Abstract
Early events in tumor development are spontaneous, microscopic, and affected by the microenvironment. We developed a mouse model of spontaneous lymphoma in which malignant transformation is coupled with light emission that can be detected noninvasively using bioluminescent imaging. The human T-cell leukemia virus (HTLV) type 1 transcriptional transactivator Tax is an oncogene sufficient to produce lymphoma in transgenic animal models. Using the granzyme B promoter to restrict Tax expression to the mature natural killer (NK)/T-cell compartment, we have reproduced many elements of HTLV-associated adult T-cell leukemia/lymphoma. Tax activates signaling cascades associated with transformation, inflammation, and tumorigenesis. Here, we report that Tax-mediated activation of luciferase in long terminal repeat-luciferase (LTR-LUC) mice serves as a reporter for imaging these processes in vivo. Using bioluminescent imaging (BLI), we discovered that microscopic intraepithelial lesions precede the onset of peripheral subcutaneous tumors, tumorigenesis progresses through early reversible stages, and Tax is sufficient for inducing tumors. Based on these findings, we propose that Tax expression in activated lymphocytes initiates a cascade of events that leads to NK/T cell recruitment, activation, and transformation. The use of BLI expands our ability to interrogate the role of Tax in tumorigenesis in vivo and has made the association of inflammation with tumor initiation amenable for study.
Introduction
Early detection of malignant tumors and identification of events that precede tumorigenesis are critical in cancer treatment and prevention strategies. Malignant transformation involves successive accumulation of genetic events that breach anticancer defense mechanisms and confer growth advantages to the mutated cell, events that are spontaneous and microscopic.1 Within the host, transformation is also affected by the tumor microenvironment. Limitations in detecting these microscopic spontaneous events in vivo have restricted the utility of animal models in the interrogation of spontaneous tumorigenesis.2 To overcome these limitations, we developed a mouse model of spontaneous lymphoma in which malignant transformation is coupled with light emission that can be detected noninvasively using bioluminescent imaging (BLI)
The Tax oncoprotein from human T-cell leukemia virus type 1 (HTLV-1), the causative agent of adult T-cell leukemia/lymphoma (ATLL) and HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP), is a potent transcriptional transactivator capable of promoting cellular transformation.3 In the context of HTLV-1 replication, Tax promotes viral gene expression by activating the 5′ long terminal repeat (LTR) of the genome. Tax activity also results in growth advantages normally acquired over time by malignant cells.4 Tax triggers cell growth and prevents apoptosis by activating pathways that signal through the cAMP responsive element binding transcription factor (CREB), nuclear factor kappa B (NFκB), and serum response factor (SRF). Inhibition of p53 and dysregulation of other checkpoint controls by Tax leads to genetic instability accelerating mutagenesis.5 Tax restricts retinoblastoma protein (Rb) activity disengaging mechanisms designed to inhibit cell growth and activates telomerase, allowing limitless replication potential.6 Placing the Tax oncogene under the human granzyme B promoter (GZB-TAX) restricts its expression to activated T cells and natural killer (NK) cells.7 Mice carrying the GZB-TAX transgene develop large granular lymphocytic (LGL) leukemia/lymphoma, osteolytic disease, neutrophilia, and splenomegaly during the first year of life.8,9 Transformed cells derived from peripheral tumors are most often CD16hi, interleukin (IL)–2 independent NK cells.10
To couple spontaneous tumorigenesis and BLI, we hypothesized that transgenic mice in which firefly luciferase (LUC) is regulated by the HTLV-1 LTR should emit light in response to Tax expression. Here, we report the production of LTR-LUC transgenic mice, confirm that double transgenic (TAX-LUC) mice recapitulate the original tumor model, and demonstrate that bioluminescence in these mice serves as a novel reporter for spontaneous tumorigenesis in vivo. Using bioluminescence to monitor Tax-mediated transformation, we discovered that (1) microscopic intraepithelial lesions precede the onset of peripheral subcutaneous tumors, (2) tumorigenesis in this model progresses through early reversible stages, (3) Tax expression is sufficient for initiating tumorigenesis in vivo, but is not required for tumor maintenance, and (4) primary tumor cells retain bioluminescent properties in tumor allograft models. Use of BLI dramatically expands our ability to investigate the role of Tax in all aspects of tumorigenesis in vivo and has made an important connection between wounding, inflammation, and tumor initiation in this model amenable for future study.
Methods
Cells
B5Luc cells were derived from B5 cells (primate kidney fibroblasts) transfected with LTR-Luc, selected in G418 (400μg/mL), and cloned by limiting dilution.11 Coculture of B5Luc cells with irradiated 293T cells transfected with a replication competent HTLV-1 molecular clone that expressed Tax (pACH) caused cell fusion, infection of B5Luc cells, and activation of LTR-Luc by Tax.12 Mirus TransIt reagent was used in all transfections according to manufacturer's instructions (Fisher Scientific, Pittsburgh, PA). CD4+CD16/32+ cell lines subsequently derived from transplanted bioluminescent tumors have been stable in culture for more than 12 months.
Transgenic mice
The LTR-Luc plasmid consists of the 0.7-kb XhoI-HindIII 5′LTR fragment of pHTE-1 and the luciferase open reading frame from pGL-3 (Promega, Madison, WI) cloned into the XhoI-NotI vector backbone of pEGFP-1 (Clontech, Mountain View, CA).13 The XhoI-AflII fragment of LTR-Luc was used to generate 2 independent transgenic founders with indistinguishable bioluminescence characteristics.14 GZB-TAX mice in which HTLV-1 Tax is regulated by the 5′ flanking region (positions −1170 to +36) of the human granzyme B gene have been previously described.8 Mice were housed under pathogen-free conditions, and animal protocols were approved by the Animal Studies Committee in accordance with the guidelines of the Washington University School of Medicine.
Flow cytometry
Cell suspensions derived from organs or tumors were stained with fluorescein isothiocyanate (FITC)–conjugated FcγR II/III antibodies (clone 2.4G2; BD Biosciences/Pharmingen, Franklin Lakes, NJ) for 30 minutes at 4°C and analyzed on a FACScan (BD Biosciences).8
Imaging
The IVIS100 imaging system (Caliper Life Sciences, Alameda, CA) was used to image bioluminescence in vivo in anesthetized mice (isoflurane inhalation).15 Standard imaging parameters included D-luciferin dose, 50 mg/mL intraperitoneal (ip; 500 mg/kg body weight); exposure time, 300 seconds; binning, 4; f/stop, 1; no optical filter. Areas of the body covered by hair required shaving or depiliation before imaging. X-ray images were obtained in a Kodak in vivo imaging system 4000MM (exposure time: 60 seconds; aperture: 2.8; focus: 12.5 mm; field of view: 60 mm). Color scale unless otherwise indicated is ×104 photons/s/cm2/sr.
Histology
Histology and tumor cell characterization in GZB-TAX tumors was described previously8,9,16-18 Malignant LGL cells are distinguishable by morphologic features including size, granularity, shape, and nuclear morphology, they typically constitute approximately 10% of tumor cells and constitutively express high levels of CD16.
Tissues were fixed in 4% paraformaldehyde (sections including bone were decalcified in 0.5 M EDTA [ethylenediaminetetraacetic acid] for 14 days) and embedded in paraffin for serial sectioning. Paraffin-embedded mouse tissue was cut at 4 μm and placed on positively charged slides. Slides with specimens were then placed in a 60°C oven for 1 hour, cooled, deparaffinized, and rehydrated through xylene-graded ethanol solutions to water. All slides were quenched for 5 minutes in a 3% hydrogen peroxide solution in water to block endogenous peroxidase. Antigen retrieval was performed by a heat method in which specimens were placed in Dako target retrieval solution, pH 6.1 for 25 minutes at 94°C using a vegetable steamer and cooled for 15 minutes in solution. Slides were then placed on a Dako Autostainer immunostaining system for use with immunohistochemistry (Dako, Glostrup, Denmark). Slides were initially blocked for endogenous biotin before the addition of biotinylated primary antibody. The biotinylated primary antibody solution was incubated for 1 hour. Next a labeled streptavidin was applied and incubated for 30 minutes. Lastly, the slides were developed with DAB (3,3′ diaminobenzidine) chromogen. Slides were then counterstained in hematoxylin, dehydrated through graded ethanol solutions, and coverslipped. Stained sections were visualized with a Nikon Eclipse E400 microscope and digital images were obtained using a Magnafire camera and software (Optronics, Muskogee, OK).
Results
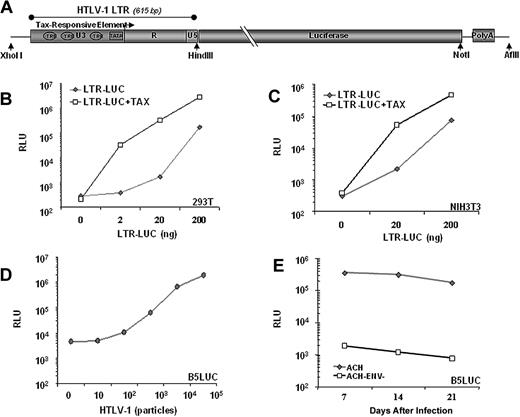
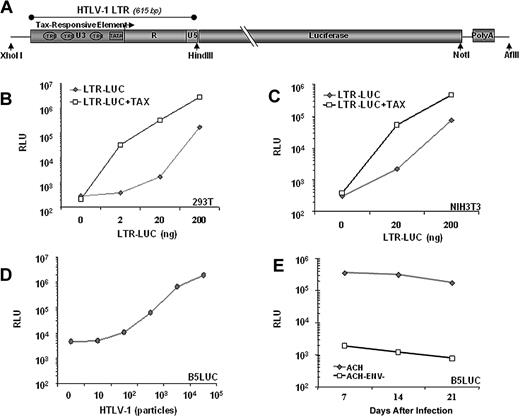
Previously, we described a transgenic model for ATLL in which Tax is expressed in activated T cells and NK cells under the regulation of the human granzyme B promotor (GZB-TAX).8 To use Tax expression as a biomarker for malignancy, we developed a second transgenic line in which firefly luciferase expression is regulated by a Tax-inducible promoter, the HTLV-1 LTR. The LTR contains 3 Tax responsive elements, which are 21 nucleotide repeats homologous to the consensus CRE (Figure 1A).19 Tax activates viral transcription by recruiting CREB and CREB-binding proteins CBP and p300 to the U3 region of the viral 5′LTR.20 In transient transfection experiments in human (Figure 1B) and mouse cell lines (Figure 1C), luciferase expression from the LTR-LUC construct was induced 50- to 100-fold by Tax. In these experiments, Tax-independent basal LTR activity also increased relative to transgene copy number. Tax-independent HTLV-1 LTR activity is mediated by the Sp1, AP1, and C/EBP families of transcription factors,21-24 can be induced by apoptosis or stress responses,25-28 and is repressed by HDAC1 and ATFx.29,30 However, when stably integrated in a primate cell line, the LTR-LUC transgene has low basal activity and 100- to 200-fold inducibility by retrovirally expressed Tax (Figure 1D,E). Similarly, in both founder LTR-LUC transgenic mice, basal luciferase activity was undetectable by BLI, and no pathologic condition was associated with the transgene.
Luciferase activity is inducible by Tax. (A) Schematic representation of the transgenic construct used. Tax activates viral transcription by recruiting CRE binding transcription factor (CREB) to the U3 region of the 5′LTR. Luciferase activity in lystates from (B) 293T cells and (C) NIH3T3 cells 2 days after cotransfection with 100 ng of CMV-Tax and indicated amounts of LTR-Luc plasmid. (D) Luciferase activity from lysates of B5Luc cells, (B5 cells containing stably integrated LTR-Luc), cocultured with indicated numbers of irradiated HTLV-1 infected (Tax-expressing) cells or with (E) 105 irradiated HTLV-1 infected cells for 3 weeks. Data represent the average of 2 experiments in duplicate.
Luciferase activity is inducible by Tax. (A) Schematic representation of the transgenic construct used. Tax activates viral transcription by recruiting CRE binding transcription factor (CREB) to the U3 region of the 5′LTR. Luciferase activity in lystates from (B) 293T cells and (C) NIH3T3 cells 2 days after cotransfection with 100 ng of CMV-Tax and indicated amounts of LTR-Luc plasmid. (D) Luciferase activity from lysates of B5Luc cells, (B5 cells containing stably integrated LTR-Luc), cocultured with indicated numbers of irradiated HTLV-1 infected (Tax-expressing) cells or with (E) 105 irradiated HTLV-1 infected cells for 3 weeks. Data represent the average of 2 experiments in duplicate.
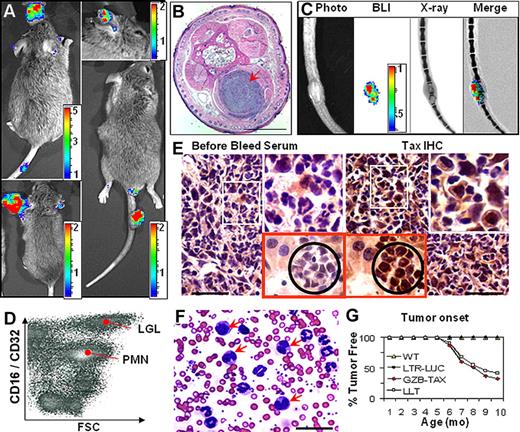
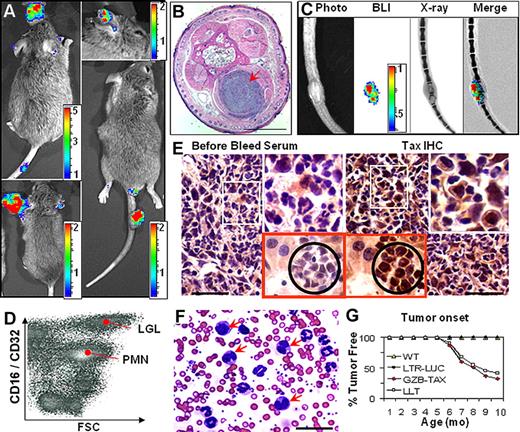
When GZB-TAX mice were bred with LTR-LUC mice, luciferase was expressed in tumors of the double transgenic (TAX-LUC) offspring and was detectable by noninvasive BLI (Figure 2A). Bioluminescent tumors arising in TAX-LUC mice, like the original GZB-TAX mice, occurred at subcutaneous sites (Figure 2B) and were often associated with osteolytic lesions (Figure 2C). Primary and disseminated tumors contain an admixture of inflammatory cells and malignant LGL cells that could be distinguished by size, morphology, and surface expression of CD16, and also included CD16lo neutrophils and CD16− lymphocytes (Figure 2D). Tax protein was detectable by immunohistochemistry in LGL cells in primary tumors and involved organs (Figure 2E).9 As in the original model, complete and differential counts of peripheral blood of TAX-LUC mice (Figure 2F) revealed an increase in total white blood cell count (32 000/μL in the example shown), dramatic neutrophilia (78% of nucleated cells), polychromasia in the erythrocyte series, and an increase in the number of circulating LGL cells, many of which contain multinucleated or multilobulated nuclei (arrows, Figure 2F).8 Finally, the presence of the LTR-LUC transgene did not affect the onset or penetrance of Tax-mediated tumorigenesis in TAX-LUC animals (Figure 2G). Taken together, these data establish that bioluminescence correlated with spontaneous tumorigenesis in TAX-LUC mice, and the spatially and temporally dynamic molecular events that precede spontaneous tumor development in this model in vivo were unaffected by introduction of the LTR-LUC transgene.
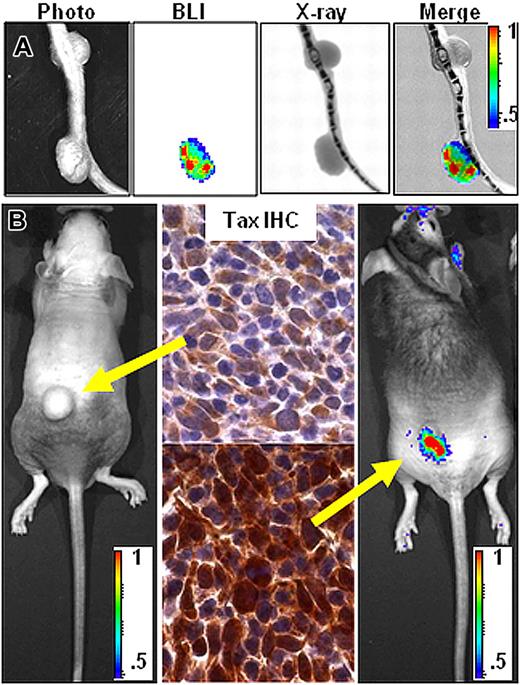
TAX-LUC mice spontaneously develop bioluminescent tumors. (A) BLI of TAX-LUC mice with peripheral tumors on the nose, ear, foot, and tail. (B) Hematoxylin and eosin (H&E)–stained section of a bioluminescent inflammatory tail nodule (red arrow). Bar = 1 mm. (C) Coregistration of photograph, BLI, and X-ray images. (D) Fluorescence activated cell sorting (FACS) histogram of a tumor homogenate. (E) Immunohistochemistry (IHC) on sequential sections of a bioluminescent tumor (white inset) and liver involvement (red inset with black circle) stained with Tax or prebleed serum. Bar = 50 μm. (F) Wright/Giemsa-stained peripheral blood smear from an animal with advanced lymphoma. Red arrows indicate multinucleated LGL cells. (G) Rate of tumor development in TAX-LUC (n = 11) and GZB-TAX (n = 9) mice. Color scale for panels A and C is ×104 photons/s/cm2/sr.
TAX-LUC mice spontaneously develop bioluminescent tumors. (A) BLI of TAX-LUC mice with peripheral tumors on the nose, ear, foot, and tail. (B) Hematoxylin and eosin (H&E)–stained section of a bioluminescent inflammatory tail nodule (red arrow). Bar = 1 mm. (C) Coregistration of photograph, BLI, and X-ray images. (D) Fluorescence activated cell sorting (FACS) histogram of a tumor homogenate. (E) Immunohistochemistry (IHC) on sequential sections of a bioluminescent tumor (white inset) and liver involvement (red inset with black circle) stained with Tax or prebleed serum. Bar = 50 μm. (F) Wright/Giemsa-stained peripheral blood smear from an animal with advanced lymphoma. Red arrows indicate multinucleated LGL cells. (G) Rate of tumor development in TAX-LUC (n = 11) and GZB-TAX (n = 9) mice. Color scale for panels A and C is ×104 photons/s/cm2/sr.
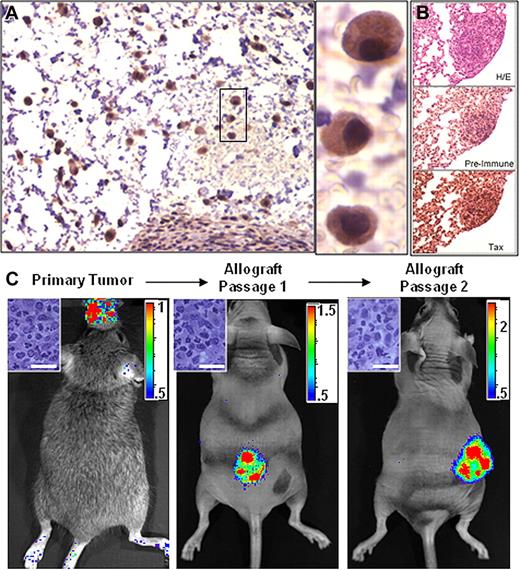
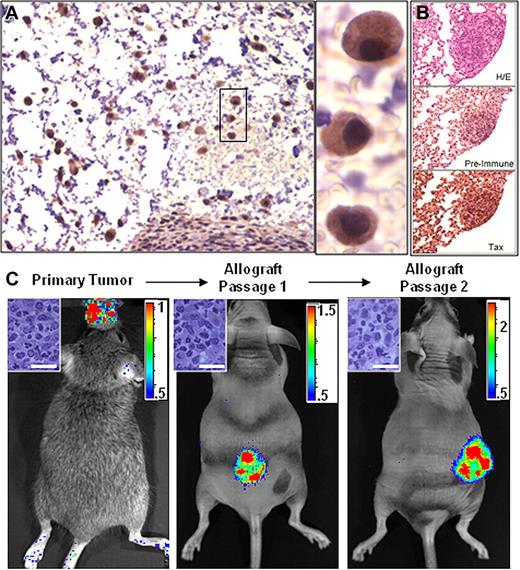
Since the granzyme B promoter is active in mature T cells and NK cells, Tax expression and subsequent luciferase activity in bioluminescent tumors originates from transformed cells or from normal cells activated within, or recruited to, the tumor microenvironment. While cell lines derived from GZB-TAX tumors constitutively express NFκB and are highly resistant to apoptosis, transformed cells constitute the minority of cells in the primary tumor in vivo and express very little Tax in culture.10,16 To determine whether bioluminescence is a property of the transformed cells within a primary tumor, cell homogenates from TAX-LUC tumors were injected subcutaneously into beige/nude/xid (BNX) mice. We found that cells derived from primary TAX-LUC tumors, when transferred to allograft recipients, propagated into tumors composed of Tax-expressing LGL cells (Figure 3A); spread to liver, lung, spleen, and bone (Figure 3B); and expressed luciferase that was observable by BLI (Figure 3C). These results indicate that luciferase activity was a property of the malignant LGL cell population in TAX-LUC mice and that BLI can be used to identify primary tumor tissue and monitor growth and dissemination of transplanted LGL tumors in allograft models.
Bioluminescence is a property of transformed LGL cells. Homogenate from a primary bioluminescent tumor implanted into and passaged in Beige/Nude/Xid (BNX) mice. (A) Tax IHC on sections of BNX tumors. (B) IHC on sequential sections of a bioluminescent lung tumor stained with H&E, Tax serum, or pre-bleed serum. (C) BLI of tumors in BNX mice confirmed that luciferase activity transferred with the malignant cell population. Bar = 20 μm. Color scale ×104 photons/s/cm2/sr. Data shown are representative of allografts in 23 mice injected with cells from fresh or frozen primary tumor tissue.
Bioluminescence is a property of transformed LGL cells. Homogenate from a primary bioluminescent tumor implanted into and passaged in Beige/Nude/Xid (BNX) mice. (A) Tax IHC on sections of BNX tumors. (B) IHC on sequential sections of a bioluminescent lung tumor stained with H&E, Tax serum, or pre-bleed serum. (C) BLI of tumors in BNX mice confirmed that luciferase activity transferred with the malignant cell population. Bar = 20 μm. Color scale ×104 photons/s/cm2/sr. Data shown are representative of allografts in 23 mice injected with cells from fresh or frozen primary tumor tissue.
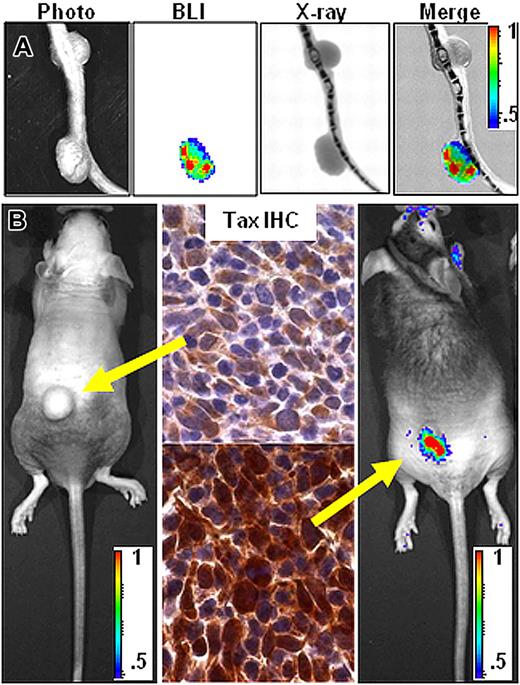
Many factors can affect bioluminescence in vivo, including availability of the D-luciferin and cofactors required for the luciferase reaction, depth and pigmentation of the tissue being imaged, and the number and transcriptional activity of the cells carrying the bioluminescent reporter.2 We observed heterogeneity in photon output among tumors over time (Figure 4A), which correlated with the level of Tax expression (Figure 4B), even though both tumors persisted and maintained a malignant phenotype. These results directly correlated bioluminescence with Tax activity in tumor cells, identified Tax as a dynamic variable during tumor development, and suggest that constitutive Tax activity is not necessary to maintain a malignant phenotype.
Bioluminescence correlates with Tax expression. (A) Coregistration of photograph, BLI, and X-ray images on tail tumors with differing bioluminescent properties. (B) Comparison of bioluminescence and TAX expression on tumors arising in 2 different BNX animals injected with homogenate from a single bioluminescent primary TAX-LUC tumor. Yellow arrows indicate correspondence between tumor BLI and TAX IHC. Color scale ×104 photons/s/cm2/sr.
Bioluminescence correlates with Tax expression. (A) Coregistration of photograph, BLI, and X-ray images on tail tumors with differing bioluminescent properties. (B) Comparison of bioluminescence and TAX expression on tumors arising in 2 different BNX animals injected with homogenate from a single bioluminescent primary TAX-LUC tumor. Yellow arrows indicate correspondence between tumor BLI and TAX IHC. Color scale ×104 photons/s/cm2/sr.
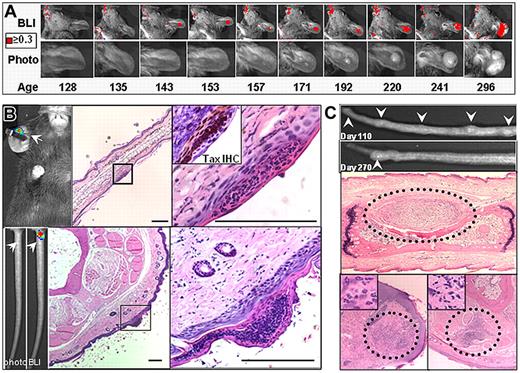
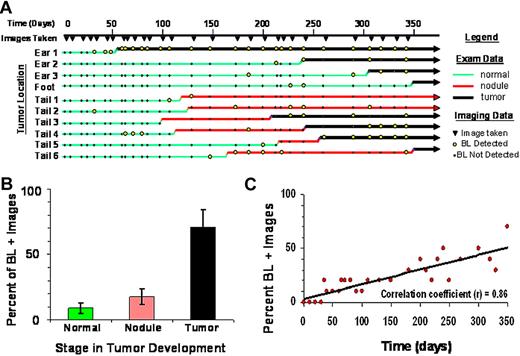
Tax is essential for HTLV-1 replication and development of ATLL, yet in HTLV-1 infected people, viral replication and gene expression is tightly restricted. In ATLL patients, Tax expression is often low or undetectable. Using TAX-LUC mice to observe Tax expression during tumor development, we conducted physical exams and obtained bioluminescent images on a cohort of animals over the course of 1 year (Figure 5A). Physical examination data were divided into 3 categories: normal, nodule, and tumor. Normal tissue had no visual or palpable mass. Nodules, which only developed in mice carrying the Tax transgene, were most clearly defined on the tail as small (< 5 mm), palpable, chronically inflamed masses, that arose rapidly but did not progress. Over time, approximately 50% of nodules resolved, 30% developed into malignant tumors, and 20% remained unchanged for the duration of the study. Tumors were defined as masses exhibiting consistent and aggressive growth, and often involved tissue damage and necrosis. Imaging data were obtained at indicated time points (Figure 5A filled inverted triangle), and bioluminescent regions (Figure 5A open circle) had a defined focus clearly distinguishable from background noise with photon flux greater than 3 × 103 photons/s/cm2/sr. Of 10 lesions that spontaneously developed during the study (Figure 5A), only 1 (Ear 1) was bioluminescent at every time point after inception. In 7 lesions (all except Ear 1, Foot, and Tail 1), bioluminescence was punctuated by intervals lacking luciferase activity. Nine of the lesions (all except Tail 3) exhibited a bioluminescent signature before aggressive tumor growth, and in 2 cases (Tail 1 and Tail 2), nodules developed but did not progress into tumors during the course of the study. While bioluminescence was most frequently observable in tumors, luciferase activity in normal tissue and nodules was stochastic and infrequent (Figure 5B). The frequency of bioluminescence in TAX-LUC mice increased over time as tumors developed with a high degree of variability at any given time point (Figure 5C). The bioluminescence profiles in these data suggest that Tax expression is dynamic. Tax expression is restricted but not permanently extinguished during tumorigenesis and can be used as a predictive reporter for spontaneous tumor development in TAX-LUC mice.
Stochastic bioluminescence correlates with spontaneous tumorigenesis. (A) Summary of physical examination and bioluminescent imaging data from 10 spontaneous lesions that developed in a cohort of TAX-LUC animals over the period of 1 year. (B) Frequency with which bioluminescence is associated with various stages in tumor development. (C) Frequency of bioluminescence within the cohort over time increases as animals develop spontaneous tumors.
Stochastic bioluminescence correlates with spontaneous tumorigenesis. (A) Summary of physical examination and bioluminescent imaging data from 10 spontaneous lesions that developed in a cohort of TAX-LUC animals over the period of 1 year. (B) Frequency with which bioluminescence is associated with various stages in tumor development. (C) Frequency of bioluminescence within the cohort over time increases as animals develop spontaneous tumors.
Development of preventative therapies requires identifying the events that promote malignant transformation and precede tumorigenesis in vivo. The ability to noninvasively detect a bioluminescent signature for weeks or months before a palpable nodule or visible tumor was detectable allowed us to investigate events that precede and promote tumor initiation, and temporally and spatially predict tumor onset. As described above, in almost all cases, small and often transient foci of bioluminescence in normal tissue preceded the onset of tumorigenesis, and one example is shown in Figure 6A. Microscopic examination of tissue sections revealed that the loci of bioluminescence preceding tumorigenesis corresponded to regions involving Tax expression within superficial inflammatory lesions within the epidermis (Figure 6B). These epidermal lesions preceded the development of palpable subcutaneous nodules and were composed primarily of an admixture of neutrophils and lymphocytes. As described above, these nodules resolved more often than they developed into a tumor, and Figure 6C shows examples of such lesions (Figure 6C). These findings render the earliest and previously inaccessible stages of tumorigenesis amenable to further study, reveal a previously unknown and variable pattern of oncogene expression within spontaneous tumors, and represent the first evidence that the initiation of spontaneous peripheral tumors in TAX-LUC animals begins with inflammation associated with a microscopic lesion.
Bioluminescence identifies early stages in tumor development. Serial images of the development of a spontaneous ear tumor in a TAX-LUC animal. Color indicates flux is greater than or equal to 0.3 × 104 photons/s/cm2/sr. (B) Bioluminescence (white arrow) generated by D-luciferin on an otherwise unremarkable ear or tail juxtaposed to corresponding histology. H&E, bar = 100 μm. Inset is TAX IHC on a serial section. (C) Palpable nodules that develop then resolve on the tails of TAX-LUC animals. Histologic sections through the nodules consistently involve inflammatory admixture but presence of malignant LGL cells is variable.
Bioluminescence identifies early stages in tumor development. Serial images of the development of a spontaneous ear tumor in a TAX-LUC animal. Color indicates flux is greater than or equal to 0.3 × 104 photons/s/cm2/sr. (B) Bioluminescence (white arrow) generated by D-luciferin on an otherwise unremarkable ear or tail juxtaposed to corresponding histology. H&E, bar = 100 μm. Inset is TAX IHC on a serial section. (C) Palpable nodules that develop then resolve on the tails of TAX-LUC animals. Histologic sections through the nodules consistently involve inflammatory admixture but presence of malignant LGL cells is variable.
Discussion
BLI has emerged as a sensitive and reliable tool for obtaining dynamic molecular information in vivo.15 Sampling errors and variability inherent in assays that require invasive procedures can easily mask subtle events and increase the number of subjects necessary for statistical significance. Using a subject as its own control, imaging allows detection of systemic changes, identifies regions of interest to guide invasive procedures, and reveals unanticipated variability within a cohort over time. While these advantages of BLI have been well established in experiments involving engraftment of bioluminescent cell lines, they remain largely untapped in animal models that develop spontaneous tumors.
Sufficient to transform primary cells in culture and produce a variety of malignancies in transgenic animal models, Tax activates NFκB and its downstream targets, promotes cell proliferation and inhibits cell-cycle arrest, genetic stability, and apoptosis.3 Using part of the human granzyme B promoter to restrict Tax expression to the mature T-cell and NK-cell compartment, we have been able to reproduce many elements of the lymphoma phenotype seen in humans with ATLL.8 Tumors that spontaneously arise in these animals during their first year of life contain malignant lymphocytes that constitutively express NFκB and NFκB target genes, including IL-6, IL-10, granulocyte macrophage colony stimulation factor (GM-CSF), and interferon gamma (IFN-γ).31 Our hypothesis proposed that the tight regulation of Tax by the granzyme B promoter, the exquisite sensitivity of bioluminescence imaging, and the unique properties of the HTLV-1 LTR would enable us to use Tax to promote malignant transformation and provide a means of detection in vivo.
We have shown that introduction of the LTR-LUC transgene did not affect disease parameters in the TAX transgenic mice, malignant LGL cells within primary tumors were bioluminescent, and bioluminescence correlated with Tax activity. While signaling pathways, surface markers, growth properties, and cytokine production in the LGL cell lines derived from these animals have been examined, several important questions regarding the earliest events in tumorigenesis remain unresolved.32 What determines when and where a tumor will form? Is Tax expressed before and during tumorigenesis, and is it required for tumor maintenance? Is it possible to identify precancerous stages during tumorigenesis? How does the tumor microenvironment affect tumorigenesis in vivo? With TAX-LUC mice and BLI, we have begun addressing these questions. We discovered a bioluminescent signature preceding tumorigenesis and corresponding to microscopic epithelial lesions. This unprecedented glimpse into the initiation events that promote tumorigenesis in this model is the first evidence that these Tax tumors are nucleated by wounds. Grossman et al10 were the first to suggest that injury to epithelial cells could start an inflammatory response that recruits and activates LGL cells, production of Tax, and further exacerbation of inflammation through activation of NFκB. Inflammation is emerging as an important promoter of many cancers including lymphoma, with NFκB serving as a master regulator.33 Chronic inflammation, a promoter of transformation perpetuated by Tax, explains the correlation seen between wounds and tumors, the occurrence of inflammatory nodules in GZB-TAX and TAX-LUC mice, and the preponderance of neutrophils at all stages of tumorigenesis.34
Based on these findings, we propose that Tax expression in activated lymphocytes transforms wounds into a chronically inflamed state, initiating a cascade of events that leads to NK cell recruitment, activation, and Tax-mediated transformation (Figure 7). Initially, CD16+ NK cells, which predominate the peripheral blood, are recruited to acutely inflamed peripheral lesions along with macrophages, neutrophils, and lymphocytes.35 Activation of the cells results in Tax expression and subsequent luciferase activity in TAX-LUC animals. The inflammatory process either resolves or expands into a palpable subcutaneous nodule which may subsequently resolve, remain stable, or progress into an aggressive tumor. We propose that nodules that do not expand into invasive tumors either do not contain transformed cells or contain cells undergoing transformation that were subsequently restricted by another mechanism. The angiostatic effect of IFN-γ retards tumor growth in this model, and may be one such mechanism of restriction.18 Alternatively, the loss of Tax expression during transformation could serve as a mechanism of restriction. Although bioluminescence precedes and is associated with tumorigenesis, our imaging data would suggest that, in the majority of tumors, Tax is not constitutively expressed. In fact, even in patients suffering from ATLL, Tax activity is variable and often undetectable.3 Paradoxically, while Tax promotes immortalization and transformation, it also promotes the expression of IFN-gamma and tumor necrosis factor (TNF)–α, it is angiostatic, it leads to tumor restriction and necrosis, under some conditions Tax can lead to cell cycle arrest, and Tax promotes cytotoxic T-lymphocyte lysis of cells in which it is expressed.3,36-38 The loss of Tax expression and bioluminescence in some tumors indicates that once transformation has occurred, Tax activity is not necessary to maintain the malignant phenotype. Extracellular Tax protein has also been reported to mediate T-cell activation and proliferation.39,40 This activity is mediated through uptake of Tax and activation of gene expression. Thus, paracrine release of Tax from inflammatory lesions could contribute to tumor initiation and maintenance in GZB-TAX mice and would be detectable by BLI.
Model overview of tumorigenesis in TAX-LUC mice. Schematic summary of the proposed mechanism of tumorigenesis and stages associated with bioluminescence. Photographs of H&E-stained tail sections representing stages of inflammation-induced tumorigenesis in this model. Bar = 1 mm.
Model overview of tumorigenesis in TAX-LUC mice. Schematic summary of the proposed mechanism of tumorigenesis and stages associated with bioluminescence. Photographs of H&E-stained tail sections representing stages of inflammation-induced tumorigenesis in this model. Bar = 1 mm.
Although ATLL is a disease of human CD4+ T cells and TAX-LUC mice develop NK leukemia and lymphoma, the mechanisms by which Tax transforms human and murine lymphocytes are of sufficient similarity to allow TAX-LUC mice to recapitulate many aspects of ATLL, including NFκB activation, delayed onset, osteolytic bone disease, hypercalcemia, hepatomegaly, splenomegaly, and pulmonary involvement. Moreover, acute, chronic, and smoldering forms of ATLL all present with skin lesions as a predominant characteristic of disease. While several TAX transgenic mouse models have been previously described and many develop inflammatory diseases, TAX-LUC animals uniquely model the link between inflammation and tumorigenesis.41-43 In models in which Tax is driven by the HTLV-1 LTR, it is expressed in a variety of tissues including fibroblasts, skeletal muscle, bone, peripheral nerves, brain, spleen, thymus, and skin.44-50 These various models developed fibroblast tumors, neurofibromas, skeletal abnormalities, and inflammatory conditions, but none of these models recapitulated human ATLL. Tax expression in other models has been driven from the immunoglobulin G (IgG) promoter/enhancer,51 CD4 promoter,43 and Thy-1 promoter.52 While these mice also develop inflammatory disorders, thymic atrophy, and nonhematopoeitic malignancies, these conditions did not lead to leukemia or lymphoma. Two recent mouse models, in which Tax is expressed from the Lck promoter in thymocytes and/or peripheral T cells, develop ATLL-like diseases.42,53 Central to the contributions of TAX-LUC mice to the literature of ATLL animal models is the ability to detect Tax activity in vivo, using BLI to identify the microenvironment involved in Tax-mediated malignant transformation. Identifying cellular and molecular factors that contribute to these events may provide new molecular targets for prevention of ATLL in HTLV-1 positive people.
We also propose that BLI and the TAX-LUC animal model could more broadly be used to investigate a range of signaling events associated with inflammation and tumor development. Inflammation promotes many forms of lymphoma including those caused by Helicobacter pylori, Epstein-Barr virus, human herpesvirus 8, and hepatitis c virus.54-56 Several forms of lymphoma with a noninfectious etiology, like Hodgkin, mantle cell, and diffuse large B cell lymphomas, are also associated with inflammation or constitutive NFκB activation57,58 We are currently using the TAX-LUC mouse model as a tool to pursue the role of T-cell activation in tumorigenesis, the effect of mediators of T-cell and NK cell development on Tax-mediated transformation, the nature of the inflammatory response associated with the onset of peripheral tumors, and the means by which tumor suppressors modulate Tax activity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to N. Campbell, D. Blanden, S. Mitra-Kaushik, D. Link, R. Kopan, and T. Tsukahara for excellent advice and technical assistance. We thank D. Novak, J. Weber, M. Colonna, and K. Weilbaecher for helpful discussion and critical reading of the manuscript.
This research was supported by grants from the National Institutes of Health to M.L. (CA10073), D.P.-W. (CA94056), and L.R. (CA10521 and CA63417).
National Institutes of Health
Authorship
Contribution: All authors designed research and analyzed data; D.R., J.H., and S.G. performed the research; S.N., D.P.-W., L.R., and M.L. contributed reagents and analytic tools; and D.R. and L.R. assembled the figures and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lee Ratner, Department of Medicine, Division of Molecular Oncology, Washington University School of Medicine, 660 S Euclid, Campus Box 8069, St Louis, MO 63110; e-mail: lratner@dom.wustl.edu.