Abstract
Bacterial plasminogen activators are commonplace among microbial pathogens, implying a central role of host plasmin in supporting bacterial virulence. Group A streptococci (GAS) secrete streptokinase, a specific activator of human plasminogen (PLG). The critical contribution of the streptokinase-PLG interaction to GAS pathogenicity was recently demonstrated using mice expressing human PLG. To examine the importance of thrombin generation in antimicrobial host defense, we challenged mice with deficiency of factor V (FV) in either the plasma or platelet compartment. Reduction of FV in either pool resulted in markedly increased mortality after GAS infection, with comparison to heterozygous F5-deficient mice suggesting a previously unappreciated role for the platelet FV pool in host defense. Mice with complete deficiency of fibrinogen also demonstrated markedly increased mortality to GAS infection relative to controls. Although FV Leiden may be protective in the setting of severe sepsis in humans, no significant survival advantage was observed in GAS-infected mice carrying the FV Leiden mutation. Taken together, our data support the hypothesis that local thrombosis/fibrin deposition limits the survival and dissemination of at least a subset of microbial pathogens and suggest that common variation in hemostatic factors among humans could affect host susceptibility to a variety of infectious diseases.
Introduction
Streptococcus pyogenes (group A streptococci [GAS]) is estimated to result in more than 700 million symptomatic human infections worldwide each year,1 such as very common but mild pharyngeal and skin infections, as well as less frequent but life-threatening diseases, such as toxic shock–like syndrome and necrotizing fasciitis.2,3 GAS is highly specific to its human host, whereas closely related bacterial species cause similar disorders in other mammals, including strangles, a common pharyngeal infection in horses.4
All pathogenic GAS isolates express streptokinase (SK, encoded by the ska gene), a bacterial activator of plasminogen (PLG), the central enzyme in the host fibrinolytic system.5-8 SK forms a stoichiometric complex with PLG, inducing a conformational change in the PLG activation pocket and nonproteolytically converting PLG into an active serine protease. This complex subsequently proteolytically activates additional PLG molecules.9 The SK from GAS is a highly specific activator of human PLG, whereas the SK generated by related bacterial species infecting other mammals can generally only activate plasminogen from the corresponding host.5,7,8 We previously reported the generation of transgenic mice (hPLG) that express human plasminogen under the control of the murine albumin gene promoter. Although wild-type mice are highly resistant to infection with human pathogenic GAS strains, hPLG-transgenic mice demonstrate markedly enhanced susceptibility and increased mortality. This dramatic difference in susceptibility is lost when the SK gene is removed to generate an SK−GAS strain, demonstrating that the SK/PLG interaction is critical for GAS pathogenicity in the mouse and that SK is a key virulence factor.10
Several other pathogens are known to produce host PLG activators, including staphylokinase from Staphylococcus aureus and the Pla protease from Yersinia pestis.11,12 Microbial pathogens have also been shown to engage other components of the hemostastic system. For example, fibrinogen can form a complex with plasminogen and streptokinase on the bacterial surface, which may be important for the initiation of infection.13
PLG activation may support GAS virulence by removing fibrin matrices surrounding local sites of infection and thereby facilitate microbial dissemination,10 limiting fibrin-dependent leukocyte activation events leading to the local implementation of antimicrobial functions, or a combination of these mechanisms. To further explore these concepts and further characterize the role of thrombin generation and PLG activation in GAS pathogenicity, we now report the analysis of GAS infection in mice with genetically engineered alterations in other hemostatic system components. Reduction in either the platelet or plasma factor V (FV) pool markedly increases susceptibility to GAS, although the presence of FV Leiden fails to provide protection in this murine model. Fibrinogen deficiency also results in a dramatic increase in mortality after GAS infection, suggesting that the primary role of FV-dependent thrombin generation in this model is supporting the formation of the fibrin matrix.
Methods
Bacterial growth and media
Streptococcal strains used for this study were derived from the GAS M type 1 strain MGAS166. UMAA2616 (previously designed as UMAA2392) contains a site-directed deletion in the covR gene and a spontaneous mutation in the covS gene as previously reported.14 Streptococci were cultured in Todd-Hewitt broth (Becton Dickinson, Sparks, MD) containing 0.2% yeast extract (THY) supplemented with streptomycin.
Genetically engineered mice
The generation of fibrinogen-deficient mice carrying an inactivating mutation of the fibrinogen α chain gene has been previously reported.15 The Fga+/− and Fga−/− mice studied here were all derived from a backcross into C57BL/6J for 6 or more generations (≥ N6). The generation of Fibγ390-396A mice expressing a mutant form of fibrinogen that is unable to support αMβ2-mediated adhesion of primary neutrophils, monocytes, and macrophages has been described previously.16 The generation of transgenic mice (Tg+) with FV expression specifically restricted to either the plasma (AlbfvB, AlbfvC) or platelet (Pf4fvA) pools, were previously reported.17 The latter animals were bred into the FV-deficient (F5−/−) background in a 2-generation mating scheme to generated mice (Tg+F5−/−) with different expression levels of FV in the plasma and platelet pools, as previously described.17 To improve the yield of Tg+F5−/− mice available for study, Tg+F5+/− mice in the C57Bl/6J background (> N6) were crossed to SJL/J mice and the Tg+F5+/− F1 offspring backcrossed to C57BL/6J F5+/− mice to generate Tg+F5−/− animals. Transgenic and nontransgenic F5+/− and F5+/+ littermates were used in all experiments to control for the effect of potential strain background differences.
The generation of transgenic mice with liver-specific expression of human PLG was previously described.10 The mice used for the current study will be denoted as hPLG+ and correspond to the AlbPLG1 transgenic line reported in Sun et al.10 Mice carrying the ortholog of human FV Leiden were previously generated by a “knockin” of the R504Q mutation into the endogenous murine F5 locus by homologous recombination.18 This allele is designated here as F5Q, with heterozygous mice indicated as F5Q/+. A cross between hPLG+ and F5Q/+ mice generated the 4 expected genotypes (hPLG-F5+/+, hPLG-F5Q/+, hPLG+F5+/+, and hPLG+F5Q/+). The hPLG+ and F5Q/+ mice used in these studies were derived from backcrosses into C57BL/6J for greater than 10 generations (> N10). All animal studies were approved by the University of Michigan Committee on Use and Care of Animals, and all procedures were in compliance with University guidelines, state and federal regulations, and the standards of the “Guide for the Care and Use of Laboratory Animals.”
GAS infection model
Mice were injected subcutaneously with GAS as previously described.10 Briefly, an overnight culture from a single GAS colony was diluted 1:10 into fresh THY medium and grown to late log phase (OD600nM = 0.75). Six- to 8-week-old mice were injected into the right flank with 100 μL culture diluted to the desired colony number in THY medium, with serial dilutions of the same bacterial cultures plated on THY plates to determine the exact inoculation titer. Mice were monitored for 9 days for mortality.
A cohort of Fga+/− and Fga−/− mice inoculated with 2 × 106 colony-forming units (CFU) GAS was studied for dissemination to the spleen as described previously.10 At 64 hours after inoculation, mice were killed, and spleens were dissected, homogenized in 5 mL THY broth, and cultured quantitatively on THY-streptomycin plates to estimate the CFU.
Prothrombin time measurement
Mouse whole blood was obtained by cardiac puncture with one-tenth volume of 3.8% sodium citrate. Platelet-rich plasma was centrifuged at 1500g for 15 minutes to generate platelet-poor plasma. Prothrombin time (PT) was measured in an Amelung KC 4A Microcoagulation analyzer according to the manufacturer's instructions (Sigma-Aldrich, St Louis, MO).
Statistical analysis
Survival data were analyzed by the log-rank test with the SigmaStat program (Systat Software, Point Richmond, CA) and the R program (http://www.r-project.org/). Differences between spleen colony counts for Fga−/− and Fga+/− mice were evaluated by the Mann-Whitney tests with the SigmaStat program.
Results
Decreased FV level increases host susceptibility to GAS infection
We previously demonstrated that the SK/PLG interaction plays a critical role in GAS pathogenicity, and hypothesized that SK functions to hijack the host fibrinolytic system to remove the fibrin matrices surrounding sites of infection and thus facilitate bacterial growth and dissemination.10 To characterize the role of thrombin generation in host defense against GAS, we took advantage of a series of genetically engineered mouse lines with altered levels and tissue specific distributions of FV, a critical component of the prothrombinase complex. We previously generated transgenic mice with decreased levels of FV restricted to either the plasma or platelet pools by crossing tissue-specific transgenes into the F5 null background (Tg+F5−/−).17 Three lines of mice (AlbfvB, AlbfvC, and Pf4fvA) were used for the current experiments.
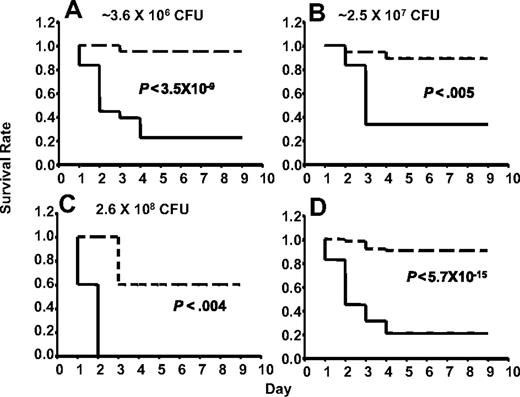
AlbfvBTg+F5−/− mice express approximately 15% of the wild-type plasma FV level, with no detectable platelet FV expression.17 No significant difference in mortality after GAS infection was observed between F5+/+ and F5+/− mice, with or without a F5 transgene, across inoculation doses ranging from 3.6 × 106 to 2.6 × 108 CFU (P > .6; Figure 1). For all subsequent experiments, all of these animals were considered together as a single control group designated F5+. All the F5+ mice (F5+/− or F5+/+, with or without a transgene) were expected to have levels of F5 expression more than or equal to 50% of wild-type F5+/+ mice.
Control mice of the FV-transgenic lines inoculated with GAS strain UMAA2616. F5+/−, Tg+F5+/−, Tg+F5+/+, and F5+/+ mice for all 3 FV-transgenic lines were infected with UMAA2616 at doses of 3.6 × 106 to 2.6 × 108 CFU. Data were pooled from 7 independent experiments using littermates, constituting a total of 18 F5+/−, 27 Tg+F5+/−, 3 Tg+F5+/+, and 7 F5+/+ mice.
Control mice of the FV-transgenic lines inoculated with GAS strain UMAA2616. F5+/−, Tg+F5+/−, Tg+F5+/+, and F5+/+ mice for all 3 FV-transgenic lines were infected with UMAA2616 at doses of 3.6 × 106 to 2.6 × 108 CFU. Data were pooled from 7 independent experiments using littermates, constituting a total of 18 F5+/−, 27 Tg+F5+/−, 3 Tg+F5+/+, and 7 F5+/+ mice.
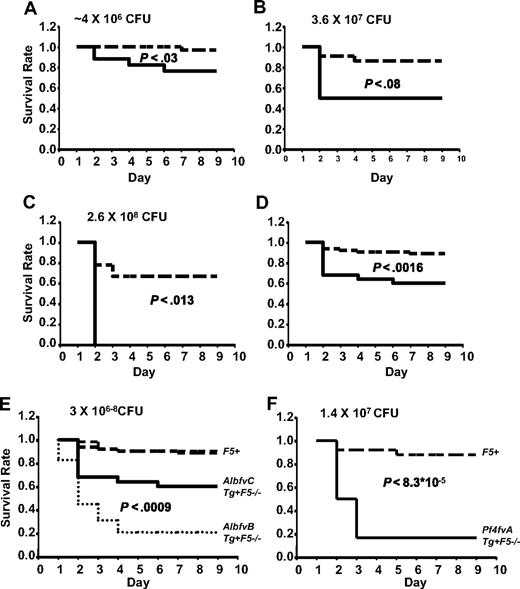
AlbfvBTg+F5−/− mice demonstrated markedly increased susceptibility to infection with GAS strain UMAA2616 compared with their sibling controls across a wide range of inoculation doses (Figure 2A-C). At a GAS infection dose of 3.6 × 106 CFU, Tg+F5−/− mice demonstrated 82% mortality by day 5, compared with 6% mortality in control F5+ mice (Figure 2A). At an approximately 100-fold higher GAS dose (2.6 × 108 CFU), mortality for Tg+F5−/− was 100% by day 2, compared with 40% in control mice (P < .004; Figure 2C). Pooling the survival data across all inoculation doses (Figure 2D), Tg+F5−/− mice demonstrated 83% mortality compared with 11% in controls (P < 6 × 10−15).
AlbfvB mice inoculated with GAS strain UMAA2616. P values for differences between the survival curves are indicated in all panels. The solid line represents Tg+F5−/−; dashed lines, F5+ littermate controls. (A) Inoculum of 3.6 × 106 CFU. Data were pooled from 4 independent experiments (totaling 17 AlbfvBTg+F5−/− mice and 32 littermate F5+ controls), at doses ranging from 1.2 × 106 to 4.9 × 106 CFU. (B) Inoculum of 2.5 × 107 CFU. Data were pooled from 2 independent experiments (totaling 6 AlbfvBTg+F5−/− mice and 18 littermate F5+ controls) at doses of 1.4 × 107 and 3.6 × 107 CFU. (C) Inoculum of 2.6 × 108 CFU. Data were from a single experiment with 5 AlbfvBTg+F5−/− mice and 5 F5+ littermate controls. (D) Pooled data from all the experiments shown in panels A to C (a total of 28 AlbfvBTg+F5−/− mice and 55 F5+ littermate controls).
AlbfvB mice inoculated with GAS strain UMAA2616. P values for differences between the survival curves are indicated in all panels. The solid line represents Tg+F5−/−; dashed lines, F5+ littermate controls. (A) Inoculum of 3.6 × 106 CFU. Data were pooled from 4 independent experiments (totaling 17 AlbfvBTg+F5−/− mice and 32 littermate F5+ controls), at doses ranging from 1.2 × 106 to 4.9 × 106 CFU. (B) Inoculum of 2.5 × 107 CFU. Data were pooled from 2 independent experiments (totaling 6 AlbfvBTg+F5−/− mice and 18 littermate F5+ controls) at doses of 1.4 × 107 and 3.6 × 107 CFU. (C) Inoculum of 2.6 × 108 CFU. Data were from a single experiment with 5 AlbfvBTg+F5−/− mice and 5 F5+ littermate controls. (D) Pooled data from all the experiments shown in panels A to C (a total of 28 AlbfvBTg+F5−/− mice and 55 F5+ littermate controls).
Thus, FV expression reduced to 15% of wild-type (F5+/+) and restricted to the plasma pool (AlbfvBTg+F5−/− mice) results in markedly increased mortality after GAS infection. To distinguish the effects of reduced plasma FV from the absence of platelet FV, a similar series of experiments were conducted in AlbfvCTg+F5−/−-transgenic mice, which also exhibit absent platelet FV but with approximately 45% of the plasma FV level observed in wild-type mice (compared with 15% in AlbfvBTg+F5−/− mice). As shown in Figure 3A, AlbfvCTg+F5−/− mice also exhibited increased mortality by day 9 (24% compared with 3% in littermate controls, P < .03, 4.0 × 106 CFU). Higher mortalities were observed at higher doses of infection (50% at 3.6 × 107 CFU and 100% at 2.6 × 108 CFU, Figure 3B,C). Combining data across all doses tested, AlbfvCTg+F5−/− mice exhibited significantly increased mortality by day 9 (40% compared with 12% in littermate controls, P < .002, Figure 3D).
AlbfvC and Pf4fvA mice inoculated with GAS strain UMAA2616. P values for survival differences are indicated in each panel. — represents Tg+F5−/−; ----, F5+ littermate controls. (A) AlbfvC mice at an inoculum of approximately 4 × 106 CFU. Data were pooled from 2 independent experiments (totaling 17 AlbfvCTg+F5−/− mice and 32 littermate F5+ controls), at doses of 3.7 × 106 and 4.4 × 106 CFU, respectively. (B) AlbfvC mice at an inoculum of 3.6 × 107 CFU. Data were derived from 1 experiment (4 AlbfvCTg+F5−/− mice and 22 littermate F5+ controls). (C) AlbfvC mice at an inoculum of 2.6 × 108 CFU. Data were derived from 1 experiment (4 AlbfvCTg+F5−/− mice and 9 littermate F5+controls). (D) Pooled data from all experiments (25 AlbfvCTg+F5−/− mice and 63 littermate F5+ controls). (E) Comparison of the AlbfvC and AlbfvB lines (combined from Figures 3D and 2D). P value of the difference between Tg+F5−/− mice from AlbfvC and AlbfvB lines is indicated. (F) Pf4fvA mice at an inoculum of 1.4 × 107 CFU. Data were derived from 1 experiment (6 Pf4fvATg+F5−/− mice and 25 littermate F5+ controls).
AlbfvC and Pf4fvA mice inoculated with GAS strain UMAA2616. P values for survival differences are indicated in each panel. — represents Tg+F5−/−; ----, F5+ littermate controls. (A) AlbfvC mice at an inoculum of approximately 4 × 106 CFU. Data were pooled from 2 independent experiments (totaling 17 AlbfvCTg+F5−/− mice and 32 littermate F5+ controls), at doses of 3.7 × 106 and 4.4 × 106 CFU, respectively. (B) AlbfvC mice at an inoculum of 3.6 × 107 CFU. Data were derived from 1 experiment (4 AlbfvCTg+F5−/− mice and 22 littermate F5+ controls). (C) AlbfvC mice at an inoculum of 2.6 × 108 CFU. Data were derived from 1 experiment (4 AlbfvCTg+F5−/− mice and 9 littermate F5+controls). (D) Pooled data from all experiments (25 AlbfvCTg+F5−/− mice and 63 littermate F5+ controls). (E) Comparison of the AlbfvC and AlbfvB lines (combined from Figures 3D and 2D). P value of the difference between Tg+F5−/− mice from AlbfvC and AlbfvB lines is indicated. (F) Pf4fvA mice at an inoculum of 1.4 × 107 CFU. Data were derived from 1 experiment (6 Pf4fvATg+F5−/− mice and 25 littermate F5+ controls).
A comparison between the pooled survival data for the 2 plasma-specific FV-transgenic lines is shown in Figure 3E. Significantly greater mortality was observed with the lower plasma FV levels of AlbfvBTg+F5−/− mice (83% mortality by day 9, compared with 40% in AlbfvCTg+F5−/−, P < .001). Thus, even a modest reduction in plasma FV from 45% to 15% (levels that would not be expected to result in significant clinical bleeding17,19 ) was associated with markedly increased mortality after GAS infection. These results demonstrate a critical dependence of host defense against GAS on the quantitative capacity for thrombin generation in plasma. PTs in platelet-poor plasma samples were also significantly longer in AlbfvBTg+F5−/− (20.9 ± 5.13 seconds) than in F5+/− (10.55 ± 0.33 seconds) (P < .013, n = 3 for each group of mice).
The increased mortality observed in AlbfvCTg+F5−/− mice (Figure 3A,D) could result either from the modest reduction in plasma FV (to ∼ 45% of wild-type), the absence of FV from the platelet pool, or a combination of these effects. As shown in Figure 1, F5+/− mice with similar plasma FV levels (50% compared with 45% in AlbfvCTg+F5−/− mice) but 50% platelet FV levels (compared with < 1% in AlbfvCTg+F5−/− mice) were resistant to GAS-associated mortality and indistinguishable from wild-type F5+/+ mice. These data suggest that the enhanced mortality observed in AlbfvCTg+F5−/− is primarily the result of the absence of the platelet FV pool and imply a critical role for platelet FV in host defense from GAS and potentially other microbial pathogens.
To further examine the role of platelet FV, mice engineered to restrict FV only to the platelet pool (Pf4fvATg+F5−/−)17 were also tested in this GAS infection model. As shown in Figure 3F, Pf4fvATg+F5−/− mice were also highly susceptible to UMAA2616 infection, with 83% mortality compared with 12% mortality in littermate controls (P < 9 × 10−5; Figure 3F). Of note, Pf4fvATg+F5−/− mice express only approximately 3% of the wild-type platelet FV level. Thus, the increased mortality in these animals could be the result of either the reduced level of platelet FV or the absence of the plasma FV pool (or a combination of both). However, taken together, these data suggest that both the plasma and platelet pools of FV are required for optimal host defense against GAS infection.
Decreasing fibrinogen level increases host susceptibility to GAS infection
The data presented in the previous section demonstrate a critical role for the prothrombinase complex, and the resulting generation of thrombin, in host defense against GAS. Although we hypothesize that the fibrin generated by thrombin action provides a critical barrier against GAS dissemination and fibrin-dependent implementation of leukocyte antimicrobial programs, thrombin has a myriad of other functions, which could also contribute to host defense, including platelet activation, activation of protein C, and initiation of cellular signaling pathways.20,21 To separate the role of fibrin generation from other functions of thrombin, we next examined the consequences of GAS infection in mice with a genetic deficiency in plasma fibrinogen.15
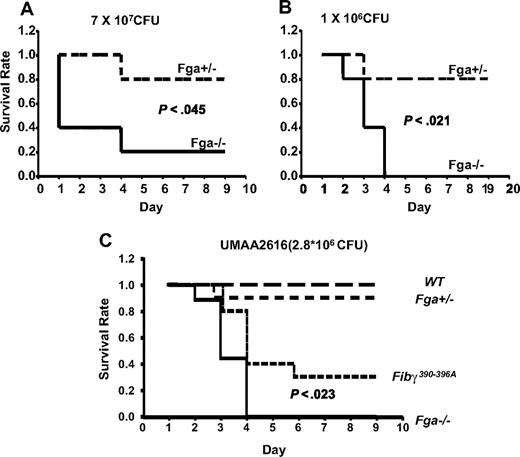
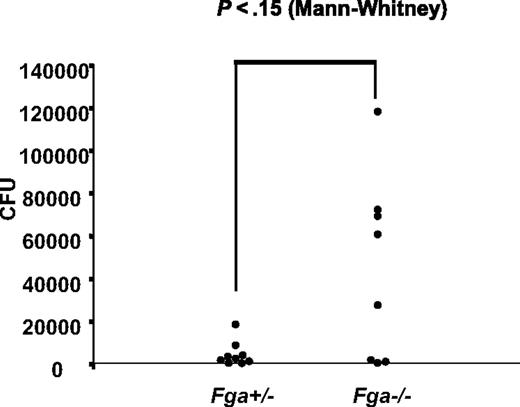
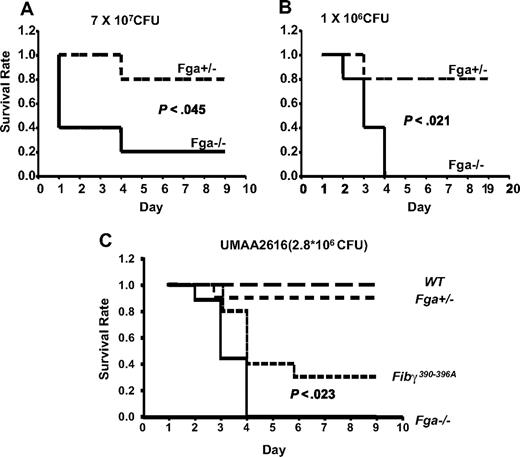
Fga−/− and littermate Fga+/− mice were infected with GAS strain UMAA2616, with the resulting survival curves shown in Figure 4. Fga−/− mice exhibited 80% mortality by day 4 compared with 20% mortality in heterozygote Fga+/− littermates (P < .05; Figure 4A). A similar survival difference was still observed at a 70-fold lower inoculation dose (P < .03; Figure 4B). Although the difference did not reach significance, we also observed a trend toward increased GAS colony counts in the spleen of infected Fga−/− mice at day 2, compared with Fga+/− littermates (P = .15; Figure 5), consistent with increased systemic spread.
Fga−/− and Fga+/− mice infected with UMAA2616. P values of survival difference are indicated in all panels. — represents Fga−/−; ----, Fga+/− littermate controls. (A) Five Fga−/− mice and 5 sibling control Fga+/− mice were infected with 7 × 107 CFU UMAA2616. (B) Five Fga−/− mice and 5 sibling control Fga+/− mice were infected with 106 CFU UMAA2616. (C) Ten Fibγ390-396A and wild-type sibling controls, 9 Fga−/−, and 10 Fga+/− sibling control were infected with 2.8 × 106 CFU UMAA2616.
Fga−/− and Fga+/− mice infected with UMAA2616. P values of survival difference are indicated in all panels. — represents Fga−/−; ----, Fga+/− littermate controls. (A) Five Fga−/− mice and 5 sibling control Fga+/− mice were infected with 7 × 107 CFU UMAA2616. (B) Five Fga−/− mice and 5 sibling control Fga+/− mice were infected with 106 CFU UMAA2616. (C) Ten Fibγ390-396A and wild-type sibling controls, 9 Fga−/−, and 10 Fga+/− sibling control were infected with 2.8 × 106 CFU UMAA2616.
GAS systemic spread in Fga−/− and Fga+/− mice infected with UMAA2616. Bacterial counts from spleens of 8 Fga−/− and 9 Fga+/− mice inoculated with 2 × 106 CFU UMAA2616 were calculated by a plating method. Data were pooled from 2 independent experiments (2.8 × 106 and 2.0 × 106 CFU). The difference of CFU between Fga−/− and Fga+/− mice was tested by the Mann-Whitney test. P value is indicated.
GAS systemic spread in Fga−/− and Fga+/− mice infected with UMAA2616. Bacterial counts from spleens of 8 Fga−/− and 9 Fga+/− mice inoculated with 2 × 106 CFU UMAA2616 were calculated by a plating method. Data were pooled from 2 independent experiments (2.8 × 106 and 2.0 × 106 CFU). The difference of CFU between Fga−/− and Fga+/− mice was tested by the Mann-Whitney test. P value is indicated.
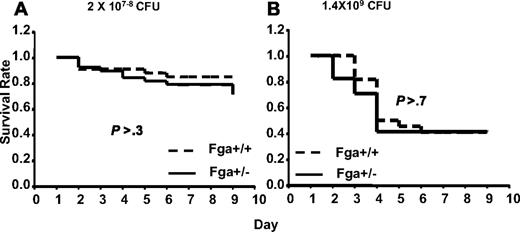
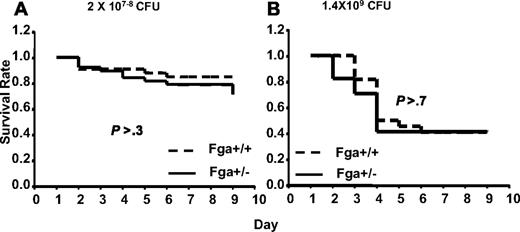
Analysis of GAS susceptibility in cohorts of Fga+/+ mice compared with Fga+/− mice carrying marginally lower (∼ 20% decrease)15 plasma fibrinogen levels revealed no significant difference (P > .3) in survival (Figure 6). Thus, complete absence of fibrinogen in Fga−/− mice results in marked increased mortality after GAS infection, whereas little or no effect is evident from an approximate 20% reduction in plasma fibrinogen (Fga+/−).
Fga+/− and Fga+/+ mice infected with UMAA2616. P values of survival difference are indicated in all panels. — represents Fga+/−; ----, Fga+/+ littermate controls. (A) Fga+/− mice and sibling control Fga+/+ mice were infected with 2 × 107 to 108 CFU UMAA2616. Data were pooled from 4 independent experiments totaling 38 Fga+/− mice and 33 Fga+/+ mice. (B) Fga+/− mice and sibling control Fga+/+ mice were inoculated with 1.4 × 109 CFU UMAA2616, totaling 17 Fga+/− mice and 22 Fga+/+ mice in 1 experiment.
Fga+/− and Fga+/+ mice infected with UMAA2616. P values of survival difference are indicated in all panels. — represents Fga+/−; ----, Fga+/+ littermate controls. (A) Fga+/− mice and sibling control Fga+/+ mice were infected with 2 × 107 to 108 CFU UMAA2616. Data were pooled from 4 independent experiments totaling 38 Fga+/− mice and 33 Fga+/+ mice. (B) Fga+/− mice and sibling control Fga+/+ mice were inoculated with 1.4 × 109 CFU UMAA2616, totaling 17 Fga+/− mice and 22 Fga+/+ mice in 1 experiment.
Fibrin(ogen) also supports leukocyte adhesion and activation events via the leukocyte integin αMβ2, an interaction that is critical for bacterial clearance.22 To distinguish between this property of fibrin(ogen) from all other functions in the GAS infection model, Fibγ390-396A mice expressing a mutant form of fibrinogen lacking the αMβ2 binding motif were tested for GAS susceptibility. Significant differences in survival were observed. Whereas all tested Fga−/− mice died of GAS infection by day 4, Fibγ390-396A mice demonstrated a delayed mortality, with improved survival (P < .023; Figure 4C).
FV Leiden does not reduce GAS-related mortality in the mouse
FV Leiden is the most common genetic predisposition to thrombosis, with prevalence of approximately 4% to 6% in European populations.23 FV carrying the R506Q Leiden mutation is resistant to inactivation by activated protein C (APC) cleavage, leading to increased thrombin generation, presumably accounting for the associated elevated risk of venous thrombosis in FV Leiden carriers.24,25 FV Leiden has been reported to protect against endotoxin challenge in mice and to confer a survival advantage for human patients with severe sepsis.26
In light of the enhanced susceptibility of FV-deficient mice to GAS infection, we next tested whether the FV Leiden gain-of-function mutation would afford protection in this model system. We previously generated a mouse model of FV Leiden by a “knockin” of the orthologous F5 R504Q substitution into the mouse F5 gene.18 Similar to humans, mice carrying the murine FV Leiden mutation are mildly prothrombotic, though otherwise phenotypically normal.
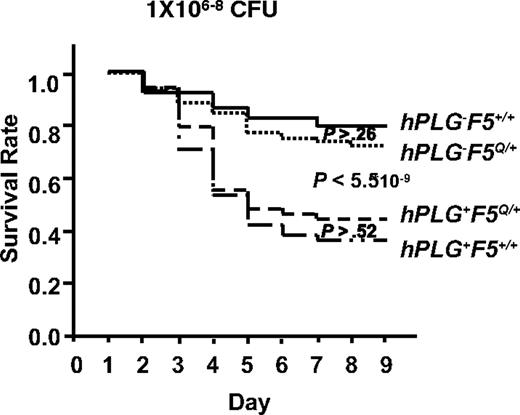
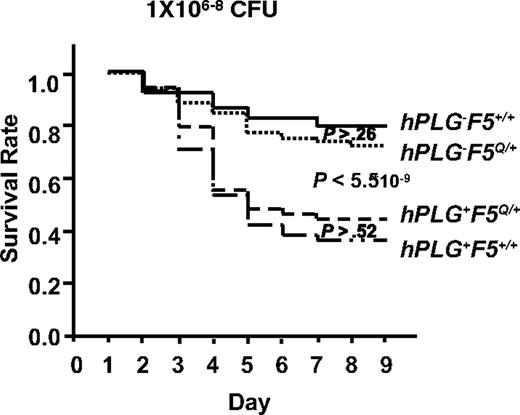
A genetic cross between hPLG-transgenic mice10 and FV Leiden mice18 was performed to generate GAS-susceptible mice carrying hPLG either in the presence (hPLG+F5Q/+) or absence (hPLG+F5+/+) of the FV Leiden allele (F5Q/+). Consistent with our previous report,10 mice carrying the hPLG transgene (hPLG+) demonstrated markedly increased mortality after GAS infection (P < 6 × 10−9; Figure 7).10 However, no significant difference in mortality is observed as a function of FV Leiden allele status, either in the presence (hPLG+) (P > .5) or absence (hPL−) (P > .2) of the hPLG transgene (Figure 7). Thus, at least in this GAS subcutaneous infection model, FV Leiden does not confer protection from mortality.
FV Leiden mice inoculated with GAS strain UMAA2616. Data were pooled from 11 independent experiments with UMAA2616 doses ranging from 106 to 108 CFU. The total number of mice in each group are 68 hPLG−F5+/+, 74 hPLG+F5+/+, 61 hPLG−F5Q/+, and 57 hPLG+F5Q/+. P values for survival difference are indicated. Among the P values indicated are difference between hPLG−F5+/+ and hPLG−F5Q/+ (P > .26), difference between combined hPLG− (hPLG−F5+/+ and hPLG−F5Q/+) and combined hPLG+ (hPLG+F5+/+ and hPLG+F5Q/+) (P < 5.5 × 10−9), and difference between hPLG+F5Q/+ and hPLG+F5+/+ (P > .52).
FV Leiden mice inoculated with GAS strain UMAA2616. Data were pooled from 11 independent experiments with UMAA2616 doses ranging from 106 to 108 CFU. The total number of mice in each group are 68 hPLG−F5+/+, 74 hPLG+F5+/+, 61 hPLG−F5Q/+, and 57 hPLG+F5Q/+. P values for survival difference are indicated. Among the P values indicated are difference between hPLG−F5+/+ and hPLG−F5Q/+ (P > .26), difference between combined hPLG− (hPLG−F5+/+ and hPLG−F5Q/+) and combined hPLG+ (hPLG+F5+/+ and hPLG+F5Q/+) (P < 5.5 × 10−9), and difference between hPLG+F5Q/+ and hPLG+F5+/+ (P > .52).
Discussion
We previously reported markedly increased mortality after GAS infection in mice genetically engineered to carry human PLG, and showed that this increased susceptibility was dependent on the species-specific interaction of SK with PLG.10 Our data further suggested that local fibrin formation was critical to the host defense against microbial spread and that SK served to coopt the host fibrinolytic system to overcome fibrin-related immune surveillance mechanisms and barrier function. The current study confirms and extends these findings and demonstrates the critical role of thrombin generation in the host defense against GAS. Our findings identify important contributions from both the platelet and plasma FV pools to this process and suggest that the primary function of thrombin in this setting is focused on the generation of the fibrin matrix.
FV is a central regulatory protein in the blood coagulation cascade, together with factor X forming the prothrombinase complex, which cleaves prothrombin into active thrombin. Factor X maintains little or no prothrombin activity in the absence of FV, and mice completely deficient in FV exhibit a mixed embryonic and perinatal lethal phenotype27 that is indistinguishable from prothrombin deficiency.28,29 In previous studies, we demonstrated that either platelet or plasma FV was sufficient for basal hemostasis, with no spontaneous hemorrhage observed in mice expressing 15% to 45% FV activity restricted to the plasma pool or mice with FV restricted to only the platelet pool (at ∼ 3% of the wild-type level).17 In the current report, we documented markedly increased mortalities after GAS infection in the same 3 groups of Tg+F5−/− mice compared with wild-type controls.
Decreased plasma FV level (ranging from 10% to 60% of wild-type) have been demonstrated to decrease thrombin formation and delay the time of onset of thrombin generation.30 Furthermore, Allen et al31 demonstrated a rapid increase in the rate of thrombin formation in an in vitro cell-based model of coagulation in samples consisting of FV-null platelets and increasing levels of FV (1%-30%), with a slower rate of increase for FV levels of 30% to 200%.31 Consistent with these reports, we observed significantly prolonged PTs in platelet-poor plasma from AlbfvBTg+F5−/− mice compared with F5+/− mice. Taken together, these observations suggest that efficient thrombin generation via the prothrombinase complex is critical for effective host defense against GAS and potentially other pathogens.
The markedly enhanced susceptibility to GAS-induced mortality of mice with a 15% plasma FV level (AlbfvBTg+F5−/−), even in the absence of human plasminogen, suggests that this host defense pathway is considerably more sensitive to small changes in thrombin generation capacity than the general hemostatic pathways necessary for the maintenance of vascular integrity. Although not statistically significant, a trend toward slightly increased mortality is observed in Figure 1 for F5+/− mice compared with F5+/+, suggesting that even a subtle approximately 50% reduction in FV (within the range of variation observed in the normal human and mouse populations) could be sufficient to impair host response to some pathogens.32 The lower reserve in animals with reduced plasma FV levels could render the host particularly sensitive to further reductions in thrombin generation capacity, resulting from the consumptive coagulopathy accompanying many bacterial infections.
Of particular note, the observation that AlbfvCTg+F5−/− mice are significantly more susceptible to GAS than F5+/− mice, despite similar levels of plasma FV (∼ 45% vs ∼ 50%), suggests a key role for the platelet pool of FV in host defense from microbial pathogens. Previous reports suggest that platelet FV is physically and functional distinct from plasma FV. Platelet FV expresses greater prothrombinase cofactor activity and is also more resistant to APC.33 These unique properties of platelet FV could account for our data and together suggest a unique role for the platelet FV pool in host defense against GAS infection. Platelets express adhesive and immune receptors on the surface to help recruitment of leukocytes, in addition to aggregating after contacting bacteria to form thrombi. Platelets also release antibacterial proteins from α-granules.34 Our data provide an additional, potential novel mechanism for the function of the platelet in both innate and adaptive immunity.35
The current data demonstrating the critical role of thrombin generation in host defense against GAS are consistent with a model in which local fibrin deposition is critical to GAS containment and clearance. This model also explains the function of bacterial SK as a key virulence factor in GAS pathogenicity, with the resulting activation of host plasminogen resulting in breakdown of immobilizing fibrin matrices.10 To more directly examine the role of fibrinogen/fibrin in this process, we took advantage of mice genetically engineered to be deficient in fibrinogen as the result of a knockout of the fibrinogen Aα chain gene.15 Markedly increased susceptibility to GAS was observed in homozygous Fga−/− mice across a range of inoculation doses. These results are consistent with our previous observation that reduction of fibrinogen levels by injection of the snake venom ancrod similarly enhances GAS-related mortality.10 Although SK binding to fibrinogen has previously been suggested as a necessary step at the first stage of bacterial invasion,13,36 our data indicate that fibrinogen is not an absolute requirement for the initiation of GAS infection and suggest that the dominant action of fibrinogen in host defense is at the level of barrier function, limiting both microbial dissemination and supporting local inflammatory clearance mechanisms. Thrombin has several other functions that undoubtedly contribute to the host response to bacterial pathogens, including platelet activation, activation of protein C, and initiation of cellular signaling pathways.20,37 Our current and previous data10 are consistent with a key barrier function for fibrin thrombus in host defense from GAS infection.
However, fibrinogen is a multifunctional protein that also plays critical roles in the inflammatory response. Fibrin(ogen) facilitates leukocyte activation events via interaction with the leukocyte integrin receptor αMβ2.22 Removal of fibrin as a ligand for αMβ2 could contribute to the defect in host defense observed in Fga−/− mice. Consistent with this hypothesis, mice with a fibrinogen gene mutation in the αMβ2-binding site (Fibγ390-396A) exhibit a defect in the clearance of S aureus.16 Our observation of significantly increased mortality in Fibγ390-396A mice compared with Fga+/− controls suggests a similar role for fibrin(ogen) leukocyte interactions in the setting of GAS infection. However, the even greater and earlier mortality in Fga−/− mice (Figure 4C) demonstrates an important role for other functions independent of leukocytes. We hypothesize that the latter protective effect is largely the result of a barrier function limiting vascular invasion and hematogenous spread.
The marked increase in susceptibility to GAS resulting from partial FV deficiency in our mouse model suggested that the gain of FV function associated with the common FV Leiden polymorphism could confer relative protection against this pathogen. Such protection would provide a balancing positive genetic selection that could potentially explain the high prevalence of FV Leiden in European populations,23 despite the 4- to 10-fold elevated risk of venous thrombosis in FV Leiden carriers.24,25 Although the data shown in Figure 7 suggest that FV Leiden does not protect against GAS infection, at least in this mouse subcutaneous model, we cannot exclude such a protective effect in humans or even other animal models of infection. However, given the ubiquitous nature of GAS infection in human populations (estimated at > 700 million cases per year),1 a polymorphism conferring significant resistance to GAS might be expected to be become rapidly fixed during human evolution, making GAS a less attractive candidate as a balancing selection for FV Leiden. Nonetheless, it seems probable that FV Leiden could provide an advantage in the setting of infection with another specific and less common bacterial pathogen, potentially through a similar hemostasis-dependent host-response mechanism. Thus, the high prevalence of FV Leiden could be the result of an infection-related advantage, similar to the balancing selection thought to result from relative resistance to Plasmodium falciparum malaria in carriers of sickle cell anemia and thalassemia.38 Consistent with this hypothesis, FV Leiden has been reported to confer a survival advantage among patients with severe sepsis.26
A large number of invasive bacterial species are known to generate proteins that interact with host coagulation factors,39-42 especially host plasminogen and fibrinogen. It seems probable that many of these organisms have adapted a similar strategy to that of GAS to circumvent this ancient vertebrate host defense system. A continuing “arms race” between microbes and their hosts could provide an evolutionary driving force for maintaining genetic variation within the genes for several hemostatic factors. Our data suggest that genetic variation in the plasma levels of FV, fibrinogen, and plasminogen could be particularly significant determinants of response among human patients to a variety of infectious agents. It is worth noting that fibrinogen levels show an approximately 3-fold variation among inbred mouse lines, very similar to the wide variation in plasma fibrinogen observed in human populations.43 These results suggest the possibility that plasma fibrinogen level might be a particularly important host susceptibility factor for human microbial infection and may also explain the evolution of plasma fibrinogen level as an acute phase reactant. These data also suggest the possibility that pharmacologic manipulation of the hemostatic system may offer opportunities for the development of novel therapeutic approaches to the treatment of infectious diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Elizabeth A. Grunz for the performing PT assay.
This work was supported by National Institutes of Health (NIH) grants R21AI076675-01 (H.S.), P01HL057346 (D.G. and H.S.), R37HL039693 (D.G.), and RO1HL85357 (J.L.D.). D.G. is a Howard Hughes Medical Institute investigator.
National Institutes of Health
Authorship
Contribution: H.S. performed research, analyzed data, and wrote the paper; X.W. performed research; J.L.D. analyzed data and wrote the paper; and D.G. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Ginsburg, Howard Hughes Medical Institute, University of Michigan, 210 Washtenaw Avenue, Room 5028, Ann Arbor, MI 48109; e-mail: ginsburg@umich.edu.