Abstract
We screened for surface proteins expressed only by the early progenitor cells present in low-passage, low-density cultures of the adult stem/progenitor cells from bone marrow referred to as mesenchymal stem cells or multipotent stromal cells (MSCs). Six proteins were identified that were selectively expressed in the early progenitors: podocalyxin-like protein (PODXL), α6-integrin (CD49f), α4-integrin (CD49d), c-Met, CXCR4, and CX3CR1. All were previously shown to be involved in cell trafficking or tumor progression. Antibodies to CD49f and PODXL, a sialomucin in the CD34 family, were the most robust for FACScan assays. PODXLhi/CD49fhi MSCs were more clonogenic and differentiated more efficiently than PODXLlo/CD49flo cells. Inhibition of expression of PODXL with RNA interference caused aggregation of the cells. Furthermore, PODXLhi/CD49fhi MSCs were less prone to produce lethal pulmonary emboli, and larger numbers were recovered in heart and kidney after intravenous infusion into mice with myocardial infarcts.
Introduction
Among the cells being used for cell therapies for nonhematopoietic tissues are the stem/progenitor cells from bone marrow that were referred to initially as fibroblast colony-forming units,1 subsequently as marrow stromal cells, then as mesenchymal stem cells2 and, most recently, as multipotent mesenchymal stromal cells or MSCs.3 Clinical trials with MSCs are now in progress,4-9 but several questions are still unresolved as to how the cells should be isolated, expanded in culture, and characterized. One view is that confluent cultures of MSCs (Figure 1A) are useful and perhaps the optimal preparations for therapy. An opposing view is that confluent cultures of MSCs are partially committed to differentiation or even senescence. Therefore, they lack some of the therapeutic potentials of low-density cultures that contain a subpopulation of rapidly self-replicating cells10-13 that display a different pattern of expressed genes,14 that have a greater capacity to generate single-cell–derived clones,10,11 and that more efficiently engraft in vivo.15
MSCs were originally described by Friedenstein et al1 and Owen and Friedenstein,16 who isolated the cells by their ready adherence to tissue culture surfaces, an isolation technique that subsequently was followed by most investigators.17 The cells were characterized primarily by their ability to generate colonies in culture and to differentiate into adipocytes, osteoblasts, and chondrocytes. Numerous attempts were made to develop more specific procedures for isolation and characterization of the cells by preparing antibodies to surface epitopes on MSC. The first antibody was the monoclonal immunoglobulin M antibody STRO-1, which was raised against confluent cultures of human MSCs that were used as feeder layers for hematopoietic stem cells.18 STRO-1 alone or in combination with other antibodies subsequently was used extensively to identify and isolate MSCs.19-26 Also, a series of additional monoclonal antibodies were prepared to MSCs.27-30 In addition, antibodies initially prepared to other cell types were used to characterize MSCs.31-35
Although the published antibodies to MSCs are useful, none distinguishes 2 major subpopulations that are present in early-passage human MSCs plated at low density: (1) spindle-shaped and rapidly self-renewing cells referred to as type I cells13 or as RS-MSCs,11 and (2) larger, slowly replicating type II cells or SR-MSCs that arise from type I or RS-MSCs as the cultures expand to confluency. Recently, we searched for antibodies to surface proteins that identify early progenitors in cultures of MSCs. We found 6 informative antibodies to proteins that were previously linked to cell trafficking and tumor progression.
Methods
Isolation and culture of human MSCs
MSCs from bone marrow aspirates were obtained from the National Institutes of Health (NIH)/National Center for Research Resources (NCRR)–funded Tulane Center for the Preparation and Distribution of Adult Stem Cells (http://www.som.tulane.edu/gene_therapy/distribute.shtml). In brief, the MSCs were prepared from 2- to 4-mL bone marrow aspirates of the iliac crest of normal adult volunteers as described previously (Table S1 and Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).10,36 Passage 1 MSCs from 8 different normal donors were used: donor 1 (no. 7009), donor 2 (no. 240L), donor 3 (no. 5064L), donor 4 (no. 5068L), donor 5 (no. 220R), donor 6 (no. 109L), donor 7 (no. 5066R), and donor 8 (no. 281).
Assays of mRNAs by microarrays
Viable passage 1 MSCs (donor 6) were plated at 100 cells/cm2 and incubated for 5, 10, or 15 days with change of medium every 2 to 3 days. RNA was isolated from 3 × 106 MSCs with the use of a commercial kit (High Pure RNA Isolation Kit; Roche Diagnostics, Indianapolis, IN). Approximately 8 μg total RNA was processed as described previously.37 Microarray chips that contained 22 283 oligonucleotides for 14 844 human genes (HG-U133A; Affymetrix, Santa Clara, CA) were analyzed with Microarray Suite 5.0 (MASS 5.0; Affymetrix) and dChip 1.3+ programs.38 All microarray databases had been deposited in Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE12800).
Reverse transcriptase–polymerase chain reaction assays
RNA was isolated from 106 cells and used to perform reverse-transcriptase polymerase chain reaction (RT-PCR) assays with a commercial kit (SuperScript III FirstStrand Synthesis System; Invitrogen, Carlsbad, CA). The cDNAs were amplified by PCR (recombinant Taq DNA polymerase; Invitrogen) with 30 cycles at 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. The primers designed by PrimerQuest software (Integrated DNA Technologies, Coralville, IA) were as follows: PODXL forward, 5′-TTT TAC TCT TGC CCT CTC-3′; PODXL reverse, 5′-CTT TCT TTC TGC CAA GAA AC-3′; α6-integrin forward, 5′-AAG GCT CCT GTT TTG CAC AG-3′; α6-integrin reverse, 5′-ATG TAA GTC AGC CAC GCC A-3′; α4-integrin forward, 5′-GGG TAG TGG CCG TTT AGT GTT GAA-3′; α4-integrin reverse, 5′-ATT GAT CAC TGA AGC GTT GGC GAG-3′; c-Met forward, 5′-CCA TGT AGG AGA GCC TTA GT-3′; and c-Met reverse, 5′-GTC TAA GGA CAC ACC TTG CT-3′. The primers for CXCR4 and CX3CR1 were as reported previously.15
FACScan
To assay cells by forward and side scatter of light, the instrument was standardized10 with microbeads (Dynosphere Uniform Microspheres; Bangs Laboratories, Fisher, IN). Approximately 3 × 105 cells MSCs were concentrated by centrifugation at 500g for 5 minutes and then suspended in 50 μL phosphate-buffered saline containing 1% (wt/vol) bovine serum albumin and the primary antibodies indicated in Table S2. The samples were incubated for 20 minutes at room temperature (RT) and then, if necessary, for 15 minutes at RT with the secondary antibody. The samples were analyzed with the use of FACScan (FACScalibur; BD Biosciences, San Jose, CA) with CellQuest software (BD Biosciences). The isotype control for each experiment was standardized to a mean value of 3.3.
CFU-F assays
Viable passage 2 MSCs (donor 1 and donor 8) were plated at initial densities of approximately 100 cells/cm2 in 174-cm2 dishes (Nunc). After incubation for 5 or 9 days, the cultures were plated about 1 cell/cm2 in 75-cm2 dishes (Nunc) containing 15 mL complete culture medium (CCM). The medium was changed every 3 to 4 days. After 2 weeks, the samples were incubated with 1% (wt/vol) crystal violet in methanol for 10 minutes and washed with water before colonies with diameters greater than 1 mm were counted.
Transfection with RNAi for PODXL
Target MSCs for the transfections were prepared with viable passage 1 MSCs (donor 1) that were plated at 100 cells/cm2 in CCM in 6-well plates with a change of medium after 3 days. After incubation for 5 days, cells were transfected with 20 nmol/L RNA interference ([RNAi]; Stealth Select 3 RNAi PODXL Set; HSS108202; HSS108203; HSS108204; Invitrogen) or RNAi negative control (Stealth RNAi negative Control; Invitrogen) by a commercial kit (Lipofectamine RNAiMAX reagent; Invitrogen). At 5 hours later, the medium was replaced with 3 mL per well of CCM lacking antibiotics. On day 7, the cells were lifted with trypsin/ethylenediaminetetraacetic acid (EDTA) for assay or replating at 1000 cells/cm2. After incubation for 24 hours, cells from regions of the replated cultures containing widely spaced cells and cells from aggregates were separated with cloning cylinders (Bel-Art Products, Pequannock, NJ) and lifted with trypsin/EDTA.
Turbidity assays
High and low PODXL and CD49f MSCs were suspended in Hanks balanced salt solution (HBSS) and incubated at RT. Turbidity was assayed by absorbance at 590 nm in a spectrophotometer under non-Rayleigh conditions commonly used in cell cytometry (BD Biosciences Training Manual, 2005, p. 52; www.bdbiosciences.com) in which extinction of forward scattered light is a reliable measure of size over a broad range of cell and particle sizes.39
Intravenous infusions and microscopy
Male immunodeficient NOD/scid mice (NOD.CB17-Prkdcscid/J; The Jackson Laboratory, Bar Harbor, ME) 7 to 8 weeks of age were anesthetized by intraperitoneal injection of a mixture of ketamine (91 mg/kg) and xylazine (9 mg/kg). High and low PODXL and CD49f MSCs were suspended in 150 μL HBSS at concentrations 6700 to 20 000 cells/μL and maintained at 4°C before they were infused slowly into a tail vein. Control mice were killed with a lethal concentration of ketamine/xylazine. Lungs were inflated by intratracheal injection of 10% neutral-buffered formalin (Richard-Allan Scientific, Kalamazoo, MI) and embedded in paraffin. Sections of 6 μm were cut, stained with hematoxylin-eosin (Richard-Allan Scientific), and then microphotographs were prepared (Eclipse TE 200; Nikon, Melville, NY). This study received Institutional Review Board Approval for the use of mice from the Tulane University Health Sciences Center.
Intravenous infusion into naive mice and mice with myocardial infarcts
Male immunodeficient NOD/scid mice (NOD.CB17-Prkdcscid/J; The Jackson Laboratory) 7 to 8 weeks of age were ventilated mechanically under anesthesia, the chest was opened, the left anterior descending artery was ligated, and the chest was closed. One million high and low PODXL and CD49f MSCs were infused into a tail vein after 1 day. Tissues were isolated by dissection after 1 day and analyzed by real-time PCR for human Alu sequences15 and by quantitative RT-PCR for human GAPDH (TaqMan Gene Expression Assays, Inventoried; Applied Biosystems, Foster City, CA).
Statistical analysis
Experimental groups were tested for normal distribution by the Kolmogorov-Smirnov test. When the distribution of both groups was normal, between-group comparisons were made with the 2-tailed Student t test. Nonparametric were compared with the Mann-Whitney U test.
Results
Microarray assays as a preliminary screen for useful epitopes
As noted previously,10,11,36 assays by phase-contrast microscopy and light scatter demonstrated that MSCs increased in size and complexity when early-passage cells were plated at low density and expanded in culture (Figure 1B,C). As an initial strategy, we queried microarray data37 for changes in transcripts for surface proteins as the cultures expanded. The results demonstrated that the steady-state levels of more than 10 transcripts decreased (> 2-fold) and an almost-equal number increased (Figure 1D). The two of the transcripts with the largest decreases coded for proteins previously shown to be linked to cell motility and tumor progression: PODXL40 and α6-integrin (CD49f).41,42 As the cultures expanded, the largest increase was for messenger RNA (mRNA) for the cell adhesion protein VCAM-1.
Microarrays as a preliminary screen for useful surface epitopes. (A) Schematic of 2 protocols used to prepare human MSCs. (B) Phase-contrast photomicrographs of viable MSCs from passage 1/donor 1 plated at 100 cells/cm2 and incubated for 5 or 9 days to generate passage 2 MSCs. (C) Assay by forward and side scatter of light of MSCs from panel B. Vertical and horizontal lines were generated with microbeads to standardize the assay.10 (D) Microarray assays of mRNAs from viable MSCs from passage 1/donor 6 plated at 100 cells/cm2 and incubated for 5 days to approximately 50% confluency, 10 days to 100% confluency, and 15 days to overconfluency. The values were normalized to mRNA signals on day 15 (left) or on day 5 (right).
Microarrays as a preliminary screen for useful surface epitopes. (A) Schematic of 2 protocols used to prepare human MSCs. (B) Phase-contrast photomicrographs of viable MSCs from passage 1/donor 1 plated at 100 cells/cm2 and incubated for 5 or 9 days to generate passage 2 MSCs. (C) Assay by forward and side scatter of light of MSCs from panel B. Vertical and horizontal lines were generated with microbeads to standardize the assay.10 (D) Microarray assays of mRNAs from viable MSCs from passage 1/donor 6 plated at 100 cells/cm2 and incubated for 5 days to approximately 50% confluency, 10 days to 100% confluency, and 15 days to overconfluency. The values were normalized to mRNA signals on day 15 (left) or on day 5 (right).
To develop assays for early progenitors, we searched for commercial sources for antibodies to PODXL and CD49f. In addition, we enlarged the screen to include antibodies to surface proteins previously reported to be involved in the migration of MSCs and that play roles in the trafficking of hematopoietic cells and cancer cells: c-Met,43 α4-integrin (CD49d),44,45 and CXCR4 and CX3CR1.15,43,44,46,47
Expression of the 6 epitopes
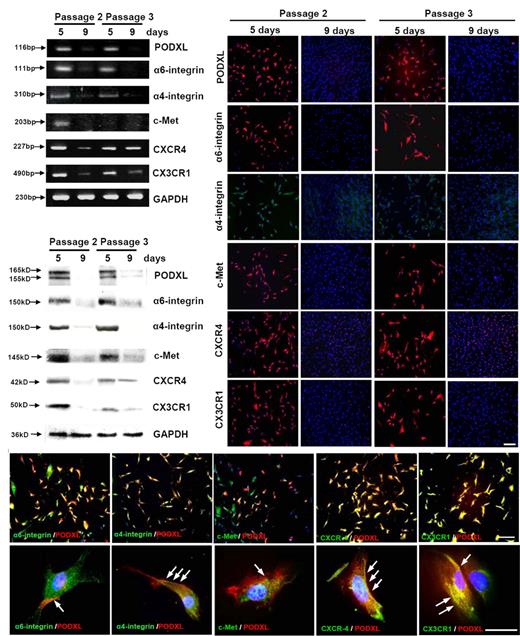
To assay for expression of the 6 surface proteins, passage 1 MSCs (donor 1) were plated at 100 cells/cm2. The cultures were then incubated for 5 days so that they were approximately 50% confluent, as judged by how extensively the surfaces of the flasks were spanned by lattices of cells that with cell-to-cell contacts (Figure 1B). Most of the cells were thin and spindle-shaped (Figure 1B).11,13,36 The yields of cells from 5-day cultures were 3400 (± 500) SD cells/cm2 (n = 5). Alternatively, the cultures were incubated for 9 days until they were approximately 90% to 100% confluent, at which point most cells had extensive cell-to-cell contacts and were broader with increased width-to-length ratios (Figure 1B).11,13,36 The yields of cells varied but were difficult to estimate because the cells readily aggregated (Table 1). Semiquantitative RT-PCR assays (Figure 2A) confirmed that mRNAs for the 6 candidate proteins were expressed at greater levels in the 5-day cultures than in the 9-day cultures. The 9-day cultures were then lifted, replated at 100 cells/cm2, and again incubated for 5 or 9 days. A total of 5 of the 6 mRNAs were again expressed at greater levels in the 5-day cultures than in the 9-day cultures. Again, the expression of CXCR4 showed a smaller change with expansion.
Assays of cultures of viable MSCs passage 1/donor 5 that were plated at 100 cells/cm2 and incubated for 5 days or 9 days to generate passage 2 MSCs. To prepare passage 3 MSCs, 9-day cultures were lifted with trypsin/EDTA and replated at 100 cells/cm2 for incubation for 5 or 9 days. (A) RT-PCR assays. (B) Western blot assays. (C) Assays by immunocytochemistry. Bar = 200 μm. (D) Double-immunostaining for PODXL (red) and the 5 other surface proteins (green). Nuclei were labeled with 4′,6-diamidino-2-phenylindole (blue). (Top panels) Bar = 200 μm. (Bottom panels) Bar = 50 μm. Arrows indicate regions of the cells in which PODXL and other proteins are colocalized.
Assays of cultures of viable MSCs passage 1/donor 5 that were plated at 100 cells/cm2 and incubated for 5 days or 9 days to generate passage 2 MSCs. To prepare passage 3 MSCs, 9-day cultures were lifted with trypsin/EDTA and replated at 100 cells/cm2 for incubation for 5 or 9 days. (A) RT-PCR assays. (B) Western blot assays. (C) Assays by immunocytochemistry. Bar = 200 μm. (D) Double-immunostaining for PODXL (red) and the 5 other surface proteins (green). Nuclei were labeled with 4′,6-diamidino-2-phenylindole (blue). (Top panels) Bar = 200 μm. (Bottom panels) Bar = 50 μm. Arrows indicate regions of the cells in which PODXL and other proteins are colocalized.
Western blot assays revealed a similar pattern of expression of the 6 candidate proteins (Figure 2B). The levels of all 6 proteins were high in 5-day cultures and decreased as the cells expanded over the course of 9 days. A similar pattern of expression reappeared when the confluent cultures were replated in that the 6 proteins were present in the low-density cultures. A total of 5 of the 6 proteins decreased as the cultures expanded. Again, the decrease in CXCR4 was less apparent than for the other 5 epitopes.
Immunocytochemistry also demonstrated a similar pattern of expression of the 6 proteins (Figure 2C). The proteins were expressed in essentially all passage 2 cells after incubation for 5 days. After the cells were expanded for 9 days, there was a marked decrease in protein expression. After the 9-day cultures were lifted and replated at low density, the proteins were again expressed in most of the cells after 5 days. However, a few cells were negative. After the cultures were expanded for 9 days, most of the cells were negative. Double labeling of day 5 passage 2 MSCs (Figure 2D) indicated that PODXL was largely colocalized with the other 5 proteins in selective regions of the cells.
Assay of the epitopes by FACScan
Because they adhere to tissue culture surfaces, confluent cultures of MSCs must be lifted from cultures by digestion with trypsin or other proteases that cleave surface proteins. To compare the effects of trypsinization, we first established that most of the cells in 5-day cultures could be lifted with incubation at 37°C for 5 minutes with 0.48 mmol/L EDTA alone. We then used FACScan to compare the candidate surface epitopes in cells from 5-day cultures that were lifted with EDTA alone and in cells from 5-day cultures lifted with a combination of trypsin/EDTA. With passage 2 MSCs from donor 1, approximately 90% of the cells lifted with EDTA were labeled with a monoclonal antibody to PODXL (Figure S1A) and most remained positive in parallel cultures lifted with trypsin/EDTA. Trypsinization reduced the number of cells positive for CD49f by approximately 17%, the number of cells positive for CD49d by approximately 24%, and the number of cells positive for c-Met by approximately 4% (Figure S1B-D). With CXCR4 and CX3CR1, the epitopes were detected in less than 30% of the cells lifted with EDTA alone (Figure S1E,F), although essentially all the cells expressed the proteins as surface epitopes when assayed by immunocytochemistry of adherent cells (Figure 2C). The discrepancy is probably explained by internalization of the proteins after lifting.48 Lifting the cells with a combination of trypsin/EDTA markedly decreased the number of cells positive for CXCR4 or CX3CR1 (Figure S1E,F).
Assays of the epitopes on MSCs from different donors
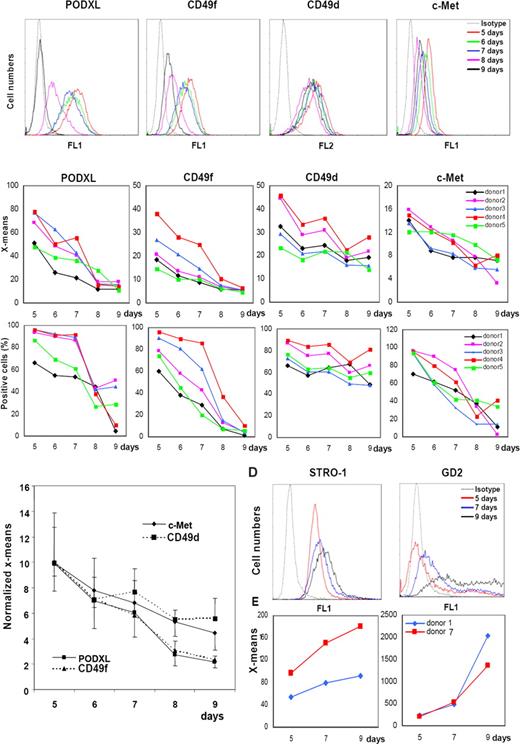
Because bone marrow aspirates from the iliac crest are taken blindly, there is considerable variation in the number and quality of MSCs that are obtained from aspirates of different donors and aspirates from different sites of the same donor during the same session (see Table S1). Also, there is considerable variation in the rates at which the cells expand (Table 1). To evaluate the variations, passage 1 MSCs from 5 different donors were plated at 100 cells/cm2 and assayed for the 4 candidate epitopes after the cells were expanded in culture for 5 to 9 days and lifted with trypsin/EDTA (Figures 3 and S2). Assays for the epitopes by FACScan indicated that there was a progressive decrease in the mean fluorescence of PODXL, CD49f, and c-Met as the cells expanded when the data were expressed as either mean peak fluorescence (Figures 3B top panels and Figure S2) or percent positive cells (Figure 3B bottom panels). The changes in CD49d were less consistent. Normalization of the mean peaks of fluorescence (X-mean) to the day 5 values, indicated that PODXL and CD49f showed the most robust decreases (Figure 3C).
FACScans of changes in the epitopes in MSCs with expansion in culture. (A) Viable passage 1/donor 4 MSCs plated at 100 cells/cm2 and incubated for 5, 6, 7, 8, or 9 days. Cells were lifted with trypsin/EDTA. (B) Data obtained with MSCs from 5 donors under conditions as in panel A. Values expressed either as mean fluorescence intensity (X-means) or percent positive cells. (C) X-mean values from panel B normalized to values for day 5. (D) FACScan from passage 1/donor 1 MSCs incubated as in panel A and assayed for STRO-1 and GD2. (E) X-means values from passage 1/donor 1 and donor 7 MSCs.
FACScans of changes in the epitopes in MSCs with expansion in culture. (A) Viable passage 1/donor 4 MSCs plated at 100 cells/cm2 and incubated for 5, 6, 7, 8, or 9 days. Cells were lifted with trypsin/EDTA. (B) Data obtained with MSCs from 5 donors under conditions as in panel A. Values expressed either as mean fluorescence intensity (X-means) or percent positive cells. (C) X-mean values from panel B normalized to values for day 5. (D) FACScan from passage 1/donor 1 MSCs incubated as in panel A and assayed for STRO-1 and GD2. (E) X-means values from passage 1/donor 1 and donor 7 MSCs.
Similar assays on cultures of MSCs from 2 donors were conducted with the antibody STRO-1 and a monoclonal antibody to the neural ganglioside GD2 that was recently suggested as a novel antibody for MSCs.35 Assays for STRO-1 were positive in 5-day cultures, and the mean fluorescence increased slightly as the cell expanded (Figure 3D). GD2 was detected at very low levels in 5-day cultures, and the levels increased as the cultures expanded (Figure 3D).
Clonogenicity of high and low PODXL/CD49f cells
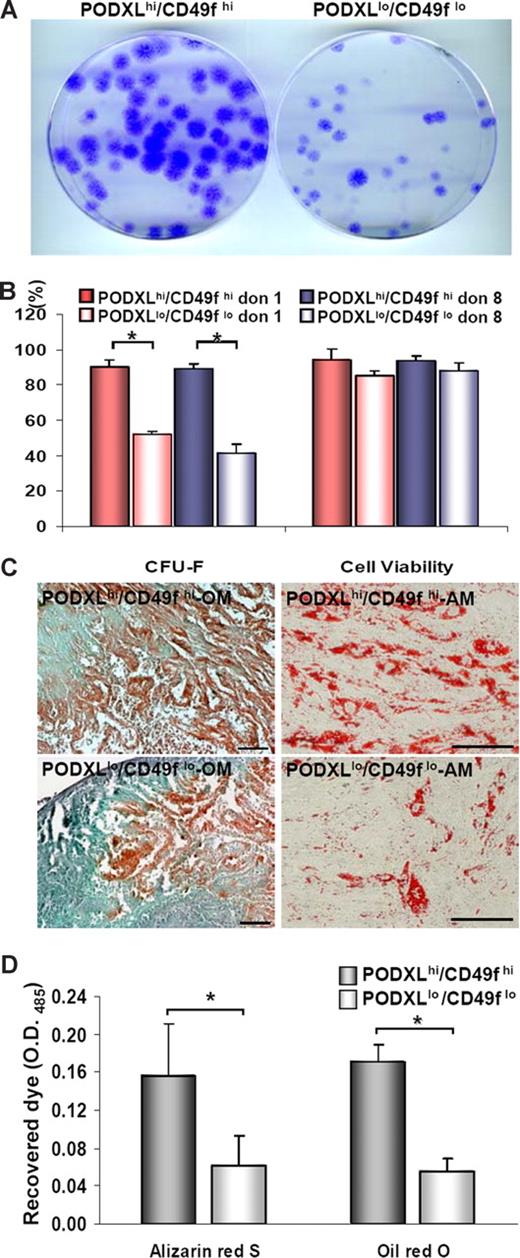
Previous observations suggested that the clonogenicity of MSCs cultures decreased as they were expanded toward confluency.12,49 To confirm the observation under the culture conditions used here, we plated MSCs at 100 cells/cm2. The cultures were expanded for 5 days to obtain MSCs that were PODXLhi/CD49fhi and for 9 days to obtain MSCs that were PODXLlo/CD49flo. The 2 subpopulations were then replated at 1 cell/cm2 to assay colony-forming units (CFU-Fs). As indicated in Figure 4A and B, the PODXLhi/CD49fhi cells were highly clonogenic with CFU-F values of 90.1% (± 6.0%), or approximately twice the CFU-F values of thePODXLlo/CD49flo cells. Also, the PODXLhi/CD49fhi cells were slightly more viable (Figure 4B). In addition, the PODXLhi/CD49fhi cells more efficiently differentiated into mineralizing cells and adipocytes (Figure 4C,D).
Assays of MSCs in vitro. Viable MSCs from passage 1/donor 1 plated at 100 cells/cm2 were incubated for 5 or 9 days to generate passage 2 MSCs that were high or low for PODXL or CD49f. (A) Passage 2/donor 1 MSCs replated at 1 cell/cm2 and incubated for 2 weeks. Colonies were stained with crystal violet. (B) CFU-F values passage 2/donor 1 and donor 8 MSCs (donor 7009 and 281). Asterisks indicate values (± SD) that differ with P < .01 by the Student t test (n = 7). (C) Alizarin Red S after osteogenic differentiation and after adipogenic differentiation with passage 3/donor 1 MSCs. Bar = 100 μm. (D) Assays of differentiation by extraction of Alizarin Red S after osteogenic differentiation and Oil Red O after adipogenic differentiation with passage 3/donor 1 MSCs. Asterisks indicate values (± SD) that differ with P < .01 by the Student t test (n = 4).
Assays of MSCs in vitro. Viable MSCs from passage 1/donor 1 plated at 100 cells/cm2 were incubated for 5 or 9 days to generate passage 2 MSCs that were high or low for PODXL or CD49f. (A) Passage 2/donor 1 MSCs replated at 1 cell/cm2 and incubated for 2 weeks. Colonies were stained with crystal violet. (B) CFU-F values passage 2/donor 1 and donor 8 MSCs (donor 7009 and 281). Asterisks indicate values (± SD) that differ with P < .01 by the Student t test (n = 7). (C) Alizarin Red S after osteogenic differentiation and after adipogenic differentiation with passage 3/donor 1 MSCs. Bar = 100 μm. (D) Assays of differentiation by extraction of Alizarin Red S after osteogenic differentiation and Oil Red O after adipogenic differentiation with passage 3/donor 1 MSCs. Asterisks indicate values (± SD) that differ with P < .01 by the Student t test (n = 4).
RNAi for PODXL causes aggregation of MSCs
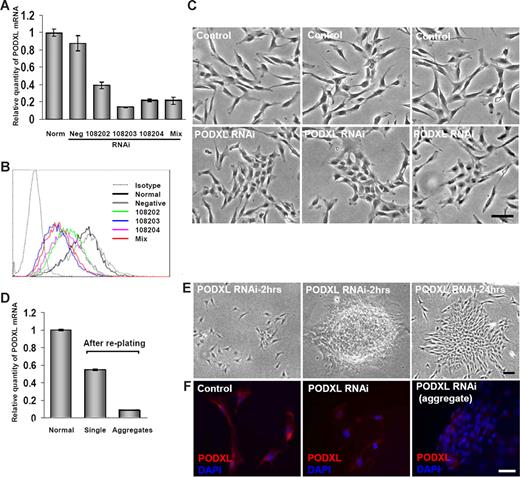
PODXL was previously shown to inhibit cell-to-cell contacts, apparently because its large glycosaminoglycans provide a highly negative charge that shields the receptors for ligand interactions.40 To test for a similar function in MSCs, the cells from 5-day cultures were transiently transfected with 3 different RNAis to PODXL or a mixture of the 3 RNAis. Real-time RT-PCR assays demonstrated a 60% to 80% decrease in the level of the transcript for PODXL (Figure 5A). There was also a decrease in the surface epitope, as assayed by FACScan (Figure 5B). Transduced cultures that were incubated in CCM for 2 days tended to form more cell-to-cell contacts than cells in control cultures (Figure 5C). When the cultures were lifted with trypsin/EDTA and replated, the transduced cells segregated into 2 subpopulations: dispersed, spindle-shaped cells with a small number of cell-to-cell contacts and large aggregates of the cells (Figure 5E). The dispersed, spindle-shaped cells expressed PODXL, as assayed by real-time RT-PCR (Figure 5D) and immunocytochemistry (Figure 5F). In contrast, the aggregates expressed much lower levels of mRNA for PODXL (Figure 5D). Also, the antibody to PODXL labeled only the cells on the periphery of the aggregates (Figure 5F).
Transfection of MSCs with RNAis for PODXL. Viable MSCs from passage 1/donor 1 were plated at 100 cells/cm2, incubated for 5 days, transfected for 5 hours, and then incubated in CCM for 2 days. (A) mRNA for PODXL assayed by real-time RT-PCR from normal control MSCs and transfected MSCs with single PODXL RNAi, a mixture of 3 PODXL RNAis or a RNAi negative control. Values are mean plus or minus SD (n = 3). (B) FACScan for PODXL of MSCs incubated with 20 nmol/L RNAis for PODXL as in panel A. (C) Phase-contrast microscopy of control MSCs and MSCs transfected with 20 nmol/L of mixture of 3 RNAis for PODXL and then incubated for 2 days. Bar = 100 μm. (D) Real-time RT-PCR assays for PODXL mRNA of cultures that were transduced with mixture of 3 RNAis and incubated as in panel C, lifted with trypsin/EDTA, replated at approximately 1000 cells/cm2, and incubated for 24 hours. Cells from regions of the plate containing single cells and aggregated cells were isolated separately by using cloning cylinders and assayed separately. Values are mean plus or minus SD (n = 3). (E) Phase microscopy of cultures taken for RT-PCR assays in panel D. Region containing single cells and 2 aggregates are shown. Bar = 100 μm. (F) Immunocytochemistry for control culture and transduced cultures shown in panel E. The dispersed single cells (middle panel) were lightly labeled with anti-PODXL. In the aggregates only the peripheral cells were labeled (right panel). Nuclei were stained with DAPI. Bar = 100 μm.
Transfection of MSCs with RNAis for PODXL. Viable MSCs from passage 1/donor 1 were plated at 100 cells/cm2, incubated for 5 days, transfected for 5 hours, and then incubated in CCM for 2 days. (A) mRNA for PODXL assayed by real-time RT-PCR from normal control MSCs and transfected MSCs with single PODXL RNAi, a mixture of 3 PODXL RNAis or a RNAi negative control. Values are mean plus or minus SD (n = 3). (B) FACScan for PODXL of MSCs incubated with 20 nmol/L RNAis for PODXL as in panel A. (C) Phase-contrast microscopy of control MSCs and MSCs transfected with 20 nmol/L of mixture of 3 RNAis for PODXL and then incubated for 2 days. Bar = 100 μm. (D) Real-time RT-PCR assays for PODXL mRNA of cultures that were transduced with mixture of 3 RNAis and incubated as in panel C, lifted with trypsin/EDTA, replated at approximately 1000 cells/cm2, and incubated for 24 hours. Cells from regions of the plate containing single cells and aggregated cells were isolated separately by using cloning cylinders and assayed separately. Values are mean plus or minus SD (n = 3). (E) Phase microscopy of cultures taken for RT-PCR assays in panel D. Region containing single cells and 2 aggregates are shown. Bar = 100 μm. (F) Immunocytochemistry for control culture and transduced cultures shown in panel E. The dispersed single cells (middle panel) were lightly labeled with anti-PODXL. In the aggregates only the peripheral cells were labeled (right panel). Nuclei were stained with DAPI. Bar = 100 μm.
Aggregation of MSCs in suspension
To assay aggregation of MSCs, passage 1 cells were expanded for 5 or 9 days to obtain preparation high and low for PODXL and CD4f. The cells were then lifted with trypsin/EDTA and suspended at 0.5 or 1 × 104 cells/μL in HBSS at RT. The PODXLlo/CD49flo cells had a greater initial turbidity (Figure 6A) that in part reflected their larger size (Figure 1B,C). When incubated at RT, the turbidity of suspensions of the PODXLlo/CD49flo cells increased (Figure 6A). In contrast, the initial turbidity of the PODXLhi/CD49fhi cells was lower, and there was a small increase over the course of 4 hours. There was no increase in turbidity with either PODXLhi/CD49fhi cells or PODXLlo/CD49flo cells when the same samples were incubated for 4 hours at 4°C (not shown).
Aggregation and formation of lethal emboli with MSCs. Viable MSCs from passage 1/donor 1 were plated at 100 cells/cm2 incubated for 5, 7, or 9 days. (A) Passage 2 MSCs from high and low of PODXL/CD49f were lifted with trypsin/EDTA, washed by centrifugation in HBBS, suspended in HBBS at a concentration of either 0.5 × 104 cells/μL or 104 cells/μL, and incubated at RT. Turbidity was assayed by absorbance at 590 nm. (B) Death rate of mice (n = 10 or 13) after infusion into a tail vein of passage 2 MSCs (donor 1) from high, medium, and low of PODXL/CD49f. (C) Photographs of lungs from euthanized control mouse and mouse that died within a few minutes of intravenous infusion of 2 × 106 of the MSCs that were PODXLlo/CD49flo in panel B. (D) Photomicrographs of sections from mice that died after infusion of PODXLlo/CD49flo in panel B. Sections stained with hematoxylin and eosin. Bar = 100 μm. Insets: Regions containing large blood vessels.
Aggregation and formation of lethal emboli with MSCs. Viable MSCs from passage 1/donor 1 were plated at 100 cells/cm2 incubated for 5, 7, or 9 days. (A) Passage 2 MSCs from high and low of PODXL/CD49f were lifted with trypsin/EDTA, washed by centrifugation in HBBS, suspended in HBBS at a concentration of either 0.5 × 104 cells/μL or 104 cells/μL, and incubated at RT. Turbidity was assayed by absorbance at 590 nm. (B) Death rate of mice (n = 10 or 13) after infusion into a tail vein of passage 2 MSCs (donor 1) from high, medium, and low of PODXL/CD49f. (C) Photographs of lungs from euthanized control mouse and mouse that died within a few minutes of intravenous infusion of 2 × 106 of the MSCs that were PODXLlo/CD49flo in panel B. (D) Photomicrographs of sections from mice that died after infusion of PODXLlo/CD49flo in panel B. Sections stained with hematoxylin and eosin. Bar = 100 μm. Insets: Regions containing large blood vessels.
Lethal pulmonary emboli
To determine whether MSCs produce pulmonary emboli, MSCs were infused into the tail veins of mice. In one series of experiments, mice were infused with increasing concentrations of MSCs cultured for 7 days that were had medium levels of PODXL/CD49f (see Figure 3B). The infusions were well tolerated in 10 mice that received infusions of 106 cells that were PODXLmed/CD49med (Figure 6B). However, 10% of mice died within minutes after the infusion of 2 × 106 cells, and 80% of mice (n = 10) died after infusion of 3 × 106 cells. In a second series of experiments, cells with high and low levels of expression of PODXL/CD49f were compared. All 10 mice tolerated infusions of 2 × 106 MSCs that were PODXLhi/CD49fhi. In contrast, 70% died after infusion of the same number of cells that were PODXLlo/CD49flo (n = 13). The lungs of mice that died after the infusions of the PODXLlo/CD49flo cells were paler than control lungs (Figure 6C) and contained blood vessels with large nucleated cell that appeared to occlude the vessels (Figure 6D). The marked tendency to aggregate of MSCs with a knockdown of PODXL (Figure 5) made them impractical to test with intravenous infusions.
Migration of MSCs to injured myocardium and kidney
To assess the fate of the MSCs trapped in the lung, we assayed DNA and RNA from lungs 1 day after the intravenous infusion of 106 human MSCs. Assays for repetitive human Alu sequences in lung demonstrated that greater numbers of PODXLhi/CD49fhi MSCs survived than PODXLlo/CD49flo MSCs (Figure 7A). Assays for human GAPDH mRNA also indicated that more of the PODXLhi/CD49fhi cells survived in lung. The C-terminal tail of PODXL forms a complex with ezrin and expression of ezrin and activation of mitogen-activated protein kinase (MAPK) was shown to provide early survival of metastatic cells in lung.50 Western blot assayed demonstrated that PODXLhi/CD49fhi expressed greater levels of ezrin, phosphorylated MAPK, and phosphorylated Akt than PODXLlo/CD49flo MSCs (Figure 7B,C). Therefore, the enhanced survival of PODXLhi/CD49fhi MSCs was correlated with increased expression of ezrin, together with activation of both MAPK and Akt.
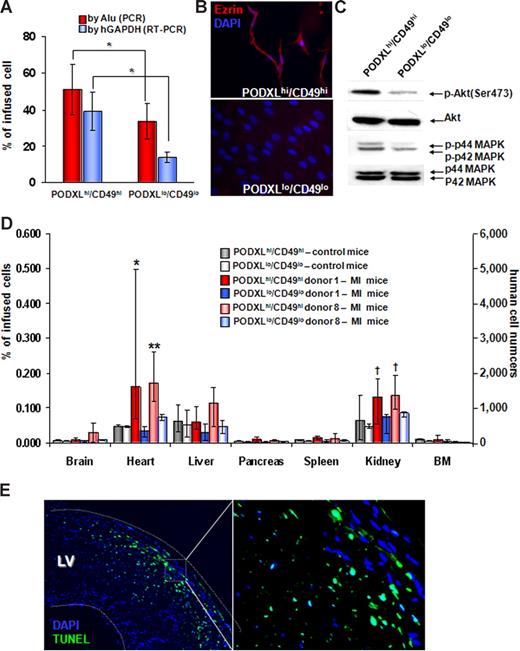
Comparisons of PODXL/CD49f high and low MSCs. (A) PODXLhi/CD49fhi MSCs survive longer in lung after intravenous infusion. One million passage 2 MSCs that were from donor 1 and 8 were infused intravenously into mice with MI. One day after the infusions, DNA was extracted for real-time PCR of Alu sequences and RNA was extracted for real-time RT-PCR for human GAPDH mRNA. Values are expressed as % values obtained with 1 million freshly isolated MSCs that were either PODXLhi/CD49fhi or PODXLlo/CD49flo. *P < .05 by Student t test (n = 5). (B) Immunocytochemistry demonstrating that PODXLhi/CD49fhi MSCs express greater levels of ezrin. The MSCs were from passage 2 of donor 8. (C) Western blot assays demonstrating that PODXLhi/CD49fhi MSCs express greater levels of phosphorylated Akt (p-Akt) and p44/42 MAPK. The MSCs were passage 2 from donor 8. (D) Tissue distributions of intravenously infused MSCs. One million passage 2 MSCs (donor 1 and donor 8) that were either PODXLhi/CD49fhi or PODXLlo/CD49flo were infused intravenously into control mice or mice with MI, and tissues were recovered 1 day after the MSC infusions. Values from real-time PCR assays for human Alu sequences are expressed either as percent of infused human cells or human cell numbers. Error bars indicate range of values. *P < .05 versus PODXLhi/CD49fhi in control mice and P < .05 versus PODXLlo/CD49flo in MI mice by nonparametric Mann-Whitney U test. **P <.05 versus PODXLhi/CD49fhi in control mice and P < .05 versus PODXLlo/CD49flo in MI mice by 2-tailed Student t test. †P < .05 versus PODXLlo/CD49flo in MI mice by 2-tailed Student t test. (E) Terminal deoxynucleotidyl transferase dUTP nick end labeling assay of heart from mice 2 days after MI induction indicating apoptotic cells in region of infarction.
Comparisons of PODXL/CD49f high and low MSCs. (A) PODXLhi/CD49fhi MSCs survive longer in lung after intravenous infusion. One million passage 2 MSCs that were from donor 1 and 8 were infused intravenously into mice with MI. One day after the infusions, DNA was extracted for real-time PCR of Alu sequences and RNA was extracted for real-time RT-PCR for human GAPDH mRNA. Values are expressed as % values obtained with 1 million freshly isolated MSCs that were either PODXLhi/CD49fhi or PODXLlo/CD49flo. *P < .05 by Student t test (n = 5). (B) Immunocytochemistry demonstrating that PODXLhi/CD49fhi MSCs express greater levels of ezrin. The MSCs were from passage 2 of donor 8. (C) Western blot assays demonstrating that PODXLhi/CD49fhi MSCs express greater levels of phosphorylated Akt (p-Akt) and p44/42 MAPK. The MSCs were passage 2 from donor 8. (D) Tissue distributions of intravenously infused MSCs. One million passage 2 MSCs (donor 1 and donor 8) that were either PODXLhi/CD49fhi or PODXLlo/CD49flo were infused intravenously into control mice or mice with MI, and tissues were recovered 1 day after the MSC infusions. Values from real-time PCR assays for human Alu sequences are expressed either as percent of infused human cells or human cell numbers. Error bars indicate range of values. *P < .05 versus PODXLhi/CD49fhi in control mice and P < .05 versus PODXLlo/CD49flo in MI mice by nonparametric Mann-Whitney U test. **P <.05 versus PODXLhi/CD49fhi in control mice and P < .05 versus PODXLlo/CD49flo in MI mice by 2-tailed Student t test. †P < .05 versus PODXLlo/CD49flo in MI mice by 2-tailed Student t test. (E) Terminal deoxynucleotidyl transferase dUTP nick end labeling assay of heart from mice 2 days after MI induction indicating apoptotic cells in region of infarction.
After intravenous infusion human MSCs into the normal mice, assays for Alu sequences indicated that the largest number of cells were detected in the lungs (Figure 7A). The levels of human DNA in other tissues were very low, with the greatest levels in heart, liver, and pancreas (Figure 7D left panel). To assess the ability of intravenously administered MSCs to engraft in injured peripheral tissues, we produced myocardial infarctions (MIs) in mice by permanent ligation of the left anterior descending artery; MSCs were infused intravenously 1 day later. Assays for Alu sequences 1 day after infusion of the MSC indicated there was increased engraftment of the PODXLhi/CD49fhi MSCs in both heart and kidney in the mice with MI (Figure 7D middle and right panels). As expected, the MI increased apoptosis of the heart (Figure 7E) and produced renal damage as reflected by an increase in serum creatinine levels (0.53 ± 0.08 SD vs 1.08 ± 0.14 SD; n = 4). Immunohistochemistry sections revealed a few cells in the border zone of the infarcts that labeled with antihuman nuclei antigen (not shown).
Discussion
By first querying microarray data, we identified a series of mRNAs for surface proteins that were expressed in low-passage, low-density cultures of MSCs enriched for early progenitor cells but that were not expressed as the cultures expand to confluency. Expression of the surface proteins was established by Western blots and immunocytochemistry. Remarkably, the surface proteins characteristic of early progenitor cells reappeared when subconfluent cultures were replated at low density. Therefore, the results confirmed the plasticity of the cells: If early-passage cells are plated at low density, they pass through lag, exponential growth, and near-stationary phases.11,12,51 If the cells are replated at low density before they reach confluency, the sequence is repeated through several passages. Commercially available antibodies identified early progenitors in the cultures even after they were lifted by partial cleavage of surface proteins with trypsin. Therefore, the antibodies, particularly the antibodies to PODXL and CD49f, provide a rapid means of identifying the early progenitors in cultures of MSCs. PODXLhi/CD49fhi cells were more efficient than PODXLlo/CD49flo cells both in generating single cell–derived colonies and in differentiating in culture. Also, PODXLhi/CD49fhi cells differed from PODXLlo/CD49flo cells in that they were less likely to produce lethal pulmonary emboli and that they survived longer in the lung after intravenous infusion. The longer survival in lung was correlated with expression of ezrin and activation of MAPK and Akt in the PODXLhi/CD49fhi cells. The observations were consistent with reports that expression of ezrin and activation of MAPK provided survival of metastatic cells in lung50 and that expression of PODXL increased the aggressive phenotype of cancer cells through its interaction with ezrin.52 In addition, after intravenous infusion into mice with myocardial infarcts, PODXLhi/CD49fhi MSCs engrafted more efficiently in both the injured heart and kidney. Therefore, our results suggested that low-density cultures of MSCs enriched for PODXLhi/CD49fhi cells may be more effective than confluent cultures for intravenous therapies.
To prepare MSCs, most investigators use protocols that are minor modifications of the protocol developed by Friedenstein et al1 and Owen and Friedenstein,16 in which mononuclear cells from bone marrow are isolated on a density gradient (Figure 1A) and then are plated at high density and incubated for several days to recover adherent cells. The adherent cells are then replated at 5000 to 6000 cells/cm2 and expanded to confluency.17 Frequently, the cells are lifted and expanded under the same conditions through several passages. However, there is a marked loss of early progenitors as MSCs are expanded at high density.12-15,36,49 The question of the optimal protocol for expanding MSCs has been difficult to resolve because of the limitations of the available assays for early progenitors. Our previous attempts to assay the early progenitors defined as RS-MSCs by cellular morphology36 or by forward and side scatter of light10 provided only semiquantitative data. The antibodies identified here to PODXL and α6-integrin are more useful. However, they also labeled a subpopulation of cells in 5-day cultures that were larger the cells previously defined as RS-MSC (Figure S1).
The 6 surface proteins expressed primarily in early progenitors in part explain the distinctive properties of the cells. All 6 proteins were previously shown to be involved in cell motility, cell mobilization, cell homing, or progression of cancers.15,40-42,47,53,54 The data provided here and previous observations in malignant cells50,52 suggest that PODXL may be the most critical surface protein for survival of intravenously infused MSCs in lung and their migration to sites of injury in vivo. It is a member of a family of sialomucins that includes CD34 and CD105 (endoglin) and that serves as antiadhesion molecules.40,55 The extracellular portion of PODXL is decorated with a negatively charged tree of large polysaccharides that shields receptors on surfaces of cells from interacting with other ligands and receptors. Podocalyxin is expressed a high levels in glomerular podocytes, mesothelial cells, vascular endothelia, platelets, erythroblasts, and hematopoietic stem cells.56,57 In canine kidney (Madin-Darby canine kidney) cells, the overexpression of podocalyxin leads to the inhibition of cell-to-cell interaction as shown by decreased cell-to-cell adhesion, decreased tight junction-dependent transepithelial resistance, and redistribution of cell junction proteins.58 As demonstrated here, PODXL also prevents MSCs from aggregating, as demonstrated by the increased aggregation in which expression was decreased by transduction with RNAis. The marked tendency of the transduced MSCs to aggregate made them impractical to test by intravenous infusion.
For therapy, intravenous infusion of MSCs is obviously simpler than any other route for administration. Promising results were reported with intravenous infusion of MSCs in animal models for disease59-62 as well as in patients.6,8,9,63 The results are surprising because a large fraction of intravenously infused MSCs are trapped in the lung.64,65 This trapping is a common phenomenon observed with many cells, including polymorphonuclear cells,66 metastatic tumors,67 and probably hematopoietic stem cells.68 MSCs from confluent cultures are more prone to produce lethal emboli, probably because they are predisposed to aggregate in larger blood vessels of the lung as a result of their larger size, the presence of cell adhesion proteins such as VCAM, and the absence of anticell adhesion proteins such as PODXL. The dose of PODXLlo/CD49flo cells that produced lethal emboli in the mice (8 × 107 cells/kg) was greater than the maximal dose of MSCs used in clinical trials (1 × 107).6-9,63 However, as also demonstrated here, PODXLlo/CD49flo MSCs lifted from subconfluent cultures with trypsin/EDTA aggregated in suspension after a few hours at RT. Therefore, pulmonary emboli are probably a concern with intravenous administration of MSCs in a clinical setting. Also, aggregated MSCs tend to generate their own microenvironments.14 Therefore, care should be taken with direct injection of the cells into tissues,69 particularly of MSCs from confluent cultures. The results here suggest the dangers can be reduced by using MSCs that are PODXLhi/CD49fhi. Also, the results suggest that PODXLhi/CD49fhi cells are more likely to migrate to injured tissues after intravenous infusion.
Expansion of MSCs at low density to obtain PODXLhi/CD49fhi cells requires larger tissue culture vessels and larger amounts of medium than expansion of confluent cultures (Figure 1A). However, 50 to 200 × 106 passage 1 MSCs were readily produced from a single bone marrow aspirate under the conditions described here (Table S1). Therefore, it should be possible to provide adequate numbers of cells for most clinical trials.6-9,63 Greater numbers of PODXLhi/CD49fhi cells can be obtained by expanding subconfluent cultures at low density through several passages. However, there is considerable variation among different preparations. Also, it is probably preferable to avoid extensive expansion of the cells that can lead to genomic instability and tumorgenecity, as was observed after prolonged culture of both human MSCs70 and murine MSCs.71
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The work was supported in part by National Institutes of Health (NIH) grants HL 073755, HL073252, and P01 HL 075161; the W. M. Keck Foundation (Los Angeles, CA); and the Louisiana Gene Therapy Research Consortium (New Orleans, LA).
National Institutes of Health
Authorship
Contribution: R.H.L., M.J.S., and D.J.P. designed the research, analyzed data, and wrote the paper; and R.H.L., M.J.S., A.A.P., C.A.G., and J.Y. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Darwin J. Prockop, Texas A & M Health Science Center, Institute for Regenerative Medicine at Scott and White, Temple, TX 76502; e-mail: Prockop@medicine.tamhsc.edu.
References
Author notes
*R.H.L. and M.J.S. contributed equally to this study.