Abstract
The morbidity and mortality associated with graft-host-disease (GVHD) is a significant obstacle to the greater use of allogeneic stem cell transplantation. Donor T cells that predominantly differentiate into TH1/Tc1 T cells and generate pro-inflammatory cytokines such as interferon-γ (IFN-γ) mediate GVHD. Although numerous studies have described a pathogenic role for IFN-γ, multiple reports have demonstrated that the lack of IFN-γ paradoxically exacerbated GVHD lethality. This has led to speculation that another subset of T cells may significantly contribute to GVHD mortality. Several groups have demonstrated a new lineage of CD4+ T helper cell development distinct from TH1 or TH2 differentiation. This lineage is characterized by production of interleukin (IL)–17A, IL-17F, IL-22, and IL-21 and has been termed TH17 cells. Here, we demonstrate that a highly purified population of TH17 cells is capable of inducing lethal GVHD, hallmarked by extensive pathologic cutaneous and pulmonary lesions. Upon transfer, these cells migrate to and expand in GVHD target organs and secondary lymphoid tissues. Finally, we demonstrate differential roles for tumor necrosis factor-α (TNF-α) and IL-17A in the clinical manifestations of GVHD induced by TH17 cells. Our studies demonstrate that cells other than TH1/Tc1 can mediate acute GVHD.
Introduction
Allogeneic stem cell transplantation (allo-SCT) is effective in the treatment of hematologic malignant diseases, bone marrow failure, or inherited immunodeficiency syndromes.1-4 However, the incidence of graft-versus-host disease (GVHD) significantly limits the applicability of allo-SCT.5-7 Two different forms of GVHD have been described.8 Acute GVHD is a proinflammatory process mediated in part by mature donor T cells present in the stem cell or marrow inoculum that are polarized toward a TH1 phenotype and recognize minor or major histocompatibility disparities between the donor and host.8-11 Chronic GVHD may be due to impaired thymic education of recent T-cell bone marrow emigrants and mediates a fibrotic process.12,13 Activation of donor T cells by host antigen-presenting cells initiates a cascade of events that eventually leads to tissue destruction in GVHD target organs such as the gastrointestinal (GI) tract, liver, and skin.8,13
Previous work has focused on the cytokine profiles of allo-reactive T-effector cells in GVHD. Here, the TH1 cytokine, interferon-γ (IFN-γ), has been shown to be important in the pathogenesis of GVHD.14-16 However, neutralization of IFN-γ surprisingly led to exacerbated disease, implying both protective and pathogenic roles for this TH1 cytokine.17,18 A recent report demonstrated that donor-derived IFN-γ, although amplifying GI tract tissue damage, was necessary for the prevention of idiopathic pneumonia syndrome (IPS).19
For the past 3 decades, CD4+ TH differentiation has been believed to be limited to 2 distinct pathways.20,21 TH1 cells were required for clearance of intracellular pathogens and distinguished by the production of IFN-γ, tumor necrosis factor-α (TNF-α), and IL-2. TH2 cells promoted humoral immune responses, important in the response to clear extracellular pathogens, and secreted IL-4, IL-5, and IL-13. Recent work has shown that the TH17 pathway of differentiation is separate from TH1 or TH2 CD4+ T-cell development.22-25 TH17 differentiation requires TGF-β124,25 and IL-6,22,25 and is enhanced by IL-1β and TNF-α.25 In addition, the transcription factors retinoid-related orphan receptor (ROR)γt26,27 and RORα27 have been shown to be critical for TH17 development. TH1 and TH2 type cytokines inhibit TH17 differentiation, notably IL-2,28 IFN-γ,23 and IL-4,23 and are potent suppressors of TH17 development. In mice, TH17 cells are enriched in the lung and GI tract and are considered important in the maintenance of mucosal host defense.29 TH17 cells have also been shown to mediate pathologic conditions in several autoimmune conditions once thought to be due to TH1 responses. Elevated levels of IL-17 have been observed in rheumatoid arthritis,30 multiple sclerosis,31 and inflammatory bowl disease.32
Considering the pathogenic role IL-17 has in autoimmune/chronic inflammatory diseases, we were interested in determining whether TH17 cells are important in the pathogenesis of acute GVHD. Here, we demonstrate that in vitro polarized TH17 cells alone mediate significant GVHD, including extensive pathologic skin and pulmonary conditions, and that these cells synergize with naive T cells to induce lethal GVHD. We show that the systemic manifestations of GVHD induced by TH17 cells were significantly dependent on the production of TNF-α, whereas the cutaneous manifestations were not dependent on TNF-α but required IL-17A. This work demonstrates that GVHD can be mediated by cells distinct from TH1/Tc1 T cells and may provide new avenues for therapy.
Methods
Mice
Donor mice were male C57BL/6J, IFN-γ−/− (B6), (H-2b; The Jackson Laboratory, Bar Harbor, ME), and enhanced green fluorescent protein (eGFP)-expressing C57BL/6J generated as described previously.33 Recipient mice were male (C57BL/6JXDBA/2) FI mice, referred to as B6D2 (H-2bxd; The Jackson Laboratory). B6/SJL (H-2b) recipient mice and miHA-mismatched C3H.SW (H-2b) donor mice were purchased from The Jackson Laboratory. Within each experiment, all recipient mice were the same age, ranging from 9 to 13 weeks of age. Donor mice also ranged from 9 to 13 weeks of age. All transplantation experiments used male mice transplanted into male recipient animals. All animal experiments were performed in accordance with protocols approved by the University of North Carolina Institutional Animal Care and Use Committee.
Antibodies and flow cytometry
Antibodies with the following specificities were purchased from eBiosciences (San Diego, CA): anti-CD4 (RM 4.5), CD62L (Mel-14), CD25 (PC61), CD8 (53-6-7), IFN-γ (XMG1.2), and IL-17F (eBio 18F10). Antibodies to IL-17A (TC11-18H10.1) and TNF-α (MP6-XT22) were purchased from Biolegend (San Diego, CA). Acquisition was performed on a FACSCalibur using CellQuest software (BD Biosciences, San Jose, CA). Analysis was performed using FlowJo (TreeStar, Ashland, OR) software. For in vivo depletion studies, animals received biweekly injections of either 200 μg anti–IL-17A (clone MM17F3; eBioscience), 500 μg anti–TNF-α (clone XT3.11; BioXCell, West Lebanon, NH), or 500 μg rat IgG isotype control (BioXCell) for 4 weeks.
Quantitative mRNA analysis of transcription factors and cytokine transcripts
RNA was extracted from cells using the RNeasy Plus Kit (QIAGEN, Valencia, CA) according to the manufacturer's recommendations. First-strand cDNA synthesis was performed under the following conditions: 1 μg RNA, 1× polymerase chain reaction (PCR) reaction buffer supplemented with 2.5 mmol/L MgCl2 (Invitrogen, Carlsbad, CA), 10 mmol/L DTT (Invitrogen), 0.5 mmol/L dNTP mix (Invitrogen), 7.5 μg random primer (Invitrogen), 50 units RNasin Ribonuclease Inhibitor (Promega), and 500 units Moloney murine leukemia virus reverse transcriptase (Invitrogen) at 37°C for 1 hour. The first-strand cDNA reaction was then heat-inactivated for 2 minutes at 95°C. Equal amounts of cDNA were analyzed by real-time quantitative PCR, in triplicate, using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) on an ABI 7000 instrument, using primer-specific standard curves. The expression level of each gene was normalized to 18S rRNA using the standard curve method before “fold activation” was calculated. The sequences of the primer pairs used were as follows: T-bet: sense primer, 5′-ACCAGAGCGGCAAGTGGG-3′; antisense primer, 5′-TGGACATATAAGCGGTTCCCTG-3′; and RORγt: sense primer, 5′-CCGCTGAGAGGGCTTCAC-3′; antisense primer, 5′-TGCAGGAGTAGGCCACATTACA-3′. Standard curves were generated for each primer set using dilutions of separate plasmids containing a cloned copy of each amplicon. IL-21 and IL-22 expression was assessed in TH17 cells after 4-hour stimulation with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and 500 ng/mL ionomycin (Sigma-Aldrich, St Louis, MO). Quantitative PCR was performed using prequalified primers and probes from Applied Biosystems. The ΔCt method was used to normalize transcripts to 18S RNA and to calculate fold induction relative to levels detected in naive CD4+/CD25− cells stimulated with PMA and ionomycin.
In vitro TH17 differentiation
Naive CD4+ (CD25−/CD62Lhi) from C57BL/6J mice were sort purified using a MoFlo cell sorter (Dako Denmark A/S, Glostrup, Denmark). Cells (5.0 × 105/mL) were stimulated with plate-bound anti-CD3 (3 mg/mL; eBiosciences) and anti-CD28 (5 mg/mL; eBiosciences) in 24-well plates (Corning Life Science, Acton, MA) with TGF-β1 (2 ng/mL; Peprotech, Rocky Hill, NJ), IL-6 (30 ng/mL; Peprotech), TNF-α (20 ng/mL; Peprotech), IL-1β (10 ng/mL; Peprotech), anti–IL-2 (10 μg/mL; clone JES6-5H4; BioXCell), and anti–IFN-γ (10 μg/mL; clone R4-6A2; BioXCell) for 4 days. We then split the cells (7.5 × 105/mL) into low cluster 24-well plates (Corning) with TGF-β1, IL-6, anti–IL-2, and anti–IFN-γ using the same concentrations given for 2 days. Cells (5.0 × 105/mL) were restimulated with anti-CD3 and anti-CD28 with TGF-β1, IL-6, TNF-α, IL-1β, IL-23 (15 ng/mL; R&D Systems, Minneapolis, MN), anti–IL-2 (15 μg/mL), and anti–IFN-γ for 3 days. Cells (7.5 × 105/mL) were removed from CD3-CD28 stimulation and cultured with TGF-β1, IL-6, IL-23, anti–IL-2 (15 μg/mL), and anti–IFN-γ. At day 11, we stimulated the cells with 50 ng/mL PMA, 500 ng/mL ionomycin, and 3 μg/mL Brefeldin A (Sigma-Aldrich) for 4 hours. Cells were then washed and stained for CD4. Intracellular cytokine staining was performed using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's recommendations. Cells not analyzed for cytokine production were infused into lethally irradiated B6D2 recipients with C57BL/6 T cell–depleted bone marrow as described.
Preparation of cells for transplant and bone marrow transplantation
T cell–depleted bone marrow cells were prepared as described previously.34 Whole naive T cells were isolated on the day of transplantation by collecting spleens from C57BL/6 mice, treating cell suspensions with ammonium chloride, potassium carbonate buffer to remove erythrocytes followed by T-cell selection using mouse T-cell recovery column kits (Cedarlane Laboratories, Burlington, ON). CD25− cells were then isolated by negative selection. The purity of selected cells was more than 90% (CD4+ + CD8+). CD4+/CD25− T cells were sorted on a MoFlo cell sorter (Dako Denmark A/S). Cells were resuspended to the appropriate concentration in sterile 0.9% saline solution, and 200 μl were administered via tail vein injection. Bone marrow transplants were performed as described previously.34
Measurement of serum TNF-α
Serum samples were obtained from mice receiving either whole naive T cells, TH17 cells, or BM only on day 12 after transplantation. Serum was collected after centrifugation and stored at −80°C. TNF-α concentrations were determined according to the manufacturer's instructions using enzyme-linked immunosorbent assay (BioLegend, San Diego CA).
GVHD grading
Mice were observed twice weekly for signs of GVHD using a previously described clinical scoring system.35
Fluorescence microscopy
Animals were anesthetized with avertin and organs were imaged with a Zeiss SteREO Lumar V12 microscope with eGFP bandpass filter (Carl Zeiss MicroImaging, Thornwood, NY). Images were acquired at room temperature with a Zeiss AxioCam MRm camera (Carl Zeiss). Image acquisition and GFP intensities were determined with AxioVision software (Carl Zeiss). Images were converted to TIFF using Adobe Photoshop (Adobe Systems, San Jose, CA).
Isolation of leukocytes from tissues
Mice were killed on day 8 after transplantation, and isolation of leukocytes was performed as described previously.34 Isolated cells were stimulated with PMA, ionomycin, and Brefeldin A as described above. Surface and intracellular staining was performed as described above.
Histopathology
Samples of each organ were removed at the time of kill (day 14), placed into Omnifix (FR Chemical, Mount Vernon, NY), and paraffin-embedded. Tissue blocks were sectioned with a microtome. The sections were stained with H&E, and individual sections were evaluated for evidence of GVHD using a quantitative assessment previously described. The sections were scored by one of us (A.P.-M.) who was blinded to the treatment given. Images were acquired at room temperature using Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI) with an RT Spot camera (Diagnostic Instruments) mounted on an Olympus BX51 microscope (Olympus, Hamburg, Germany). Images were converted to TIFF files using Adobe Photoshop (Adobe Systems).
Statistical analysis
For GVHD scoring, we used the Student t test and for median survival we used the Mann-Whitney log rank test. P values less than or equal to .05 were considered significant.
Results
Novel in vitro culture conditions yield a highly enriched population of TH17 cells
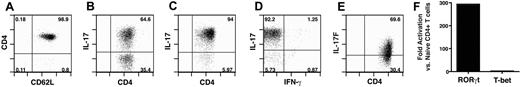
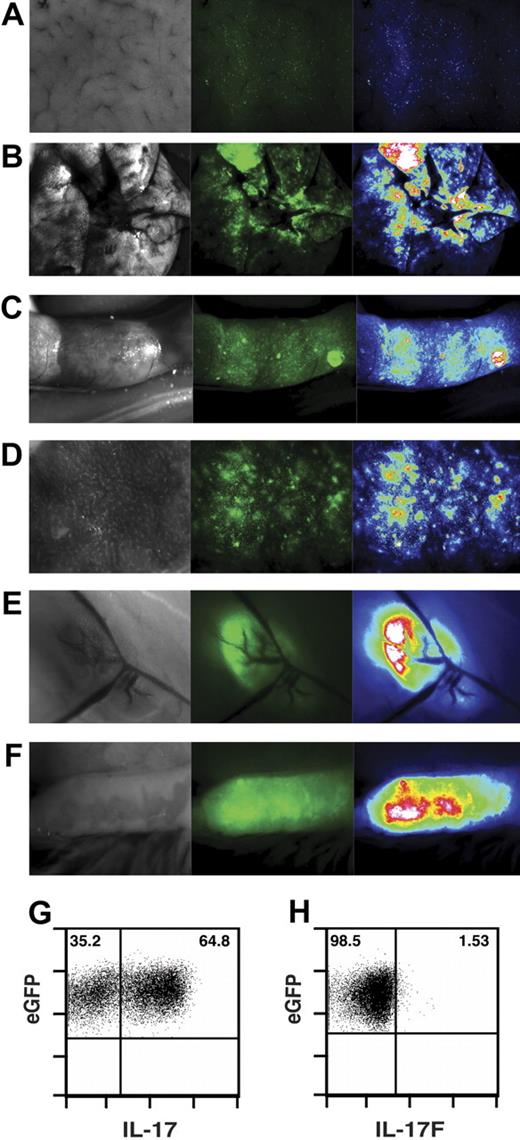
Studies examining TH17 function in vivo have been hampered by an inability to generate a purified IL-17–expressing population in vitro. Thus, we sought to optimize conditions to obtain a highly enriched population of TH17 cells. Naive CD4+ T cells (Figure 1A) were differentiated in vitro using a combination of TH17 polarizing cytokines. After 6 days in culture, approximately 60% of the T cells were generating IL-17A (Figure 1B). At day 11, 94% of the cells produced IL-17A, only 2.1% producing IFN-γ (Figure 1C,D). TH17 cells produce another member of the IL-17 family, IL-17F.36 Although the expression of IL-17F was less than IL-17A in these in vitro–generated TH17 cells, the majority produced IL-17F protein (Figure 1E).
TH17 in vitro differentiation. TH17 cells were generated as described in “Methods.” (A) Initial histogram of CD4+ T cells isolated after sorting. (B) Flow cytometric analysis of T cells differentiated for 6 days. Evaluation of T cells differentiated for 11 days: IL-17A (C,D), IFN-γ (D), and IL-17F (E). (B-E), plots derived from CD4+ gate. (F) Aliquots of cells from TH17 culture (day 11 in culture) and sorted naive CD4+ T cells were harvested, and RNA was extracted. RNA samples were subjected to quantitative PCR for RORγt and T-bet.
TH17 in vitro differentiation. TH17 cells were generated as described in “Methods.” (A) Initial histogram of CD4+ T cells isolated after sorting. (B) Flow cytometric analysis of T cells differentiated for 6 days. Evaluation of T cells differentiated for 11 days: IL-17A (C,D), IFN-γ (D), and IL-17F (E). (B-E), plots derived from CD4+ gate. (F) Aliquots of cells from TH17 culture (day 11 in culture) and sorted naive CD4+ T cells were harvested, and RNA was extracted. RNA samples were subjected to quantitative PCR for RORγt and T-bet.
To confirm that the T cells polarized over 11 days in culture had minimal contamination by TH1 cells, real-time PCR analysis for the expression of RORγt and T-bet in the in vitro–differentiated TH17 cells was evaluated. Compared with naive CD4+ T cells, there was approximately a 300-fold induction of RORγt, whereas the transcription factor T-bet, which is required for TH1 differentiation,37 displayed minimal up-regulation (Figure 1F). Together, these results demonstrate that the differentiation protocol used yielded a highly purified population of TH17 cells with limited contamination by TH1 cells.
TH17 cells mediate lethal acute GVHD with extensive pathologic cutaneous lesions
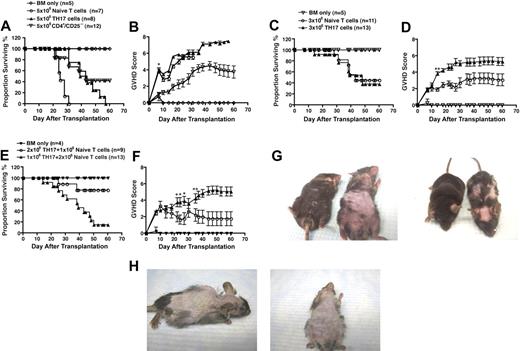
To examine the capacity of TH17 cells to induce GVHD in vivo, various doses of TH17 cells, CD4+/CD25−, or whole T cells from WT C57BL/6 mice were transferred into lethally irradiated B6D2 hosts. Although the median survival time of recipients of TH17 cells and whole T cells was not significantly different (P = .1), animals receiving TH17 cells had significantly worse overall survival compared with recipients of CD4+/CD25−T cells (P < .01) (Figure 2A). As further evidence that animals treated with TH17 cells died of GVHD, we used a clinical score shown previously to discriminate between GVHD and other causes of mortality.35 GVHD scores between mice receiving TH17 cells were not statistically different from those receiving whole T cells and were greater than those given naive CD4+/CD25− T cells (Figure 2B). These data indicated that TH17 cells could mediate significant clinical GVHD symptoms.
In vitro-differentiated TH17 cells induce lethal acute GVHD. Whole splenic T cells (5 × 106; n = 7) or CD4+/CD25− sorted cells (5 × 106; n = 12) or TH17 cells (n = 8; differentiated as in Figure 1) were transferred with T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients. Animals were monitored for survival (A) and signs of GVHD (B). *P values at all of the statistically significant time points were at most .04. Whole splenic T cells (3 × 106; n = 11) or TH17s (3 × 106; n = 13) were transferred with T-cell-depleted bone marrow cells into lethally irradiated B6D2 recipients. Animals were monitored for (C) survival and (D) signs of GVHD. (*P < .04; **P values remained significant from day 14 on.) TH17 cells were transferred with whole T cells in different doses and T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients (2 × 106 whole T cells plus 106 TH17 cells, n = 13; 106 whole T cells plus 2 × 106 TH17 cells, n = 9). Animals were monitored for (E) survival and (F) signs of GVHD. (*P < .04; **P values remained significant from day 35 on.) For each experiment animals receiving bone marrow only (n = 4) served as controls for GVHD. (G) Comparison of pathologic skin lesions in recipients of 5 × 106 whole splenic T cells (left animal) versus recipients of TH17 cells (right animal) at day 25 after transplantation. (H) Pathologic skin lesions in recipients of TH17 cells at day 38 after transplantation.
In vitro-differentiated TH17 cells induce lethal acute GVHD. Whole splenic T cells (5 × 106; n = 7) or CD4+/CD25− sorted cells (5 × 106; n = 12) or TH17 cells (n = 8; differentiated as in Figure 1) were transferred with T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients. Animals were monitored for survival (A) and signs of GVHD (B). *P values at all of the statistically significant time points were at most .04. Whole splenic T cells (3 × 106; n = 11) or TH17s (3 × 106; n = 13) were transferred with T-cell-depleted bone marrow cells into lethally irradiated B6D2 recipients. Animals were monitored for (C) survival and (D) signs of GVHD. (*P < .04; **P values remained significant from day 14 on.) TH17 cells were transferred with whole T cells in different doses and T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients (2 × 106 whole T cells plus 106 TH17 cells, n = 13; 106 whole T cells plus 2 × 106 TH17 cells, n = 9). Animals were monitored for (E) survival and (F) signs of GVHD. (*P < .04; **P values remained significant from day 35 on.) For each experiment animals receiving bone marrow only (n = 4) served as controls for GVHD. (G) Comparison of pathologic skin lesions in recipients of 5 × 106 whole splenic T cells (left animal) versus recipients of TH17 cells (right animal) at day 25 after transplantation. (H) Pathologic skin lesions in recipients of TH17 cells at day 38 after transplantation.
In acute GVHD in patients, TH17 cells would not mediate their effects independently but would work in concert with other T cells. Thus, we evaluated whether the administration of TH17 cells could cooperate with whole splenic T cells in the induction of GVHD. At a lower dose (3 × 106), we demonstrated that recipient mice transplanted with TH17 cells or whole T cells did not differ in overall long-term survival (∼ 40%), median survival time; (∼ 6 weeks; Figure 2C), or mean clinical GVHD scores (Figure 2D). Recipient mice given the same lower dose (3 × 106) of T cells composed of 2 × 106 whole T cells plus 106 TH17 cells had a statistically significant increase in mortality (85%; P < .001) compared with recipient mice given the reciprocal dose of 106 whole T cells plus 2 × 106 TH17 cells, which resulted in 20% of the mice succumbing to disease (Figure 2E). Consistent with greater lethality, there was a significant difference in clinical GVHD scores (P < 0.04; Figure 2F).
In humans, acute GVHD is often manifested clinically by extensive cutaneous involvement. The administration of whole C57BL/6 T cells to lethally irradiated B6D2 recipients leads to very modest GVHD-induced cutaneous changes manifested predominantly as skin ulcerations. However, transfer of TH17 cells resulted in significant fur loss and pathologic skin conditions, which was considerably more extensive than found using whole T cells (Figure 2G). Over time, the skin manifestations worsened, resulting in nearly complete loss of fur and significant skin ulcerations (Figure 2H). Collectively, these results illustrate that in vitro– differentiated TH17 cells alone can cause lethal acute GVHD, that these T cells cooperate with naive T cells to mediate GVHD, and that GVHD induced by TH17 cells was characterized by extensive cutaneous involvement similar to clinical GVHD.
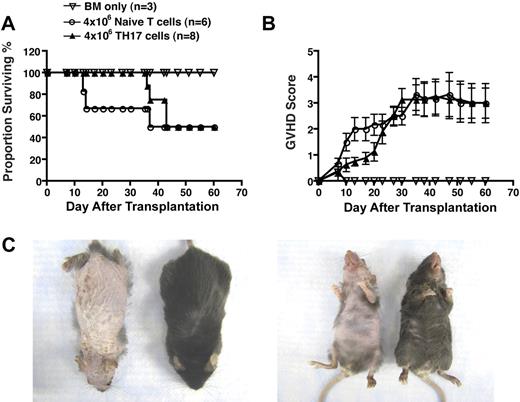
To determine whether the pathologic condition observed was unique to the strain combination used, we assessed the ability of TH17 cells to induce acute GVHD in a major histocompatibility complex (MHC)–identical, multiple minor histocompatibility antigen-mismatched model of allo-SCT analogous to allogeneic matched human transplantation. The mortality rate of animals receiving TH17 cells was the same as those receiving whole splenic T cells (Figure 3A), and GVHD scores were also similar (Figure 3B). It is noteworthy that recipients of TH17 cells displayed prominent cutaneous manifestations as demonstrated by extensive fur loss and skin ulcerations, whereas recipients of whole splenic T cells displayed only mild fur ruffling without skin ulcerations (Figure 3C). These results demonstrated TH17 cell–mediated pathologic lesions were not strain-dependent and, importantly, that TH17 cells were capable of inducing disease in a MHC-matched model of allo-SCT that was previously shown to be CD8+ T cell–dependent.
In vitro differentiated TH17 cells mediate extensive pathologic cutaneous lesions in a MHC-match, multiple minor mismatch model of acute GVHD. Whole splenic T cells (4 × 106; n = 6) or TH17 cells (4 × 106; n = 8; differentiated as in Figure 1) from C3H.SW mice were transferred with T cell–depleted bone marrow cells into lethally irradiated B6/SJL recipients. Animals were monitored for survival (A) and signs of GVHD (B). (C) Comparison of pathologic skin lesions in recipients of 5 × 106 TH17 cells (left animal) versus recipients of whole splenic T cells (right animal) at day 37 after transplantation.
In vitro differentiated TH17 cells mediate extensive pathologic cutaneous lesions in a MHC-match, multiple minor mismatch model of acute GVHD. Whole splenic T cells (4 × 106; n = 6) or TH17 cells (4 × 106; n = 8; differentiated as in Figure 1) from C3H.SW mice were transferred with T cell–depleted bone marrow cells into lethally irradiated B6/SJL recipients. Animals were monitored for survival (A) and signs of GVHD (B). (C) Comparison of pathologic skin lesions in recipients of 5 × 106 TH17 cells (left animal) versus recipients of whole splenic T cells (right animal) at day 37 after transplantation.
TH17 cells mediate extensive skin and pulmonary pathologic lesions
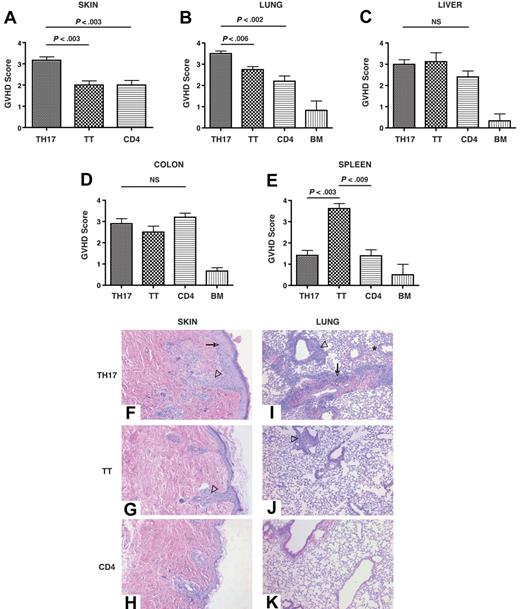
Histopathologic examination was carried out in GVHD target organs and spleen in recipients of WT TH17 cells, total splenic T cells, or splenic CD4+/CD25− T cells. Consistent with the clinical evaluations, the histopathology results demonstrated significantly increased pathologic cutaneous lesions in recipients of TH17 cells (Figure 4A). In addition, TH17 cells also mediated more severe pathologic lesions in the lung (Figure 4B). All T-cell types induced equivalent pathologic lesions in the liver (Figure 4C) and colon (Figure 4D). It is noteworthy that the animals receiving total splenic T cells showed significantly worse pathologic lesions in the spleen compared with TH17 cells or CD4+/CD25− T cells (Figure 4E). Kidney sections from all transplant groups demonstrated little histologic evidence of GVHD, indicating that the type of GVHD pathologic lesion induced by TH17 cells was not different from that induced by total T cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Representative skin images from recipients of TH17 cells (Figure 4F) displayed significant epidermal hyperplasia compared with recipients of whole T cells (Figure 4G) or CD4+/CD25− cells (Figure 4H). In addition, TH17 cells mediated more severe pathologic lesions in the lung (Figure 4I-K), characterized by perivascular cuffing, vasculitis, peribronchiolar cuffing, and alveolar hemorrhage. These results indicated that TH17 cells mediate substantial pathologic lesions in typical GVHD target organs with more severe disease in the skin and pulmonary tissues.
TH17 cells induce severe pathologic skin and pulmonary lesions. TH17 cells (5 × 106; n = 6), whole splenic T cells (5 × 106; n = 4), or CD4+/CD25− (5 × 106; n = 5) cells were transferred with T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients. At day 14 after transplantation organs were harvested and processed as described in “Methods.” Histopathologic assessment of the skin (A), lung (B), liver (C), colon (D), and spleen (E) was performed by one author (A.P.-M.) who was blinded to the identity of the transplanted mice. Values presented are the mean plus or minus SEM for each group. Score range for each tissue, 0-4. Representative pictures of skin from animals receiving TH17 cells (F), total T cells (G), or CD4+/CD25− cells (H). → denotes epidermal hyperplasia, ▿ denotes follicular obliteration. Representative pictures of lung from animals receiving TH17 cells (I), total T cells (J), or CD4+/CD25− cells (K). → denotes perivascular cuffing and vasculitis, ▿ denotes peribronchiolar cuffing, ★ denotes area of alveolar hemorrhage. TT = total T cells, CD4 = CD4+/CD25− cells. Original magnification: skin = ×200, lung = ×100.
TH17 cells induce severe pathologic skin and pulmonary lesions. TH17 cells (5 × 106; n = 6), whole splenic T cells (5 × 106; n = 4), or CD4+/CD25− (5 × 106; n = 5) cells were transferred with T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients. At day 14 after transplantation organs were harvested and processed as described in “Methods.” Histopathologic assessment of the skin (A), lung (B), liver (C), colon (D), and spleen (E) was performed by one author (A.P.-M.) who was blinded to the identity of the transplanted mice. Values presented are the mean plus or minus SEM for each group. Score range for each tissue, 0-4. Representative pictures of skin from animals receiving TH17 cells (F), total T cells (G), or CD4+/CD25− cells (H). → denotes epidermal hyperplasia, ▿ denotes follicular obliteration. Representative pictures of lung from animals receiving TH17 cells (I), total T cells (J), or CD4+/CD25− cells (K). → denotes perivascular cuffing and vasculitis, ▿ denotes peribronchiolar cuffing, ★ denotes area of alveolar hemorrhage. TT = total T cells, CD4 = CD4+/CD25− cells. Original magnification: skin = ×200, lung = ×100.
TH17 cells migrate to and expand in GVHD target organs and secondary lymphoid tissues
Because TH17 cells could mediate GVHD, the trafficking and accumulation of TH17 cells was assessed in secondary lymphoid and GVHD target organs by in vivo imaging of in vitro-differentiated TH17 cells generated from C57BL/6 eGFP transgenic donors. Seven days after transplantation, eGFP+ TH17 cells had migrated to the liver (Figure 5A), lung (Figure 5B), colon (Figure 5C), spleen (Figure 5D), and inguinal (Figure 5E) and mesenteric lymph nodes (Figure 5F and Figure S2). Moreover, the total number of cells isolated from the lung alone was approximately 3-fold higher than the number of cells transferred indicating substantial expansion (data not shown).
In vitro–differentiated TH17 cells traffic to GVHD target organs and secondary lymphoid organs. Naive CD4+ T cells from eGFP+ mice were differentiated into TH17 cells as in Figure 1. TH17 cells (1.35 × 106) were transferred with T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients. Seven days after transfer, animals were anesthetized with avertin, and organs were imaged with a Zeiss SteREO Lumar.V12 microscope with eGFP bandpass filter. Brightfield images (left) and GFP images (middle) were taken for each organ. GFP intensities (right) were determined by software analysis: liver (A), lung (B), colon (C), spleen (D), inguinal lymph node (E), and mesenteric lymph node (F). eGFP+ TH17 cells were transferred into irradiated B6D2 recipients. Eight days after transplantation, lymphocytes were extracted from the lung and stimulated as in Figure 1 followed by intracellular cytokine staining for IL-17A (G) and IL-17F (H). Plots derived from eGFP+ gate. We verified that the eGFP signal documented was not due to background autofluorescence by transfer of non–GFP-expressing T cells and imaging of GVHD target organs and lymphoid tissues as described previously (Figure S2). Original magnification liver = ×40, lung = ×25, colon = ×20, spleen = ×40, ILN = ×45, MLN = ×45.
In vitro–differentiated TH17 cells traffic to GVHD target organs and secondary lymphoid organs. Naive CD4+ T cells from eGFP+ mice were differentiated into TH17 cells as in Figure 1. TH17 cells (1.35 × 106) were transferred with T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients. Seven days after transfer, animals were anesthetized with avertin, and organs were imaged with a Zeiss SteREO Lumar.V12 microscope with eGFP bandpass filter. Brightfield images (left) and GFP images (middle) were taken for each organ. GFP intensities (right) were determined by software analysis: liver (A), lung (B), colon (C), spleen (D), inguinal lymph node (E), and mesenteric lymph node (F). eGFP+ TH17 cells were transferred into irradiated B6D2 recipients. Eight days after transplantation, lymphocytes were extracted from the lung and stimulated as in Figure 1 followed by intracellular cytokine staining for IL-17A (G) and IL-17F (H). Plots derived from eGFP+ gate. We verified that the eGFP signal documented was not due to background autofluorescence by transfer of non–GFP-expressing T cells and imaging of GVHD target organs and lymphoid tissues as described previously (Figure S2). Original magnification liver = ×40, lung = ×25, colon = ×20, spleen = ×40, ILN = ×45, MLN = ×45.
To determine whether TH17 cells retained TH17 protein expression, donor eGFP+ cells were isolated from the lung (Figure 5G) on day 8 after transplantation. Approximately 60% of the isolated cells produced IL-17A, indicating that the majority of in vitro–differentiated TH17 cells present in the lung on day 8 after transplantation retained the ability to produce IL-17A in vivo. It is noteworthy that the IL-17A negative fraction contained a population of IFN-γ producing cells (Figure S3). Whereas the majority of in vitro–cultured cells produced IL-17F (Figure 1E), in striking contrast, eGFP+ cells isolated 8 days after transplantation did not express IL-17F protein (Figure 5H), demonstrating discordant expression of IL-17A and IL-17F. These data illustrated that in vitro–differentiated TH17 cells infiltrated and expanded robustly in lymphoid and GVHD target organs in the first week after transplantation. Furthermore, we show that IL-17A production was maintained in vivo, whereas IL-17F was not. Finally, the lung had a minority of highly polarized TH17 cells that could generate IFN-γ in vivo, which has been observed in other models.38
TH17-mediated pathologic conditions are independent of IFN-γ
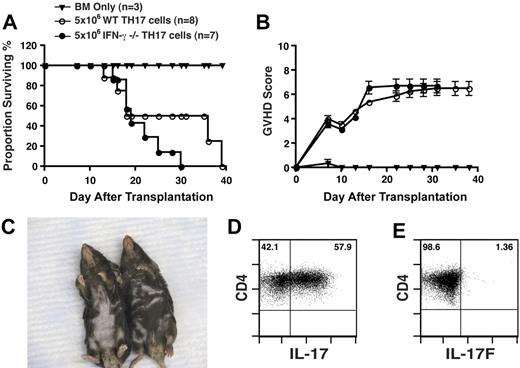
Because we detected a population of polarized TH17 cells capable of producing IFN-γ upon transfer, there was the possibility that the pathologic lesion observed was due solely to the production of IFN-γ. To address this question, CD4+ T cells from IFN-γ−/− or WT mice were differentiated as in Figure 1, followed by adoptive transfer into lethally irradiated B6D2 recipients. Both WT and IFN-γ−/− TH17 cells mediated lethal acute GVHD with similar kinetics (Figure 6A). GVHD scores between the groups were also equivalent (Figure 6B). It is noteworthy that the recipients of IFN-γ−/− TH17s also displayed prominent pathologic skin lesions and fur loss (Figure 6C). We also assessed cytokine production of transferred cells in the lung on day 8 after transplantation and found that the majority of cells still produced IL-17A (Figure 6D) and consistent with the results obtained from WT TH17 cells, there were few cells producing IL-17F (Figure 6E). Taken together, these data demonstrated that the pathologic skin lesion observed with WT TH17 cells was not due to the production of IFN-γ.
TH17-mediated pathologic conditions are independent of IFN-γ. TH17 cells (5 × 106) from WT (n = 8) or IFN-γ−/− mice (n = 7; differentiated as in Figure 1) were transferred with T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients. Animals were monitored for survival (A) and signs of GVHD (B). (C) Comparison of pathologic skin lesions in recipients of 5 × 106 IFN-γ−/− TH17 cells (left animal) versus recipients of WT TH17 cells (right animal) at day 25 after transplantation. IFN-γ−/− TH17 cells were transferred into irradiated B6D2 recipients. Eight days after transplantation, lymphocytes were extracted from the lung and stimulated as in Figure 1 followed by intracellular cytokine staining for IL-17 (D) and IL-17F (E). Plots derived from CD4+ gate.
TH17-mediated pathologic conditions are independent of IFN-γ. TH17 cells (5 × 106) from WT (n = 8) or IFN-γ−/− mice (n = 7; differentiated as in Figure 1) were transferred with T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients. Animals were monitored for survival (A) and signs of GVHD (B). (C) Comparison of pathologic skin lesions in recipients of 5 × 106 IFN-γ−/− TH17 cells (left animal) versus recipients of WT TH17 cells (right animal) at day 25 after transplantation. IFN-γ−/− TH17 cells were transferred into irradiated B6D2 recipients. Eight days after transplantation, lymphocytes were extracted from the lung and stimulated as in Figure 1 followed by intracellular cytokine staining for IL-17 (D) and IL-17F (E). Plots derived from CD4+ gate.
Mechanism(s) of TH17-mediated pathologic manifestations
In addition to IL-17A and IL-17F, TH17 cells produce other cytokines, including IL-22,39,40 IL-21,41-43 and TNF-α.44 Given the profound weight loss observed in recipients of TH17 cells, we evaluated whether TNF-α was important in the clinical manifestations found in recipient animals. First, we determined whether in vitro–polarized TH17 cells generated TNF-α. As shown (Figure 7A left), a significant majority of the in vitro–differentiated TH17 cells displayed high-level expression of TNF-α. We then determined whether TH17 cells isolated from the lung 8 days after transfer continued to generate TNF-α. TH17 cells generated from WT (Figure 7A middle) or IFN-γ−/− (Figure 7A right) donors retained substantial TNF-α production in vivo. In addition, serum levels of TNF-α were significantly higher in recipients of TH17 cells compared with recipients of whole splenic T cells (Figure 7B).
Mechanism(s) of TH17-mediated pathologic lesions. TH17 cells were generated as described in “Methods.” (A) TNF-α expression in in vitro–differentiated cells (left), TH17 cells isolated from the lung 8 days after transplantation (WT TH17 cells; middle and IFN-γ−/− TH17 cells; right). (B) Serum TNF-α concentrations on day 12 after transplantation from recipients of TH17 cells, total T cells, and BM only. TH17 cells (5 × 106) from WT mice were transferred with T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients. Animals were treated with 500 μg of anti–TNF-α (n = 6), 200 μg of anti–IL-17A (n = 6), or 500 μg rat IgG (n = 7) biweekly for 4 weeks. Animals were monitored for survival (C), signs of GVHD (*P < .01 [anti–TNF-α vs rat IgG] and remained significant from day 7 on, D), and weight loss *P < .01 [anti–TNF-α vs rat IgG] (**P values remained significant from day 18 on) (E). (F) Comparison of pathologic skin lesions in recipients of 5 × 106 TH17 cells receiving anti–IL-17A (left animal), anti–TNF-α (middle animal), or rat IgG (right animal). (G) TH17 cells were generated as described in “Methods.” Cells were then stimulated with PMA and ionomycin for 4 hours followed by RNA extraction. IL-21 and IL-22 expression were determined by quantitative PCR. Data are presented as fold change compared with naive PMA and ionomycin stimulated CD4+/CD25− cells.
Mechanism(s) of TH17-mediated pathologic lesions. TH17 cells were generated as described in “Methods.” (A) TNF-α expression in in vitro–differentiated cells (left), TH17 cells isolated from the lung 8 days after transplantation (WT TH17 cells; middle and IFN-γ−/− TH17 cells; right). (B) Serum TNF-α concentrations on day 12 after transplantation from recipients of TH17 cells, total T cells, and BM only. TH17 cells (5 × 106) from WT mice were transferred with T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients. Animals were treated with 500 μg of anti–TNF-α (n = 6), 200 μg of anti–IL-17A (n = 6), or 500 μg rat IgG (n = 7) biweekly for 4 weeks. Animals were monitored for survival (C), signs of GVHD (*P < .01 [anti–TNF-α vs rat IgG] and remained significant from day 7 on, D), and weight loss *P < .01 [anti–TNF-α vs rat IgG] (**P values remained significant from day 18 on) (E). (F) Comparison of pathologic skin lesions in recipients of 5 × 106 TH17 cells receiving anti–IL-17A (left animal), anti–TNF-α (middle animal), or rat IgG (right animal). (G) TH17 cells were generated as described in “Methods.” Cells were then stimulated with PMA and ionomycin for 4 hours followed by RNA extraction. IL-21 and IL-22 expression were determined by quantitative PCR. Data are presented as fold change compared with naive PMA and ionomycin stimulated CD4+/CD25− cells.
Because TNF-α production by donor TH17 cells was maintained in vivo, we assessed the impact of blocking TNF-α by the administration of neutralizing antibody. TNF-α neutralization significantly improved recipient median survival time compared with isotype control treatment (P < .01; Figure 7C). Consistent with the increased survival, GVHD scores in mice receiving anti–TNF-α were significantly improved (Figure 7D), with a marked improvement in weight loss (Figure 7E). However, despite these improved outcomes, anti–TNF-α treatment had no effect on pathologic skin lesions induced by TH17 cells.
Thus, we evaluated whether IL-17A itself might play a significant role in the pathologic skin lesions induced by TH17 cells. Blockade of IL-17A markedly decreased fur loss and skin manifestations (Figure 7F) mediated by TH17 cells, although it had no impact on overall survival or GVHD score. It is noteworthy that, as described by others, we found that our in vitro–generated TH17 cells produced substantial quantities of cytokines associated with skin disease such as IL-21 and IL-22 (Figure 7G). These results strongly support differential roles for TNF-α and IL-17A in TH17-mediated clinical pathologic lesions.
Discussion
Previous work investigating a role for TH17 cells in processes such as GVHD have been difficult because of the inability to generate a highly enriched population of TH17s. Obtaining a very highly enriched population of TH17 cells has been a challenge because an algorithm of surface markers unique to TH17s has not been identified, and culture conditions have not been optimized. Thus, using IL-6 and TGF-β, typically under polarizing culture conditions, approximately 30% to 60% of the T cells will generate IL-17.23-25 Although this approach has been critical in our understanding of the cytokines that mediate TH17 polarization, the presence of a large number of cells that are not generating IL-17 has made it difficult to use these conditions to investigate the effects of TH17 cell administration in vivo. We have developed a protocol in which naive CD4+ T cells can be differentiated into TH17 cells of significant purity. The critical aspects of this approach include (1) highly purified population of naive T cells to initiate the culture, (2) neutralization of IL-2 during differentiation, and (3) addition of IL-23 for expansion. The ability to generate a highly enriched population will provide opportunities for studying the biology of TH17 cells in vitro and in vivo.
Our findings show that purified TH17 cells mediated lethal GVHD that included extensive pathologic skin lesions and was independent of IFN-γ production. We demonstrated that TH17 cells traffic to and expand within GVHD target organs as well as lymphoid tissues. Quantitative histopathologic analysis confirms the GVHD scoring data that TH17 cells promote significant cutaneous and pulmonary disease. Consistent with our data, TH17 cells have been shown to be involved in a model of autoimmunity resembling chronic GVHD.45
The haploidentical model (C57BL/6 into [C57BL/6J × DBA/2] FI) of acute GVHD rarely displays any significant pathologic skin lesions. Damage to the liver, GI tract, and lung results in animals succumbing to disease before the generation of extensive pathologic skin lesions. Here, we found that TH17 cells mediated severe cutaneous GVHD in B6D2 recipients with pathology scores higher than those found using naive whole T cells. In addition, extensive pathologic lung lesions were demonstrated, suggesting that TH17 cells may play a role in pulmonary processes found after allogeneic transplantation. In support of our observations, IPS has recently been demonstrated to be associated with TH17 cell accumulation in the lung regulated by IFN-γ.46
TH1 differentiation is dependent upon the signal transducer and activator of transcription-4 (STAT4) molecule. STAT4 renders cells responsive to IL-12, which is required for initial IFN-γ production. Animals deficient in STAT4 have impaired TH1 responses and have enhanced TH2 function.47,48 Although in vitro–polarized TH2 cells were unable to induce GVHD49 a role for TH2 T cells in mediating GVHD has been inferred from the ability of STAT4−/− T cells to induce GVHD and the increased production of TH2 cytokines such as IL-4 in recipients of STAT4−/− donor T cells.50 One potential explanation for our findings, especially when using TH17 cells from IFN-γ−/− mice, is the potential for TH2 polarization and a role for T cells generating IL-4 in the pathophysiology observed by in vitro–generated TH17 cells. Our data strongly refute this premise. IFN-γ−/− TH17 cells retained TNF-α production in vivo, which is inconsistent with a shift to a TH2 response. In addition, the predominant TH2 cytokine, IL-4, was undetectable in cell cultures from WT TH17 cells (data not shown). These data suggest that TH2 cells do not play a significant role in the pathologic lesions observed after the transfer of TH17 cells to lethally irradiated recipients. It is intriguing to speculate that TH17 cells could contribute to the pathologic lesions observed with STAT4−/− donor T cells.
Yi et al51 recently found that the administration of IL-17−/− donor T cells to lethally irradiated MHC mismatched recipients led to enhanced GVHD as a result of robust TH1 expansion and IFN-γ production by donor cells. Interestingly, we have found the administration of anti–IFN-γ mAb to lethally irradiated B6D2 recipients given B6 T cells markedly increased the number of TH17 cells in lymphoid sites and GVHD target organs (M.J.C. and J.S.S., unpublished data, November 2007). These observations suggest that in the context of a blunted TH1 response enhanced TH17 differentiation may significantly contribute to GVHD. Future work will address a specific role for TH17 cells in mediating GVHD in the absence of IFN-γ, and the interactions between IL-17 and IFN-γ in the reciprocal control of TH1 and TH17 polarization.
The mechanisms by which TH17 cells mediate GVHD pathologic conditions are not completely clear. Given that TH17 cells produce several inflammatory cytokines, including IL-17A,22-25 IL-17F,36 TNF-α,44 IL-2141-43 and IL-2239,40 we sought to determine the relative contributions of these effector proteins. Our data suggested that IL-17F was not a significant factor in GVHD pathologic conditions, because transferred TH17 cells displayed minimal production of IL-17F in vivo. IL-17A or cytokines induced by IL-17A played a critical role in skin GVHD induced by TH17 cells. However, the exact mechanism by which TH17 cells induced pathologic lesions in organs other than the skin was not determined. Neutralization of TNF-α greatly diminished systemic disease in mice receiving TH17 cells, as demonstrated by superior survival time, diminished GVHD scores, and minimal weight loss. Studies from previous investigators implicate both IL-17A and TNF-α in pathologic pulmonary lesions in GVHD-like models.19,52 It is interesting that the predominant sites for TH17 activity are the lung, GI tract, and skin, and that GVHD found at these sites or IPS have been amenable to therapy in patients using soluble TNF-α receptor (etanercept)53,54 or a monoclonal antibody to TNF-α (infliximab).55 TH17 cells could play a role in the clinical manifestations of GVHD mediated by TNF-α at these sites.
The results of histopathologic examination demonstrated significantly enhanced tissue damage in the lungs and skin of recipients of TH17 cells compared with whole T cells or CD4+/CD25− cells. There are at least several possible explanations for these findings. One potential hypothesis is that effector cytokines within the TH17 axis are particularly deleterious to the skin and lung. A likely candidate for enhanced pathologic cutaneous conditions would be IL-22. IL-22 has been described as having proinflammatory activities in the liver, colon, small bowel, and skin.40,56,57 More recently, a report has shown that TH17-produced IL-22 was the immunologically dominant cytokine involved in a mouse model of psoriasis.58 Although IL-17A blockade substantially attenuated skin manifestations, a role for IL-22 in TH17-mediated pathologic conditions cannot be excluded. A second hypothesis is that TH17 cells have a migration bias to traffic to the lungs and skin compared with other CD4+ T cells. IL-17A induces expression of the chemokine CCL20 in human keratinocytes59 and both human60 and murine61 TH17 cells express CCR6, the receptor for CCL20. In addition, several different groups have demonstrated that pulmonary epithelial cells generate CCL20 in response to infectious and inflammatory stimuli.62-64 Although our data would support either hypothesis, we favor differences in cell trafficking as the hypothesis that best explains our findings. Future will work address these hypotheses.
The induction of cutaneous GVHD by TH17 cells was not model specific as it occurred in both a haploidentical and a minor mismatch model. Thus, it is tempting to speculate that TH17 cells may mediate cutaneous clinical GVHD. However, confirming this finding may be difficult. We have found that after adoptive transfer of in vitro–differentiated TH17 cells, the percentage of cells that generate IFN-γ increased over time, whereas the number of cells generating IL-17A decreased (M.J.C. and J.S.S., unpublished data, October 2007). Thus, identification of TH17 cells solely on the basis of immunohistochemistry or intracellular cytokine staining specific for IL-17 would not account for these “late” TH17 cells that have turned off IL-17 production. We have observed that the addition of IL-12 to in vitro differentiated TH17 cells also induced a switch to IFN-γ production (M.J.C. and J.S.S., unpublished data, November 2007). These results are consistent with those observed in human TH17 cells.60 Moreover, microarray analysis of host murine antigen-presenting cells after irradiation has indicated a substantial increase in the transcription of IL-12 (C. Wysocki and J.S.S., unpublished data, April 2007). Thus, the conditioning therapy used to induce engraftment may enhance the production of IFN-γ by TH17 cells after transplantation by up-regulating IL-12 expression. A switch from IL-17 to IFN-γ production, however, does not alter TNF-α production by TH17 cells. Despite the evidence that TH17 cells can generate IFN-γ, we confirmed that the pathologic condition mediated by TH17 cells did not require IFN-γ, because TH17 cells generated from IFN-γ−/− donors induced similar cutaneous disease as WT TH17 cells. Evaluating for the production of IL-21 or IL-22 may be more useful for a clinical assessment of the role of TH17 cells in patients with GVHD.
Our work demonstrates that in vitro–differentiated TH17 T cells mediate significant pathologic cutaneous and pulmonary conditions during GVHD. These data support the role of TH17 cells in IPS and psoriasis. Specifically targeting TH17 differentiation or function, especially in cases of severe cutaneous or pulmonary disease, may provide a new approach for the treatment of GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Joseph Burgents for technical assistance.
This research was supported by National Institutes of Health grants R01-CA102052, R01-AI064363 to J.S.S., R01-AI034495, R37-HL56067, P01-065299 to B.R.B., and T32-HL007149 to M.J.C. This work was also supported by the Mary Elizabeth Thomas Endowment fund (J.S.S).
National Institutes of Health
Authorship
Contribution: M.J.C. designed and performed research and wrote the manuscript; M.L.W. and J.M.C. performed experiments; A.P.-M. performed experiments and reviewed the manuscript; B.R.B. wrote the manuscript; and J.S.S. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan S. Serody MD, Department of Microbiology and Immunology, The University of North Carolina, Chapel Hill, NC 27599; e-mail: jonathan_serody@med.unc.edu.

![Figure 7. Mechanism(s) of TH17-mediated pathologic lesions. TH17 cells were generated as described in “Methods.” (A) TNF-α expression in in vitro–differentiated cells (left), TH17 cells isolated from the lung 8 days after transplantation (WT TH17 cells; middle and IFN-γ−/− TH17 cells; right). (B) Serum TNF-α concentrations on day 12 after transplantation from recipients of TH17 cells, total T cells, and BM only. TH17 cells (5 × 106) from WT mice were transferred with T cell–depleted bone marrow cells into lethally irradiated B6D2 recipients. Animals were treated with 500 μg of anti–TNF-α (n = 6), 200 μg of anti–IL-17A (n = 6), or 500 μg rat IgG (n = 7) biweekly for 4 weeks. Animals were monitored for survival (C), signs of GVHD (*P < .01 [anti–TNF-α vs rat IgG] and remained significant from day 7 on, D), and weight loss *P < .01 [anti–TNF-α vs rat IgG] (**P values remained significant from day 18 on) (E). (F) Comparison of pathologic skin lesions in recipients of 5 × 106 TH17 cells receiving anti–IL-17A (left animal), anti–TNF-α (middle animal), or rat IgG (right animal). (G) TH17 cells were generated as described in “Methods.” Cells were then stimulated with PMA and ionomycin for 4 hours followed by RNA extraction. IL-21 and IL-22 expression were determined by quantitative PCR. Data are presented as fold change compared with naive PMA and ionomycin stimulated CD4+/CD25− cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/6/10.1182_blood-2008-06-162420/4/m_zh80030929770007.jpeg?Expires=1769090729&Signature=xsc3cD-~dfxe53G6vz-NoY1xwjU9~kay4VguOBRpiFB4O58lHvjqi-iIqWUUTehp6ecRFvxixREfOzIRsq7iboyLU0Z9Y6WRKbO2qILuRJXEACKy5shHtiPx5HBlP~8jtds1qoSSyPXRPkSyh-CgZzf~J~Drd6Lyqsdsn-bDU7MmVcEQm-6EW2PWpn6OeD-Rjx6Z6z2Eypff0fRNRmSBoEcjJKZoWJp3OyBNajv8H9r-4U82cbK0ft-XWEEnSB9hrlbsmnVrKnQdhMEGqKhrhKenJSseEjM36YaDFOpQ7Cz16nM1MJkAy8ckdv9sZLVQs7K6FifwnnZKz3-fxr1ndA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
