not CLDN1 (Figure 5). In contrast to a previous report,34 greater than 90% of cells analyzed expressed SR-BI by flow cytometry and Western blotting (Figure 5). Consistent with other findings,28 a small proportion of B cells expressed DC-SIGN and L-SIGN (Figure 5C), which increased after CD40L/IL-4 stimulation. To explore the role of CD81, SR-BI, and C-type lectins in B-cell binding to HCV, we tested a range of inhibitors and specific antibodies for their effect on B-cell transinfection (Figure 6). The scavenger receptor inhibitors poly-I and fucoidin and polyclonal anti–SR-BI sera reduced transinfection by 50%, suggesting a role for SR-BI in particle binding. SR-BI has been reported to be an important factor defining HCV particle association with liver-derived cells37 and more recently for the internalization and processing of HCV-like particles by dendritic cells.34,38
Similar levels of inhibition were observed with EDTA, mannan, and antibodies specific for DC-SIGN and L-SIGN, both alone and in combination (Figure 6). Although DC-SIGN and L-SIGN expression are not sufficient to render a cell permissive to HCV entry, they have been reported to capture particles and transfer HCVpp to hepatoma cells.22 DC-SIGN expression at the B-cell surface may be transient, with a greater proportion of tonsillar B cells expressing DC-SIGN than cells from the peripheral blood.39 B cells in the parafollicular area(s) of lymphatic tissue express varying levels of DC-SIGN, and expression is stimulated by B cell–activating factor (BAFF).39 A recent report showing increased levels of BAFF in HCV-infected subjects suggests a mechanism for the expansion of DC-SIGN/L-SIGN+ cells in the periphery.40 Several viruses such as Ebola, severe acute respiratory syndrome coronavirus, HIV, Dengue, and Sindbis have been reported to use DC-SIGN to transinfect permissive cell types (reviewed in Cambi et al41 ).
B cells are activated through engagement of the surface Ig/B-cell antigen receptor complex and coreceptor comprising CD81, CD19, and CD21 molecules. Costimulation lowers the threshold for B-cell activation and proliferation. Several laboratories have hypothesized that HCV ligation of CD81 may perturb B-cell proliferation.12 However, anti-CD81 antibodies had no effect on the association or B-cell transfer of JFH-1 to Huh-7.5 cells (Figure 6B), suggesting a minimal role for CD81 in this process. B cell–depleted peripheral blood lymphocytes, which express high levels of CD81, and the monocytic cell line U937 with or without CD81 expression (data not shown) failed to transfer virus to Huh-7.5 cells, lending further support to this conclusion. CD81 expression is essential for HCV infection of hepatoma cells, and reports that CD81-specific antibodies and recombinant soluble forms of CD81 inhibit cell-associated particle infectivity suggest a role for CD81 postvirus attachment.42 Recent data highlighting the importance of CD81-CLDN1 complexes in HCV particle internalization offer a potential explanation for the minimal role of CD81 in B-cell association with HCV.43
To investigate whether HCV is internalized by B cells we compared the sensitivity of B cell–associated virus with the proteolytic effects of trypsin or nAbs. Incubation of cell-associated virus at 37°C for 2 hours or more protected the virus from trypsin and nAbs, suggesting particle internalization. The partial resistance to trypsin (Figure 7A) may reflect multiple pathways for B-cell transfer of HCV to hepatocytes, some of which may depend on virus internalization. Alternatively, trypsin may affect other undefined aspects of the cellular machinery that mediate transinfection. The observation that bafilomycin A1, an ATPase inhibitor that prevents acidification of endosomal structures, reduced B-cell transinfection in a dose-dependent manner lends further support for a pH-dependent internalization process of HCV (Figure 2B).
The transfer of virus from B cells to hepatocytes is likely to require a close interaction between the 2 cell types, and our data suggest that viral infection may promote this interaction. HCV significantly increased B-cell adherence to Huh-7.5 cells in all donors studied (Figure 3B). HCV promotion of B-cell adhesion to hepatoma cells may explain why lymphoid aggregates containing B cells are found in the liver parenchyma in chronic HCV infection7,8 and provide a mechanism to stabilize B-cell/hepatocyte interactions allowing viral transmission between the 2 cell types.
Sequestration of virus by B lymphocytes in vivo is potentially important for several reasons: first, B cells have the ability to move through all compartments of the body, including the liver, thereby facilitating viral transmission; second, the pool of virus that binds B cells may be large, with 0.5 to 2.0 × 106 total B cells in the peripheral blood, representing approximately 2% of the total B cells in the body. The resistance of B cell–associated virus to neutralization by viral-specific antibodies provides a mechanism for HCV immune evasion and thus persistence in the periphery and tissue-associated B cells. Under appropriate conditions B cell–associated virus could traffic to the liver and transinfect hepatocytes by crossing sinusoidal endothelium or by direct interactions with hepatocyte extensions that protrude through endothelial fenestrations into the sinusoidal lumen.44 Such a mechanism may be particularly important after liver transplantation, when preservation/reperfusion injury of the donor liver may facilitate interactions with circulating lymphocytes.45
In conclusion, this study shows that peripheral blood B cells can bind, internalize, and transfer infectious JFH-1 to hepatoma cells. Although we found no evidence for B cells supporting HCVcc replication, we show that JFH-1 significantly increased the ability of naive healthy blood B cells to associate with hepatoma cells in vitro, which may have important implications for viral transmission and persistence.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Takaji Wakita for JFH-1; Charles Rice for Huh-7.5 cells, J6/JFH, H77/JFH, and anti-NS5A 9E10 mAb; Joe Grove for purified HCV-specific IgG; and Fedor Berditchevski for anti-CD81 mAb M38. We thank Michelle Farquhar and Jennifer Timpe for critical reading of the manuscript.
This work was supported by Public Health Service (PHS) (grants AI50798 and AI40034-14), the Medical Research Council (MRC), and the Wellcome Trust.
National Institutes of Health
Wellcome Trust
Authorship
Contribution: Z.S. designed and performed research and wrote the paper; C.S-L., J.S., and D.M. provided essential reagents; A.B.R., J.G., and D.H.A. helped design the research; and P.B. and J.A.M. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jane A. McKeating, Institute for Biomedical Research, University of Birmingham, Birmingham B15 2TT, United Kingdom; e-mail: j.a.mckeating@bham.ac.uk.

![Figure 3. Effect(s) of CD40/IL-4 and HCV on B-cell adhesion to Huh-7.5 hepatoma cells. Untreated and CD40L/IL-4–stimulated L3055-Bcl-2 (□) and PBMC-derived () B cells were labeled with CMFDA and added to a confluent monolayer of Huh-7.5 cells for 15 minutes at 37°C. Nonbound cells were removed by gentle washing, and the adherent cells were enumerated (A). Alternatively, PBMC-derived B cells from 7 healthy donors were incubated with mock media or JFH-1 overnight, labeled with CMFDA, and quantified for their adhesion to Huh-7.5 cells (B). The data are presented as adhered lymphocytes per 10 000 Huh-7.5 cells. The first panel depicts the median values for mock (○) and HCV-exposed B cells (●), and the second panel indicates the response per donor and shows a significant increase in binding after HCV exposure (P = .0156, nonparametric paired t test [Wilcoxon]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/3/10.1182_blood-2008-05-158824/5/m_zh80240828430003.jpeg?Expires=1771282897&Signature=f-SjmtfnbxKNFPH~BFZsF19X4~rDybJunlw2errAjbKUhzCHJ~-A~T78Ax9uNmEF34xtFOl79KrpixZNE3JfMU5Z23twyz05q6-XhON9uUCRn2dfRPY2q9ML6eupLpAp~Ze2tu-aW20LVWIRWPic3iXIasHTrTyYuNTIAcs9QDECMjpPClz4Q0D1Jjfyc~-KKoYqDPIijaOriRStogH0BijhVL~9HCj7Sk4bIdi3luwtoIBv4d54UUq4zjt1vNUMjDA7eeHCDFqErajTMm~YdFmZK2uVEluxDxjwDw9uCF1x6kkEhPxQVoFRhqUifMpZZqkvmtJHGt-4z2PA8LHV3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)