Abstract
Primitive quiescent CD34+ chronic myeloid leukemia (CML) cells are more biologically resistant to tyrosine kinase inhibitors than their cycling counterparts; however, graft-versus-leukemia (GVL) effects after allogeneic stem cell transplantation (SCT) probably eliminate even these quiescent cells in long-term surviving CML transplant recipients. We studied the progeny of CD34+ cells from CML patients before SCT, which were cultured 4 days in serum-free media with hematopoietic growth factors. BCR-ABL expression was similar in both cycling and quiescent noncycling CD34+ populations. Quiescent CD34+ cells from CML patients were less susceptible than their cycling CD34+ and CD34− counterparts to lysis by natural killer (NK) cells from their HLA-identical sibling donors. Compared with cycling populations, quiescent CD34+ CML cells had higher surface expression of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) receptors DR4 and DR5. Bortezomib up-regulated TRAIL receptor expression on quiescent CD34+ CML cells, and further enhanced their susceptibility to cytotoxicity by in vitro expanded donor NK cells. These results suggest that donor-derived NK cell–mediated GVL effects may be improved by sensitizing residual quiescent CML cells to NK-cell cytotoxicity after SCT. Such treatment, as an adjunct to donor lymphocyte infusions and pharmacologic therapy, may reduce the risk of relapse in CML patients who require treatment by SCT.
Introduction
The effectiveness of tyrosine kinase inhibitors (TKIs) in the treatment of chronic myeloid leukemia (CML) has revolutionized the management of this disease.1-3 Imatinib is now the first-line treatment for chronic-phase CML, and several newer TKIs such as dasatinib4 and nilotinib5 have been added to the pharmacologic compendium. Despite the proven efficacy of TKIs to induce hematologic and cytogenetic remission,3,6,7 the large majority of patients still have molecularly detectable disease.8,9 Biologically, detectable BCR-ABL transcripts in patients who achieve cytogenetic remission after TKI treatment have been attributed to the persistence of quiescent leukemic stem cells, which have been shown to be resistant to several TKIs.10-12 Conversely, allogeneic stem cell transplantation (SCT) remains the only treatment that can conclusively eradicate CML.13,14 The curative effect of SCT is attributed to the allogeneic graft-versus-leukemia (GVL) effect, to which CML is particularly susceptible, as evidenced by the success of donor lymphocyte infusions (DLI) in treating disease relapse, and improved leukemia-free survival in patients with graft-versus-host disease.15,16 The biologic mechanism of GVL in humans has been studied in vitro using donor cytotoxic T lymphocytes (CTLs) as effectors against patient leukemia cells.17-19 In these studies, leukemic CD34+ cells were shown to be variably susceptible to CTL cytotoxicity, and in particular, immature progenitor cells were found to be more resistant to CTL lysis compared with mature myeloid and monocytic cells.20
We recently showed that in T cell–depleted SCT, a rapid recovery of the natural killer (NK) subset of lymphocytes after transplantation is associated with early molecular remission and improved survival.21,22 We therefore hypothesized that NK cells participate in the GVL effect. As quiescent leukemic stem cells are a necessary target for effective GVL, we studied pre-SCT CD34+ quiescent cells from CML patients and found that although these were lysed by NK cells from their human leukocyte antigen (HLA)–identical sibling donors, they were less susceptible than their cycling CD34+ and CD34− counterparts. Previously, bortezomib was observed to increase surface expression of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) death receptors on tumor cell lines, enhancing their susceptibility to both recombinant TRAIL and NK-cell TRAIL–mediated cytotoxicity.23 Here we observed that bortezomib treatment of primary CML CD34+ cells up-regulated the surface expression of TRAIL receptors on quiescent leukemic CD34+ cells, enhancing their susceptibility to killing by expanded donor NK cells. Our results suggest that the adoptive transfer of in vitro expanded donor NK cells with concomitant sensitization of residual leukemia cells in the patient by administration of bortezomib may improve NK cell–mediated GVL effects.
Methods
Patient and donor samples
Fourteen CML patients (8 in chronic phase, 4 in accelerated phase, and 2 in blast crisis), including 10 patient-donor pairs, with sufficient biological material, were studied (Table 1). One patient was studied at diagnosis, while the other 13 patients were studied before T cell–depleted SCT according to myeloablative transplantation protocols, details of which have been reported previously.21,24 Written informed consent was obtained from patients and their healthy donors in accordance with the Declaration of Helsinki for the use of cells from leukapheresis products collected within 1 month before SCT for research according to the requirements of the Institutional Review Board of the National Heart, Lung, and Blood Institute. Donor leukaphereses were collected before administration of granulocyte colony-stimulating factor (G-CSF) to mobilize stem cells for transplantation. Mononuclear cells (MNCs) were isolated by density gradient centrifugation (Organon Teknika, Durham, NC) and cryopreserved in RPMI 1640 supplemented with 20% fetal calf serum (FCS) and 10% dimethyl sulfoxide. CD34+ cells from patients or NK cells from donors were selected by binding to immunomagnetic beads (MiniMACS; Miltenyi Biotec, Auburn, CA) from thawed MNCs, according to the manufacturer's instructions.
Flow cytometry and cell cultures
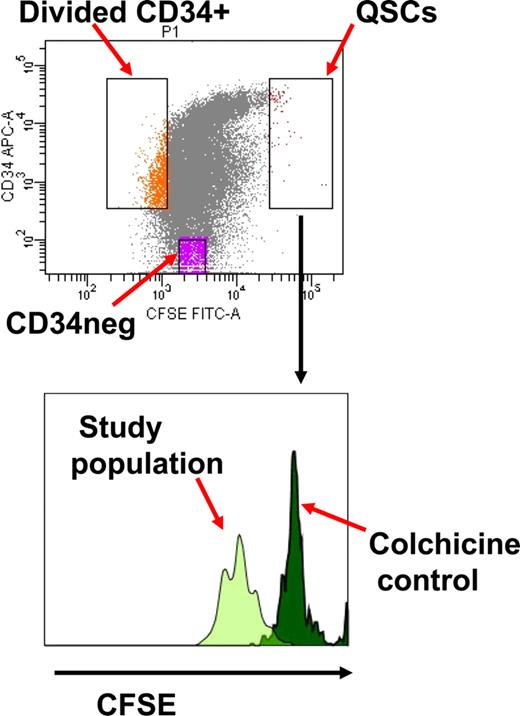
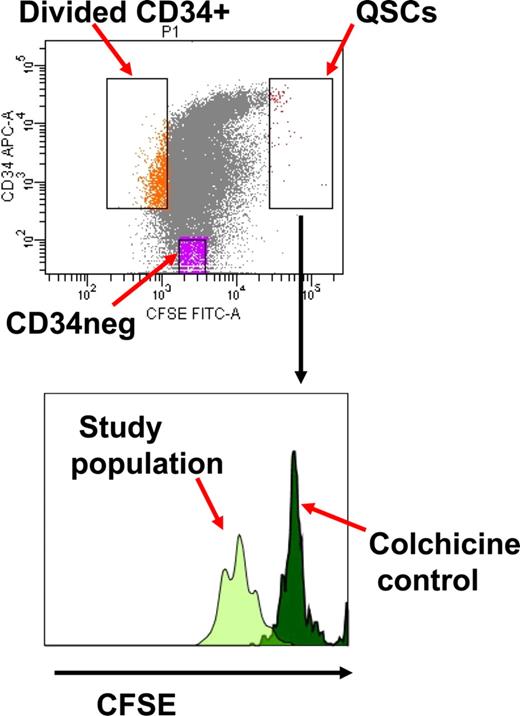
CD34+ cells were stained with 1 μM carboxy-fluorescein diacetate succinimidyl diester (CFSE; Molecular Probes, Eugene, OR) and cultured overnight at 37°C and 5% CO2 in serum-free media (SFM) with 5 growth factors (stem cell factor, G-CSF, interleukin [IL] 3, IL-6 [all from Biosource, Camarillo, CA], and flt3 ligand [PeproTech, Rocky Hill, NJ]) as previously described.10,25,26 Cells were stained with allophycocyanin (APC)–conjugated CD34 (BD Biosciences, San Jose, CA) and CFSE+ CD34+ cells collected after fluorescence-activated cell sorting (FACS) with the FACSVantage sorter using the FACSDiva software (BD Biosciences), and cultured in SFM with or without 5 growth factors, and with or without colchicine (Colcemid; Invitrogen, Carlsbad, CA) 100 ng/mL for 96 hours.10 At the end of this time, cells were stained with CD34-APC and sorted into CD34+ divided (dim CFSE+) and quiescent undivided (bright CFSE+) cells, and CD34− divided populations (Figure 1). Cells cultured in colchicine were used to establish the range of fluorescence emitted by cells that had not divided at the end of the culture time period. The BCR-ABL–positive leukemia cell line K562 (ATCC, Manassas, VA), which does not express HLA class I, was cultured in RPMI 1640 supplemented with 10% FCS and used as a positive control for cytotoxicity assays.27,28
Isolation of quiescent noncycling CD34+ cells (QSCs) and cycling CD34+ and CD34− cells from bulk CML CD34+ cells after 4 days culture in serum-free media with 5 growth factors. The QSC gate is determined by the CFSE fluorescence of noncycling CD34+ cells following treatment with colchicine.
Isolation of quiescent noncycling CD34+ cells (QSCs) and cycling CD34+ and CD34− cells from bulk CML CD34+ cells after 4 days culture in serum-free media with 5 growth factors. The QSC gate is determined by the CFSE fluorescence of noncycling CD34+ cells following treatment with colchicine.
In experiments to assess the effect of bortezomib (Velcade, PS-341; Millennium Pharmaceuticals, Cambridge, MA) on CD34+ divided (dim CFSE+) and undivided (bright CFSE+) cells, and CD34− divided populations from CML patients, sorted CFSE+ CD34+ cells were separated into bortezomib treatment versus no bortezomib arms and cultured in SFM with 5 growth factors for 96 hours. The bortezomib treatment arm was incubated with 10 nM bortezomib23 for 16 to 18 hours before the second sort and washed; both arms were stained with CD34-APC and the leukemic subpopulations (CD34+ divided [dim CFSE+] and quiescent undivided [bright CFSE+] cells and CD34− cells) collected and immediately used as targets for cytotoxicity by the effector donor NK cells. The cell sorting procedure lasted 2 to 3 hours.
The viability of cultured cells was assessed before the cytotoxicity assay using trypan blue exclusion on the Vi-Cell XR cell viability analyzer (Beckman Coulter, Fullerton, CA).
NK effector cells
NK cells were isolated from thawed MNCs collected before G-CSF administration, from HLA-identical sibling donors of the respective CML patients, using negative selection with the MiniMACS NK isolation kit II (Miltenyi Biotec), according to the manufacturer's instructions. NK cells were cultured in X-VIVO 20 supplemented with 10% human AB serum and penicillin, streptomycin, and glutamine (Invitrogen), in the presence of 200 U/mL IL-2 and 50 ng/mL IL-15 (all from PeproTech)29 at 37°C and 7% CO2 for 3 days. NK phenotype was assessed using fluorescein isothiocyanate—conjugated CD16, phycoerythrin (PE)–conjugated CD56, and PE-Cy5–conjugated CD3 monoclonal antibodies (all from BD Biosciences). To assess viability, 50 nM TO-PRO-3 (Molecular Probes) was added to the cells immediately before acquisition using the LSRII flow cytometer (BD Biosciences), and analyzed with the FACSDiva software.
Donor NK cells were expanded in vitro by coculturing with irradiated (100 Gy) EBV-transformed lymphoblastoid cell lines as feeder cells in X-VIVO 20 supplemented with 10% human AB serum and penicillin, streptomycin, and glutamine (Invitrogen), in the presence of 500 U/mL IL-2, as previously described.23,30 For all experiments with expanded NK cells, we used cells that had been expanded over 11 to 13 days. Donor NK cells expanded with this method under good manufacturing practice were being used in ongoing clinical trials, and thus would be more applicable for practical and translational purposes.
In experiments to inhibit TRAIL-mediated cytotoxicity, 10 μg/mL mouse anti–human TRAIL antibody (Biolegend, San Diego, CA) or isotype control was incubated with NK cells before cytotoxicity assays.
Cytotoxicity assay
The cytotoxicity assay was performed as previously described31 with modifications. Positive control K562 cells and negative control donor autologous cells were labeled with 1 μM CFSE and rested overnight at 37°C and 5% CO2. Autologous cells consisted of donor MNCs that had been depleted of NK cells and were obtained from the same batch and isolation procedure as the donor NK cells used in the experiments. Cells were washed once with RPMI 1640 and then resuspended in RPMI 1640 supplemented with 10% FCS at 104/mL before the cytotoxicity assay. Sorted CD34+ divided (dim CFSE+) and quiescent undivided (bright CFSE+) cells, and CD34− divided populations from the CML patient were adjusted to 104/mL with SFM supplemented with 5 growth factors. NK effectors were prepared by adjusting to 4 × 105/mL and preparing additional dilutions for 40:1, 20:1, 10:1, 5:1 effector-target (E:T) ratios. One thousand target cells were used for each E/T ratio and all conditions were assessed in triplicate. Cytotoxicity assays were performed in 12 × 75 mm polystyrene round bottom tubes, and after the addition of effector cells to target cells, tubes were agitated gently and the cells pelleted (200g for 1 minute). The samples were then incubated at 37°C with 5% CO2 for 4 hours or 16 to 18 hours. Although standard cytotoxicity assays use 4 hours of incubation28 in experiments to assess the effect of bortezomib on cytotoxicity efficiency, we used 16 hours of incubation, as it was previously reported to be more informative.23 Subsequently, 100 μL cold 0.5% bovine serum albumin/PBS was added to quench cytotoxicity, and the tubes were kept on ice until acquisition on the LSRII flow cytometer. Immediately before acquisition, 10 μL FlowCount Fluorospheres (Beckman Coulter) were added to allow for quantitative analysis. The number of beads in 10 μL was determined and one-fourth of this number was used as the stop gate. To assess viability, 50 nM TO-PRO-3 was added to the cells before acquisition. For each sample, a predetermined number of microbeads were acquired (usually 2500-3000). The absolute number of surviving target cells was determined for each condition by calculation of the ratio between the number of cells and the number of beads. The difference between targets alone acquired at the completion of the cytotoxicity assay (4 or 16 hours), and the number of targets remaining which had been incubated with effector donor NK cells was used to calculate the percentage lysis as given by the formula:
% Lysis = 100 − [(absolute number viable CFSE+ target cells at completion of incubation with effector NK cells / absolute number viable CFSE+ target cells alone) × 100].
Background lysis of target cells alone was determined by comparing the absolute number of target cells alone that were present at the onset of the assay with the absolute number of target cells alone remaining after incubation.
Analysis of surface apoptosis–related receptors on target patient cells
In experiments to determine the surface expression of NK-cell cytotoxicity–associated molecules and death receptors on CML cells, aliquots of 1 × 106 CFSE+ CD34+ cells that had been cultured for 96 hours and incubated with or without 10 nM bortezomib for 16 hours before cytotoxicity assays were stained with DR4-PE and DR5-PE (Biolegend); CD34-APC; CD95-PE-Cy5; HLA A-, B-, and C-PE-Cy5; and MICA/B-PE (BD Biosciences). CD34+ divided (dim CFSE+) and quiescent undivided (bright CFSE+) cells, and CD34− divided populations were then analyzed for coexpression of CD95 (fas); DR4 and DR5 (TRAIL receptors); HLA A, B, and C; and MICA/B.
Real-time quantitative polymerase chain reaction
Total RNA was extracted from FACS-sorted CD34+ cells using the RNeasy kit (QIAGEN, Valencia, CA). Reverse transcription was performed using the Advantage RT-for-PCR kit (Clontech Laboratories, Mountain View, CA). Primers and TaqMan probes for BCR-ABL32 and ABL expression as the endogenous cDNA quantity control for all samples was measured as previously described.33 All reactions were performed in triplicate on 10 μL volume using standard conditions with 40 cycles of amplification using the ABI PRISM 7900 sequence detection system.
Fluorescence in situ hybridization
FACS-sorted cycling and quiescent CD34+ cells were analyzed for the proportion of BCR-ABL positive cells by fluorescence in situ hybridization (FISH) using standard techniques with the BCR-ABL LSI dual-color dual-fusion translocation probes (Vysis, Abbott Laboratories, Des Plaines, IL).
Killer immunoglobulin-like receptor typing
Killer immunoglobulin-like receptor (KIR) genotyping was performed on genomic DNA from patient and donor MNCs using the sequence specific primer–polymerase chain reaction (SSP-PCR) with the KIR Genotyping Kit (Dynal Biotech; Pel-Freez Clinical Systems, Brown Deer, WI), according to the manufacturer's instructions to detect the presence or absence of KIR genes, as previously described.22
Statistical methods
Data between groups were compared using the Mann-Whitney U test or Student t test as appropriate using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA), or Welch modified 2-sample t test using the S-plus 8.1 statistical package (TIBCO Software, Palo Alto, CA). All quoted P values are from 2-sided tests with values less than .05 considered significant.
Results
Characteristics of target quiescent and divided CML CD34+cells and NK effectors
After 4 days culture in SFM with or without 5 growth factors, CD34+ cells from CML patients were sorted into CD34+ divided (dim CFSE+), CD34+ quiescent undivided (bright CFSE+), and CD34− populations (Figure 1). Under these conditions, all sorted populations expressed similar levels of BCR-ABL mRNA irrespective of the presence or absence of growth factors (data not shown). In 7 representative patients, FISH demonstrated greater than 80% BCR-ABL positivity in sorted populations. NK cells expanded for 11 to 13 days from healthy HLA-identical sibling donors had a viability of 90% (± 5.7%), and mean purity of 91% CD56+ CD3− cells. The majority of these NK cells (mean, 69%) coexpressed CD16.
Differential susceptibility of divided and quiescent CML CD34+cells to NK-cell cytotoxicity
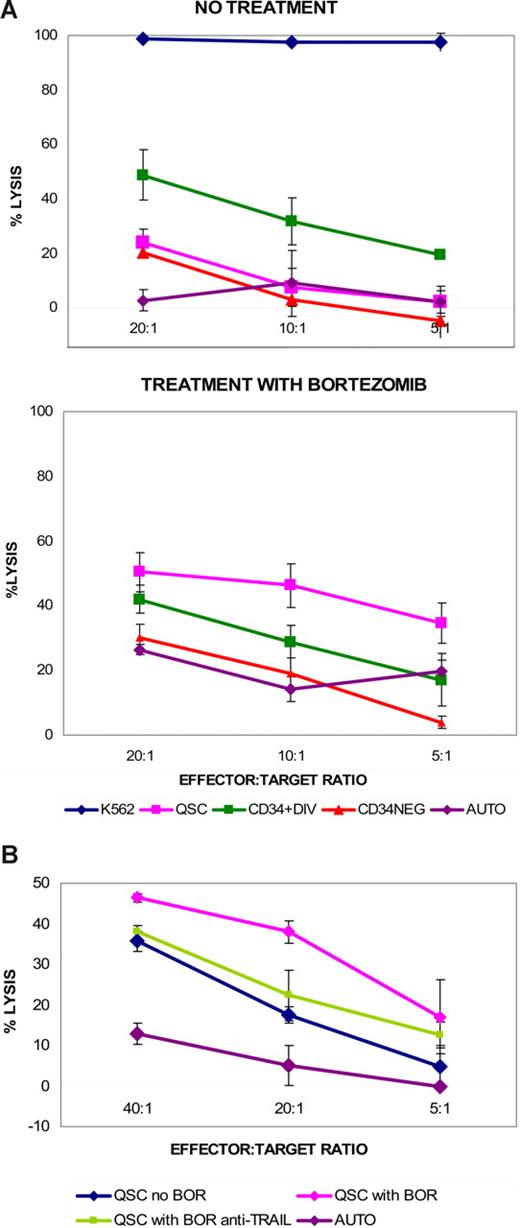
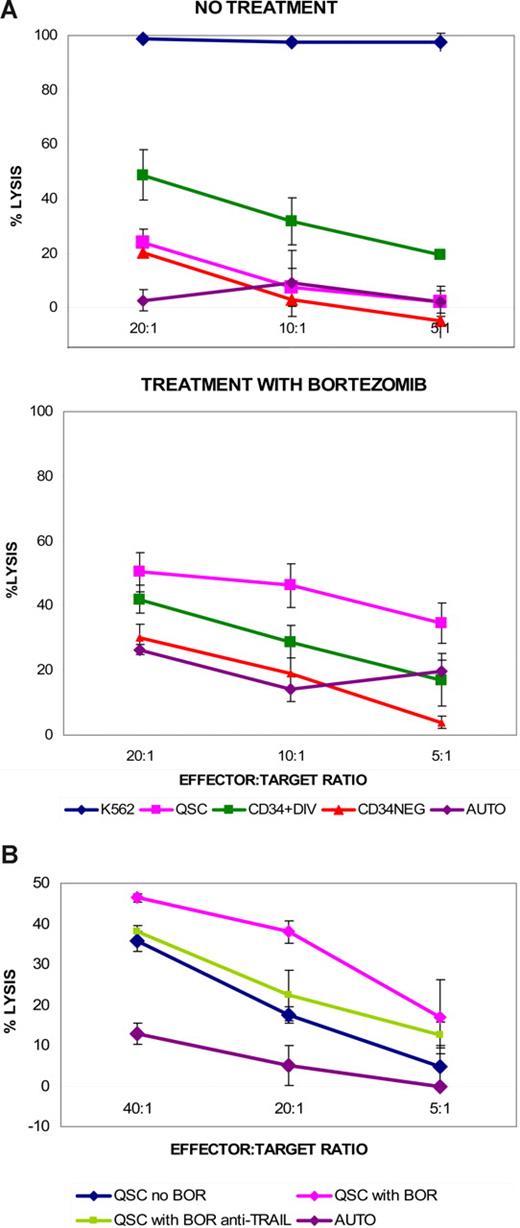
We tested the ability of allogeneic HLA-identical sibling donor NK cells to lyse the progeny of cultured CD34+ cells from CML patients and found that, compared with the divided CD34+ and CD34− populations, quiescent CD34+ cells were the least susceptible to cytotoxicity using NK cells that had been cultured for 3 days in IL-2 and IL-1529 (Figure 2A). The same pattern of reduced susceptibility of quiescent CD34+ cells from CML patients to NK-cell lysis compared with other sorted populations was seen in cytotoxicity assays from 3 additional patient-donor pairs.
Differential susceptibility of cycling and quiescent CD34+ CML cells to HLA-identical sibling donor NK-cell cytotoxicity. (A) Quiescent CD34+ CML cells (QSC) are less susceptible to NK-cell cytotoxicity than cycling CD34+ (CD34+DIV) and CD34− (CD34NEG) progeny from the same bulk CML CD34+ culture. (Unique patient identifier [UPI] 360, accelerated-phase CML; 4-hour cytotoxicity using NK cells cultured for 3 days with IL-2 and IL-15; AUTO = autologous control). Data representative of cytotoxicity assays from 4 separate patient-donor pairs. (B) Donor NK cells expanded after 11 to 13 days are more cytotoxic after 4-hour incubation with control K562 cells, but QSCs are still less susceptible than other cycling cell populations (UPI 400, accelerated-phase CML). Data representative of cytotoxicity assays from 4 separate patient-donor pairs.
Differential susceptibility of cycling and quiescent CD34+ CML cells to HLA-identical sibling donor NK-cell cytotoxicity. (A) Quiescent CD34+ CML cells (QSC) are less susceptible to NK-cell cytotoxicity than cycling CD34+ (CD34+DIV) and CD34− (CD34NEG) progeny from the same bulk CML CD34+ culture. (Unique patient identifier [UPI] 360, accelerated-phase CML; 4-hour cytotoxicity using NK cells cultured for 3 days with IL-2 and IL-15; AUTO = autologous control). Data representative of cytotoxicity assays from 4 separate patient-donor pairs. (B) Donor NK cells expanded after 11 to 13 days are more cytotoxic after 4-hour incubation with control K562 cells, but QSCs are still less susceptible than other cycling cell populations (UPI 400, accelerated-phase CML). Data representative of cytotoxicity assays from 4 separate patient-donor pairs.
To extend these results to a clinically applicable context, we subsequently repeated cytotoxicity assays using donor NK cells expanded in vitro with techniques that are currently being developed for clinical trials.30 We found that expanded allogeneic sibling donor NK cells were also more cytotoxic toward divided CD34+ and CD34− CML cells than quiescent CD34+ CML cells (Figure 2B). This pattern of differential susceptibility to NK-cell cytotoxicity using in vitro expanded donor NK cells, with the greater resistance to killing of quiescent CD34+ CML cells, was consistently found in 3 additional patient-donor pairs.
Differential expression of death receptor proteins on quiescent and divided CD34+ cells and up-regulation with bortezomib
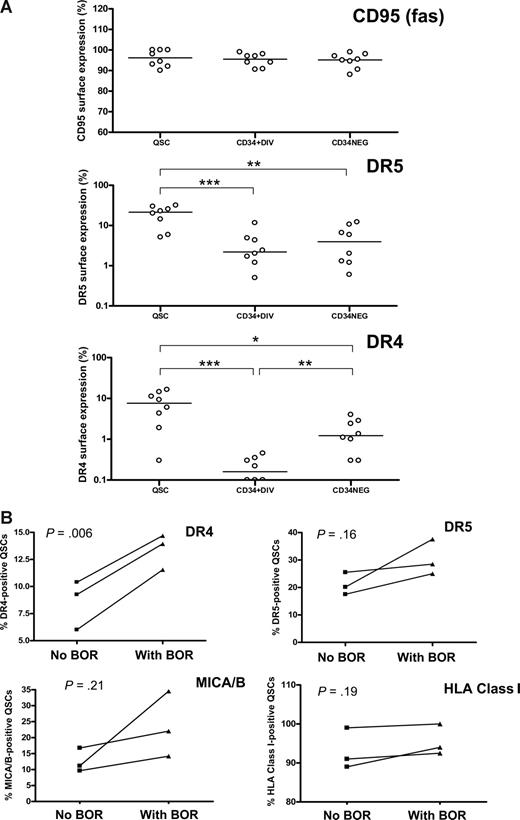
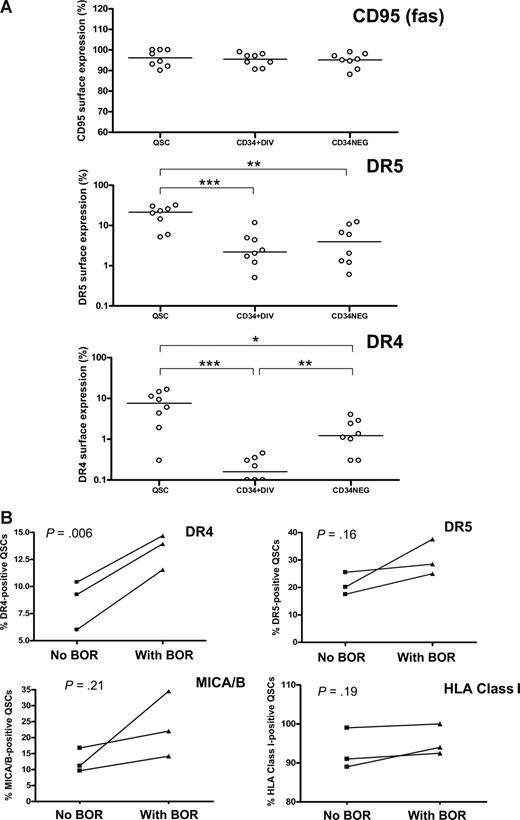
To further investigate the cause of the differential susceptibility of CML dividing and quiescent CD34+ cells and CD34− cells to NK cell–mediated cytotoxicity, we studied the surface expression of death receptor proteins. All subpopulations studied had equally high expression of Fas (CD95). However, there was a significantly higher expression of TRAIL receptors DR4 and DR5 on the surface of quiescent CD34+ cells than on divided CD34+ or CD34− cells (Figure 3A). There was no significant difference in the surface expression of MICA/B or HLA A, B, or C between the populations sorted (data not shown).
Surface expression of death receptor proteins on quiescent CD34+ and cycling CD34+ and CD34− CML cells. (A) Expression of Fas (CD95), TRAIL receptors DR4 and DR5 on quiescent CD34+ (QSC), cycling CD34+ (CD34+DIV) and CD34− (CD34NEG) CML cells. *P < .05, **P < .01, ***P < .001 (n = 3 chronic phase, n = 4 accelerated phase, n = 1 blast crisis) (B) Effect of 10 nM bortezomib (BOR) treatment on the surface expression of TRAIL receptors DR4 and DR5, MICA/B, and major histocompatibility complex class I (HLA A,B, and C) on QSCs (mean of 2 separate experiments in each patient-donor pair; UPI 360, UPI 452, accelerated-phase CML and UPI 481, blast-crisis CML).
Surface expression of death receptor proteins on quiescent CD34+ and cycling CD34+ and CD34− CML cells. (A) Expression of Fas (CD95), TRAIL receptors DR4 and DR5 on quiescent CD34+ (QSC), cycling CD34+ (CD34+DIV) and CD34− (CD34NEG) CML cells. *P < .05, **P < .01, ***P < .001 (n = 3 chronic phase, n = 4 accelerated phase, n = 1 blast crisis) (B) Effect of 10 nM bortezomib (BOR) treatment on the surface expression of TRAIL receptors DR4 and DR5, MICA/B, and major histocompatibility complex class I (HLA A,B, and C) on QSCs (mean of 2 separate experiments in each patient-donor pair; UPI 360, UPI 452, accelerated-phase CML and UPI 481, blast-crisis CML).
Furthermore, as the expanded donor NK cells have been shown to have enhanced surface expression of TRAIL,30 and bortezomib up-regulated TRAIL receptor expression in tumor cell lines,23 we tested the effect of bortezomib on CML CD34+ cells. Addition of 10 nM bortezomib to cell cultures overnight (16-18 hours) up-regulated DR4 expression on quiescent CD34+ cells in particular (Figure 3B). Bortezomib had no significant effect on the surface expression of any other death receptor proteins studied. The dose of bortezomib at 10 nM for 16 to 18 hours did not significantly affect the viability of cultured CML CD34+ cells (viability 88% ± 8.8% for bortezomib treatment vs 90% ± 9.4% with no bortezomib). NK cell–mediated lysis of bortezomib-treated quiescent CML CD34+ cells was enhanced compared with untreated controls (Figure 4A). In contrast, the cytotoxic effects of NK cells on divided CD34+ or CD34− cells were minimally affected by bortezomib treatment. When NK cells were incubated with anti-TRAIL antibody before cytotoxicity assays, there was a modest reduction of NK-cell cytotoxicity on bortezomib-treated quiescent CML CD34+ cells. This suggested that the observed enhanced cytotoxicity was in part dependent on TRAIL activity (Figure 4B).
Effect of bortezomib treatment on quiescent CD34+ and cycling CD34+ and CD34− CML cells. (A) Enhancement of killing of quiescent CD34+ CML cells (QSC), but not cycling CD34+ (CD34+DIV) or CD34− (CD34NEG) cells following bortezomib treatment. AUTO = autologous control (B) Anti-TRAIL antibody diminishes the enhanced NK-cell cytotoxicity of QSC following bortezomib treatment. Bortezomib treatment versus no treatment, P = .019; bortezomib treatment with anti-TRAIL antibody versus no treatment, P = .448, at 20:1 E/T ratio, using Welch modified 2-sample t test. (Data using cells from UPI 481, blast-crisis CML; 16-hour cytotoxicity using expanded HLA-identical donor NK cells, representative of cytotoxicity assays from 3 separate patient-donor pairs, n = 2 accelerated-phase, n = 1 blast-crisis CML.)
Effect of bortezomib treatment on quiescent CD34+ and cycling CD34+ and CD34− CML cells. (A) Enhancement of killing of quiescent CD34+ CML cells (QSC), but not cycling CD34+ (CD34+DIV) or CD34− (CD34NEG) cells following bortezomib treatment. AUTO = autologous control (B) Anti-TRAIL antibody diminishes the enhanced NK-cell cytotoxicity of QSC following bortezomib treatment. Bortezomib treatment versus no treatment, P = .019; bortezomib treatment with anti-TRAIL antibody versus no treatment, P = .448, at 20:1 E/T ratio, using Welch modified 2-sample t test. (Data using cells from UPI 481, blast-crisis CML; 16-hour cytotoxicity using expanded HLA-identical donor NK cells, representative of cytotoxicity assays from 3 separate patient-donor pairs, n = 2 accelerated-phase, n = 1 blast-crisis CML.)
Donor KIR genotype and leukemia susceptibility to NK-cell cytotoxicity
Ten HLA-identical sibling patient-donor pairs had sufficient biological material available for cytotoxicity assays. We previously found that higher numbers of inhibitory KIR genes in donors correlated with higher peripheral blood NK counts in CML patients 30 days after T cell–depleted HLA-identical SCT and were associated with improved transplantation outcome.22 Based on this finding, we analyzed KIR genotyping in the patient-donor pairs for which in vitro cytotoxicity data were performed. The median number of donor inhibitory KIR genes was 7 (range, 5-8), with 3 of 10 donors having fewer than 7 inhibitory KIR genes (Table 1). With the caveat of the limited number of patient-donor pairs tested, quiescent CML CD34+ cells were the least susceptible population to NK-cell cytotoxicity compared with cycling populations, regardless of patient-donor KIR status.
Discussion
The success of TKIs as single-agent treatment in CML has resulted in a major change in management for most patients in chronic phase, compared with a decade ago. There is accumulating evidence that patient survival is significantly prolonged with achievement of complete cytogenetic remission on imatinib therapy.3 These promising results are offset by the fact that despite achieving complete cytogenetic remission, a substantial proportion of patients with clinically satisfactory responses still harbor disease, as evidenced by the detection of BCR-ABL transcripts and Philadelphia chromosome–positive CD34+ cells.3,8,9 A proportion of these BCR-ABL–positive cells may be quiescent and insensitive to imatinib or other TKIs.10,11 Furthermore, nonproliferating quiescent CD34+ cells in CML have been found to be more resistant than proliferating leukemic cells to apoptosis after treatment with several chemotherapeutic agents, including cytarabine and arsenic.34 Recently, the farnesyltransferase inhibitor BMS-214662 has shown promise in vitro in inducing apoptosis in quiescent CML CD34+ cells, but its in vivo clinical efficacy remains untested.35 In contrast, SCT has been proven to be curative in many CML patients, with the most potent antileukemic activity being attributed to the GVL effect. We sought to dissect out the components of cellular mechanisms that may target quiescent CML CD34+ cells in the setting of SCT and to propose new strategies to enhance and improve the cytotoxicity of GVL effectors.
We chose to use the same model for quiescent CML CD34+ cells as that used to demonstrate their resistance to TKIs.10,11 The noncycling CD34+ population remaining after 4 days of culture under serum-free media conditions with a high concentration of growth factors has been characterized extensively by others10,25,26 and shown to consist of quiescent cells. The strength of our study lies in the use of patient CML cells paired with NK cells from their HLA-identical sibling donors to simulate in vivo conditions after SCT. The majority of patients included in this study were part of a previously described cohort, in which a superior outcome after SCT was associated with improved NK-cell recovery and specific donor KIR genotypes.22 Furthermore, all cytotoxicity assays using cells from advanced-phase CML patients were from nonresponders to imatinib treatment. The use of CFSE to isolate quiescent CML CD34+ cells also enabled us to perform cytotoxicity assays31 on this vanishingly small population of cells and compare them with their dividing counterparts within the same experiment, to minimize experimental variation.
We found that the expression of BCR-ABL and minor histocompatibility antigens (data not shown) was the same for both quiescent and cycling CML CD34+ and CD34− cells in this culture system. Jamieson and colleagues had previously found that the most primitive CD34+ progenitors from chronic-phase CML patients had higher BCR-ABL expression than more mature common myeloid or granulocyte-macrophage CD34+ progenitors, although the converse was found in blast-crisis CML patients, who exhibited higher BCR-ABL expression in more mature CD34+ progenitors.36 On the other hand, Copland and colleagues found no difference in BCR-ABL expression between total CD34+ cells and CD34+ CD38− cells from CML patients, but BCR-ABL expression was lower in MNCs than in CD34+ populations.11 With the caveat of the small sample size, in our experiments, the BCR-ABL expression of CD34− cells, following a 4-day culture of parental CD34+ cells in SFM with 5 growth factors, was lower than the expression of quiescent CD34+ cells, but this difference did not reach statistical significance (P = .097). Our BCR-ABL results are not directly comparable to those obtained from phenotyped populations of unstimulated CML CD34+ cells. There is no published data comparing BCR-ABL expression in quiescent cells with cycling CML CD34+ and CD34− cells. However, recent microarray profiling of quiescent and cycling CML CD34+ cells revealed very little difference between the transcriptional profiles of these 2 populations, except for cell-cycle genes,26 suggesting that their BCR-ABL levels are not that disparate, because their downstream target genes are not differentially expressed. Recently, quiescent CML CD34+ cells have been found to be resistant to the cytotoxic effects of potent minor histocompatibility antigen–specific CTLs.37 These results indicate a need for new techniques to target this small but important cell population. Thus, we used NK cells from HLA-identical sibling donors of CML patients as GVL effectors in our studies.
We found that quiescent CML CD34+ cells were more resistant to NK-cell cytotoxicity than cycling CD34+ or CD34− cells. The greater resistance of quiescent, primitive, leukemic cells to elimination by either chemotherapy10,11,34 or cell-mediated cytotoxicity37 may be an innate property that is part and parcel of the metabolic quiescence that defines this population. However, the identification of a higher surface expression of TRAIL receptors on these cells than on their cycling counterparts suggests that they may be susceptible to TRAIL-mediated killing, presenting an opportunity for TRAIL enhancing agents to operate. Bortezomib is a proteasome inhibitor widely used in the treatment of multiple myeloma. It has been shown to up-regulate the surface expression of death receptors on cancer cells, augmenting NK-cell cytotoxicity of tumor cell lines both in vitro and in vivo.23,38 Proteasome inhibitors induce expression of NKG2D ligands on human fibroblast cell lines, with subsequent enhancement of susceptibility to NK-cell cytotoxicity.39 Although BCR-ABL+ cells have high levels of proteasome activity and expression, bortezomib does not directly affect BCR-ABL expression in CML.40 Bortezomib did not significantly affect viability of CML CD34+ cells after 16 to 18 hours of incubation, as assessed by trypan blue exclusion in our experiments, or after a further 16-hour incubation after sorting, when target cells alone were assessed as controls in cytotoxicity assays. However, antiproliferative and apoptotic effects were reported after a longer period of culture in bortezomib, but no synergism was demonstrated with TKIs.40 In our study, short-term bortezomib treatment of CML CD34+ cells significantly up-regulated the expression of the TRAIL receptor DR4 on the surface of quiescent CML CD34+ cells. There was less up-regulation of DR5 and MICA/B, but no effect on HLA class I or Fas (CD95) surface expression. The enhanced cytotoxic effects on quiescent CML CD34+ cells observed after bortezomib treatment may be exerted in part through its ability to increase TRAIL receptor expression in this particular cell population. TRAIL has been shown to induce apoptosis in BCR-ABL+ cells in CML cell line models, with augmentation in the presence of proteasome inhibition, primarily determined by the expression of TRAIL receptors DR4 and DR5 on leukemia cells.41 Although quiescent CML CD34+ cells have higher expression of TRAIL receptors than cycling progeny, this unique cell population nevertheless eludes killing by activated NK cells. The specific sensitization of quiescent CML CD34+ cells to in vitro expanded NK-cell cytotoxicity by bortezomib may be due to bortezomib-induced caspase activation and retention within target cells,42,43 which especially potentiates TRAIL-associated cytotoxicity in target cells that strongly express TRAIL receptors. The ability of bortezomib to reduce levels of the antiapoptotic protein c-FLIP in leukemia cells may also contribute to the sensitization of TRAIL-associated NK-cell cytotoxicity.44 Although TRAIL- and Fas-mediated pathways are used by NK cells to lyse tumor cells, the main effector pathway of NK-cell cytotoxicity is through perforin/granzyme release, which is unaffected by bortezomib45 and probably accounts for the residual cytotoxicity observed after TRAIL blockade. Fas surface expression was unchanged by bortezomib treatment in all the cell populations studied, as reported in previous studies.23
The role of KIR mismatching as a prognostic indicator of transplantation outcome in T cell–depleted HLA-identical sibling allografts is still contentious. We found that KIR incompatibility was not essential for an antileukemic effect29 in HLA-identical sibling patient-donor pairs and we reported improved survival for CML patients who had a rapid NK-cell recovery by Day +30 after SCT.21,22 In univariate analysis, a higher number of donor inhibitory KIR genes (>7) was associated with improved outcome as a consequence of reduced relapse rates and subsequent prolongation of overall survival.22 In this study, we were limited by the availability of biological material from patient-donor pairs on which to perform cytotoxicity assays. However, in the 10 patient-donor pairs available, we observed similar insensitivity of quiescent CML CD34+ cells to NK-cell cytotoxicity irrespective of donor or patient KIR status. As we only had 3 patient-donor pairs with sufficient biological material to test enhanced killing of quiescent CML CD34+ cells with bortezomib, we could not draw conclusions about the role of donor KIR status on this effect. The absolute number of inhibitory donor KIR genes may influence in vivo NK-cell cytotoxic efficacy against leukemia cells in T cell–depleted HLA-identical sibling allografts, however, this requires substantiation with further studies involving greater patient-donor numbers.
Our results suggest that expanded NK cells from HLA-identical sibling donors can be used as effectors of GVL to eliminate quiescent CML CD34+ cells and that their efficacy may be enhanced by short-term treatment of residual leukemia cells with bortezomib at standard clinical doses. With the change in clinical transplantation practice after the advent of TKIs, a greater proportion of CML patients now receive SCT after failure of TKI treatment, and in later stages of the disease, which historically have been associated with a greater risk of relapse and refractoriness to DLI. It seems likely that effective GVL requires a combination of NK-cell and CTL cytotoxic effects in vivo. Furthermore the failure of DLI in some cases to eliminate leukemia despite causing graft-versus-host disease suggests that CTLs alone are not always effective. Addition of activated donor NK cells to DLI might be more effective at preventing or treating relapses in patients who receive transplants in advanced-phase CML. Furthermore, although our observations demonstrate that bortezomib enhances allogeneic NK-cell killing of quiescent CML CD34+ cells, it is possible that autologous expanded NK cells, which have been shown to kill metastatic renal cell carcinoma,23 could also have this effect, and may be useful in CML patients who have minimal residual disease after imatinib treatment. Further studies and clinical trials to address this are planned.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Heather Jorgensen and Prof Tessa Holyoake from the Section of Experimental Hematology, University of Glasgow, United Kingdom, for technical advice on quiescent CML CD34+ cell cultures, and Dr Giuseppe Sconocchia from the National Research Council Institute for Organ Transplantation and Immunocitology, Rome, Italy, for advice on NK-cell cultures. The authors are grateful to Dr Colin Wu from the Office of Biostatistics Research at the National Heart, Lung, and Blood Institute (NHLBI) for providing formal statistical review.
This research was supported by the Intramural Research Program of the National Institutes of Health at the NHLBI.
National Institutes of Health
Authorship
Contribution: A.S.M.Y. conceived and designed the study, processed samples, performed experiments, analyzed data, and wrote the report; K.K. performed experiments and commented on the manuscript; N.H. performed experiments, analyzed data, and commented on the manuscript; R.E. processed samples and performed experiments; B.N.S. collected patient data, provided clinical care, and commented on the manuscript; M.B. developed NK expansion technique and commented on the manuscript; A.L., J.M.G., and R.C. advised study design and commented on the manuscript; S.A. performed KIR genotyping on samples; E.M.S. performed experiments and commented on the manuscript; and A.J.B. supervised the study, provided clinical care, and wrote the report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.N.S. is Vanderbilt University and VAMC Transplant Program, Nashville, TN; the current affiliation for J.M.G. is Department of Haematology, Imperial College London, Hammersmith Hospital, London, United Kingdom.
Correspondence: Agnes S. M. Yong, MD, PhD, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Building 10, Hatfield CRC, Room 3-5140, 10 Center Drive, MSC 1202, Bethesda, MD 20892-1202; e-mail: yonga@nhlbi.nih.gov.

![Figure 2. Differential susceptibility of cycling and quiescent CD34+ CML cells to HLA-identical sibling donor NK-cell cytotoxicity. (A) Quiescent CD34+ CML cells (QSC) are less susceptible to NK-cell cytotoxicity than cycling CD34+ (CD34+DIV) and CD34− (CD34NEG) progeny from the same bulk CML CD34+ culture. (Unique patient identifier [UPI] 360, accelerated-phase CML; 4-hour cytotoxicity using NK cells cultured for 3 days with IL-2 and IL-15; AUTO = autologous control). Data representative of cytotoxicity assays from 4 separate patient-donor pairs. (B) Donor NK cells expanded after 11 to 13 days are more cytotoxic after 4-hour incubation with control K562 cells, but QSCs are still less susceptible than other cycling cell populations (UPI 400, accelerated-phase CML). Data representative of cytotoxicity assays from 4 separate patient-donor pairs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/4/10.1182_blood-2008-05-158253/4/m_zh80010928840002.jpeg?Expires=1769801505&Signature=Ps0nZKzKOTjTnmFFtQIpsrn-S7Vc2YdwIaEmrnIh4t50BJaj4b-9BYVDlAJYqeCeM1UTByLSCHDLQYiHW8UvzgrcCOURaal6IyK0yghBR1YTH5gCfXVvyUKHqRnWU5BsOymRzCwOvFfMUZfIPPaSBu5GR1qW5tg3BfB~DyHbXhBeCFU7dPIdDRrNkllI3qflEkRxVLlu6bcLrIL4MqJjWqmqt5EFJp9zKl4NYs8uRRIuQP3EzmZ0o0ihcfGwWkDmSRgJIi8o5IxOs4zkBF9BgQlMnccEpGF6kyIKZCCxM38sI7AMh7MuWUZsJI1zILIpZjujlwsAnrOt-IQP9UydeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Differential susceptibility of cycling and quiescent CD34+ CML cells to HLA-identical sibling donor NK-cell cytotoxicity. (A) Quiescent CD34+ CML cells (QSC) are less susceptible to NK-cell cytotoxicity than cycling CD34+ (CD34+DIV) and CD34− (CD34NEG) progeny from the same bulk CML CD34+ culture. (Unique patient identifier [UPI] 360, accelerated-phase CML; 4-hour cytotoxicity using NK cells cultured for 3 days with IL-2 and IL-15; AUTO = autologous control). Data representative of cytotoxicity assays from 4 separate patient-donor pairs. (B) Donor NK cells expanded after 11 to 13 days are more cytotoxic after 4-hour incubation with control K562 cells, but QSCs are still less susceptible than other cycling cell populations (UPI 400, accelerated-phase CML). Data representative of cytotoxicity assays from 4 separate patient-donor pairs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/4/10.1182_blood-2008-05-158253/4/m_zh80010928840002.jpeg?Expires=1769807196&Signature=1W7pbdZk7uv15CEZrLdWA4auuaturnduQredpCs-SUS09RgbBgalmTItv0bqlanyjbSJxBvmBKSsaGaWMph2UXtt65bdNrtKrQHLFKfCnK-RDTPVZ-vR3UxuYleW6v8zf3VTISNdFvuL-rLr-1ac41pkHHI2Y2mQYJR7Q2AwBaUc3mRmUrf6slH0gQWuacTfQTJB4fpjZPZrzjOnfTzItJdD3x8WstwU7LO8rdpDLj1gWEbAtulLB~BHZ-slFYJ2LjLZr7YULKZq74AwHNskixAiRs5-Mtbo54tlmzbjDTWa84Cm2dIdVxCerbYsNYIICbZPsgjXaZEipGybneD8jg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)