Abstract
An increased expression of antiapoptotic molecules is often found in malignant cells, where it contributes to their clonal expansion by conferring an improved survival ability. We found that erythroid precurors derived from patients with polycythemia vera (PV) with medium and high JAK2V617F mutation rates often express elevated levels of the antiapoptotic molecules Bcl-2 and Bcl-XL (5 of 12 patients with 3 to 7 times Bcl-2 and 3 of 12 patients with 4 to 7 times Bcl-XL than average normal controls) and are more resistant to myelosuppressive drugs than normal erythroblasts. ABT-737, a small-molecule inhibitor of Bcl-2, Bcl-XL, and Bcl-W, induced apoptosis preferentially in JAK2V617F-high PV erythroid precursors as compared with JAK2V617F-low or normal erythroblasts. ABT-737 inhibited also the proliferation of PV erythroblasts and interfered with the formation of endogenous erythroid colonies by PV hematopoietic progenitors. Altogether, these results suggest that small-molecule inhibitors of Bcl-2/Bcl-XL may be used in the treatment of patients with PV with high JAK2V617F allele burden.
Introduction
Neoplastic cells are often characterized by ineffective death pathways, resulting in enhanced resistance to apoptosis, which contributes to the expansion of the abnormal clone. The detection of apoptotic defects in malignant hematopoietic cells paved the way for the development of therapeutic agents that kill cancer cells or reset their apoptotic threshold.1 ABT-737 is a synthetic small-molecule inhibitor that binds Bcl-2, Bcl-XL, and Bcl-W and promotes apoptosis as a single agent in several malignant hematopoietic and nonhematopoietic cells.2,3 Regulators of apoptotic pathways play a key role in the control of erythroid cell expansion.4,5 Above all, Bcl-XL is essential for erythroid cell development and, together with Bcl-2, controls erythroblast survival to cytotoxic stimuli.6-9 Landmark studies on erythroid cells derived from patients with polycythemia vera (PV) revealed an increased expression of Bcl-XL associated with cell survival in the absence of erythropoietin.10 The discovery of the V617F mutation in the JAK2 tyrosine kinase (JAK2V617F) present in the vast majority of patients with PV opened new opportunities to understand the molecular pathogenesis of PV.11-14 Recently, an association between the presence of the JAK2V617F mutation and an increased resistance to apoptosis induced by death receptors was found in differentiating PV erythroblasts.15 As activated JAK2 can induce both Bcl-XL and Bcl-2 expression,6,16 we analyzed the levels of these 2 proteins in erythroblasts derived from patients with PV patients. In this report, we show that Bcl-2 and Bcl-XL are often expressed in PV erythroid precursor cells in relation to JAK2V617F allele burden, and we investigate the effect of the BH3 mimetic ABT-737 on PV erythroblast survival and expansion, with the hypothesis that it may represent a new inhibitor of PV erythropoiesis.
Study design
Cell culture and reagents
CD34+ hematopoietic progenitor cells (HPCs) were purified from the peripheral blood of healthy donors and patients with PV (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), and cultured in serum-free, stem cell factor–free erythroid medium15 with 3 U/mL erythropoietin (for normal HPCs) or 0.3 U/mL erythropoietin (for PV HPCs). Informed consent was obtained in accordance with the Declaration of Helsinki. In 6 patients with PV, the percentage of JAK2V617F alleles was assessed at day 0 and day 6 of culture and indicated that these culture conditions maintained or increased the initial percentage of JAK2V617F alleles (Table S2). Apoptosis induced by ABT-737 (kindly given by Abbott Laboratories, Abbott Park, IL) was evaluated by annexin V FITC/7-amino-actinomycin D staining (Invitrogen Molecular Probes, Eugene, OR). Endogenous erythroid colonies were obtained by culturing 2 × 105 peripheral blood mononucleated cells from patients with PV in the presence of 30% fetal bovine serum (FBS). BFU-E (burst-forming unit erythroid) colonies were obtained from normal and PV HPCs by plating 3 × 102 CD34+ cells with 1% FBS and 3 U/mL erythropoietin. Anti–Bcl-XL, anti–Bcl-2, anti–Mcl-1, and anti–GATA-1 (clone N1) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Cytosine arabinoside, hydroxyurea, and anti-actin antibodies were from Sigma-Aldrich (St Louis, MO). The intensity of bands was quantified using Scion Image (Scion Corporation, Frederick, MD). The research reported in this manuscript was approved by the Institutional Review Board of each participating institution as part of a project called “Regulation of Survival and Lineage Commitment of Hematopoietic Progenitor Cells.”
JAK2 mutational analysis
Granulocyte genomic DNA was analyzed for the presence of the JAK2V617F mutation by quantitative real-time polymerase chain reaction (PCR)–based allelic discrimination as described.17 Patients were classified as JAK2V617F-low/null, JAK2V617F-medium, or JAK2V617F-high based on the percentage of mutant alleles (< 1%, 1%-75%, and > 75% respectively; see also Table S1). A total of 7 patients with PV classified as JAK2V617F-low/null had a percentage of JAK2V617F alleles between 0.1% and 0.88%, while 2 were apparently “true” JAK2V617F-negative patients.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). The percentage of inhibition in Figure 2A was determined as % inhibition = 100 − [(B2 × 100)/B1], where B1 is the number of vehicle (DMSO)–treated cells and B2 is the number of cells treated with different doses of ABT-737.
Results and discussion
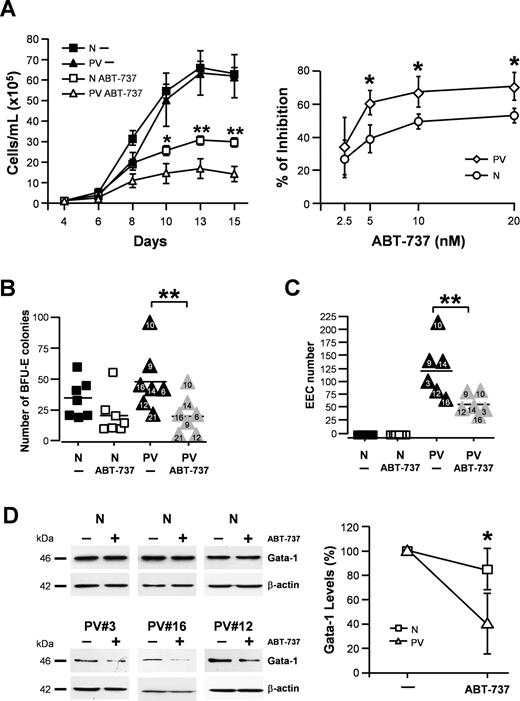
Pure populations of primary erythroid precursors were obtained from CD34+ cells of healthy donors and patients with PV and used to analyze the expression of Bcl-2, Bcl-XL, and Mcl-1, 3 antiapoptotic Bcl-2 family members that play a major role in hematopoietic cell survival. Immunoblotting experiments performed on erythroblasts derived from healthy donors and patients with PV showed that the expression of Bcl-XL was increased in patients with PV compared with controls, thus confirming early observations made by Silva et al.10 The expression of Bcl-2 was also increased in PV erythroblasts, whereas Mcl-1 levels did not significantly differ between control erythroblasts and 6 patients with PV examined (Figure 1A; data not shown). Then, we analyzed Bcl-2 and Bcl-XL expression in a larger number of patients clustered on the basis of a different JAK2V617F allele burden. We found that, on average, the expression of both Bcl-2 and Bcl-XL was comparable to controls in PV erythroblasts with low/null and medium JAK2V617F allele burden, whereas Bcl-2 and Bcl-XL levels increased in patients with a high percentage of mutated JAK2 alleles (P < .001 and P < .05, respectively; Figure 1B). As a likely consequence of increased Bcl-2 and Bcl-XL levels, we found that JAK2V617F-medium and -high PV erythroblasts were more resistant than normal erythroblasts to apoptosis induced by the myelosuppressive drugs cytosine arabinoside and hydroxyurea (P = .006 and P = .002, respectively; Figure 1C). No strict correlation between Bcl-2 and Bcl-XL expression and drug resistance was found at the individual patient level, suggesting that multiple mechanisms may dictate the outcome of myelosuppressive drug treatment. ABT-737 has been shown to induce apoptosis in leukemic cells by activating the mitochondrial apoptotic pathway.18-20 Therefore, we investigated whether ABT-737 was effective in inducing apoptosis of normal and PV erythroblasts. ABT-737 was responsible for a dose-response induction of apoptosis, which was higher in PV erythr oblasts with elevated JAK2V617F allele burden than in normal control erythroblasts or in JAK2V617F-low erythroblasts starting from 0.5 μM (P < .05 for .5 μM and 1 μM; Figure 1D). Bcl-XL levels and ABT-737-induced apoptosis did not vary significantly if PV erythroblasts were cultivated with high levels of Epo (Figure S1A,B), thus confirming previous observations that survival pathways in PV erythroblasts are maximally active at low Epo concentrations.15 ABT-737 was able to induce higher levels of mitochondrial depolarization in PV erythroblasts than in normal erythroblasts, suggesting a differential activation of the mitochondrial apoptotic pathway (Figure S2A). However, caspase cleavage was not significantly different between PV erythroblasts and controls (Figure S2B). To assess whether ABT-737 could inhibit the proliferation of erythroid precursors, we analyzed the effect of low doses of this compound on cell growth in liquid or semisolid culture. We found that a prolonged treatment with ABT-737 (but not a short treatment; Figure S3A) inhibited the expansion of JAK2V617F-high PV erythroblasts in liquid culture, whereas it had a significantly lower effect on the proliferation of normal erythroblasts (Figure 2A; left panel: day 10, P < .05; day 13: P < .01; day 15, P < .01; right panel: P = .028 for 5, 10, and 20 nM). The presence of ABT-737 significantly reduced BFU-E colony formation by PV and normal CD34+ cells in semisolid culture with a preferential inhibitory activity of this compound toward PV HPCs (P = .007; Figure 2B). ABT-737 was also able to effectively inhibit the production of endogenous erythroid colonies by PV HPCs (P = .008; Figure 2C). Treatment with ABT-737 did not produce significant alterations in differentiation nor in cell-cycle distribution of normal and PV erythroblasts (Figure S3B,C). To investigate the mechanism underlying ABT-737–mediated inhibition of erythroblast expansion, we analyzed GATA-1 levels4 in normal and PV erythroblasts treated with low doses of ABT-737. We found that GATA-1 significantly decreased upon ABT-737 treatment in PV erythroblasts but not in normal erythroblasts (P < .05; Figure 2D), indicating a differential extent of caspase-mediated degradation of GATA-1, which may ultimately determine the effect of ABT-737 on cell growth. Taken together, these results indicate that ABT-737, differently from traditional myelosuppressive drugs, is more active on PV erythroblasts than on normal erythroblasts and may find a therapeutic application in the treatment of PV.
PV erythroblasts overexpress Bcl-2 and Bcl-XL and are sensitive to ABT-737–induced apoptosis. CD34+ hematopoietic progenitors isolated from healthy donors and patients with PV were grown in liquid culture for 7 days in medium containing 3 U/mL erythropoietin (for normal HPCs) or 0.3 U/mL erythropoietin (for PV HPCs) as described in “Study design.” The numbers assigned to PV samples correspond to patients described in Table S1. (A) Western blot analysis of Bcl-2, Bcl-XL, and Mcl-1 levels in erythroblasts of healthy controls (N) and PV patients (PV). The right panel shows the quantification of protein levels normalized to beta-actin. (B) Levels of Bcl-2 and Bcl-XL expression in erythroblasts derived from 15 healthy donors or 18 patients with PV with different JAK2V617F allele burdens. Values correspond to the quantification of Western blot bands normalized to beta-actin. Data were analyzed by means of nonparametric Kruskal-Wallis test and the Dunn post-test to compare each group (***P < .001 [Bcl-2]; *P < .05 [Bcl-XL] between normal and JAK2V617 high erythroblasts). (C) Cell death induced by treatment of normal and PV erythroblasts for 16 hours with 75 μM cytosine arabinoside (AraC) or 150 μM hydroxyurea as evaluated by ethidium bromide/acridine orange staining and fluorescence microscopy. Mann-Whitney nonparametric test showed statistical significance (**P = .006 [AraC]; **P = .002 [hydroxyurea] between normal and JAK2V617F-medium/high erythroblasts). (D) Annexin V/7-AAD staining of erythroblasts derived from 5 healthy donors (N), 5 patients with PV with low JAK2V617F allele burden (PV Low/Null), and 5 patients with PV with high JAK2V617F allele burden (PV High). Bars represent the mean and SD of values obtained by treating normal and PV erythroblasts with ABT-737 at the indicated doses or with an equivalent volume of DMSO (−) for 48 hours. The left panel shows a representative experiment from each of the categories analyzed, with numbers indicating the total percentage of Annexin V+ cells. Data were analyzed by means of 2-way analysis of variance (ANOVA) with Bonferroni post-tests and showed a statistically significant difference (*P < .05 at doses of 500 nM and 1 μM between normal and JAK2V617F-high PV samples). Experiments shown in panels C and D were conducted in erythroid medium containing 0.3 U/mL Epo for both normal and PV erythroblasts.
PV erythroblasts overexpress Bcl-2 and Bcl-XL and are sensitive to ABT-737–induced apoptosis. CD34+ hematopoietic progenitors isolated from healthy donors and patients with PV were grown in liquid culture for 7 days in medium containing 3 U/mL erythropoietin (for normal HPCs) or 0.3 U/mL erythropoietin (for PV HPCs) as described in “Study design.” The numbers assigned to PV samples correspond to patients described in Table S1. (A) Western blot analysis of Bcl-2, Bcl-XL, and Mcl-1 levels in erythroblasts of healthy controls (N) and PV patients (PV). The right panel shows the quantification of protein levels normalized to beta-actin. (B) Levels of Bcl-2 and Bcl-XL expression in erythroblasts derived from 15 healthy donors or 18 patients with PV with different JAK2V617F allele burdens. Values correspond to the quantification of Western blot bands normalized to beta-actin. Data were analyzed by means of nonparametric Kruskal-Wallis test and the Dunn post-test to compare each group (***P < .001 [Bcl-2]; *P < .05 [Bcl-XL] between normal and JAK2V617 high erythroblasts). (C) Cell death induced by treatment of normal and PV erythroblasts for 16 hours with 75 μM cytosine arabinoside (AraC) or 150 μM hydroxyurea as evaluated by ethidium bromide/acridine orange staining and fluorescence microscopy. Mann-Whitney nonparametric test showed statistical significance (**P = .006 [AraC]; **P = .002 [hydroxyurea] between normal and JAK2V617F-medium/high erythroblasts). (D) Annexin V/7-AAD staining of erythroblasts derived from 5 healthy donors (N), 5 patients with PV with low JAK2V617F allele burden (PV Low/Null), and 5 patients with PV with high JAK2V617F allele burden (PV High). Bars represent the mean and SD of values obtained by treating normal and PV erythroblasts with ABT-737 at the indicated doses or with an equivalent volume of DMSO (−) for 48 hours. The left panel shows a representative experiment from each of the categories analyzed, with numbers indicating the total percentage of Annexin V+ cells. Data were analyzed by means of 2-way analysis of variance (ANOVA) with Bonferroni post-tests and showed a statistically significant difference (*P < .05 at doses of 500 nM and 1 μM between normal and JAK2V617F-high PV samples). Experiments shown in panels C and D were conducted in erythroid medium containing 0.3 U/mL Epo for both normal and PV erythroblasts.
ABT-737 inhibits PV erythroblast proliferation and endogenous erythroid colony production. (A) ABT-737 inhibits the proliferation of PV erythroblasts. Erythroblasts at day 4 of liquid culture derived from CD34+ cells of 3 healthy donors (N) or of 3 JAK2V617F-high patients (PV) were plated in medium containing 3 U/mL erythropoietin (for normal HPCs) or 0.3 U/mL erythropoietin (for PV HPCs) in the presence of 20 nM ABT-737 (ABT-737) or an equivalent volume of DMSO (−). Cell growth is expressed as the mean and SEM of samples counted at different days of culture (left panel). Statistical analysis performed by means of 2-way ANOVA with Bonferroni post-tests showed statistical significances (*P < .05 at day 10; **P < .01 at day 13; and **P < .01 at day 15 between ABT-737–treated normal and PV samples). The percentage of growth inhibition exerted by increasing doses of ABT-737 was assessed by cultivating day-4 erythroblasts from 5 JAK2V617F-positive patients (PV) or 5 healthy donors (N) in medium containing 3 U/mL erythropoietin (for normal HPCs) or 0.3 U/mL erythropoietin (for PV HPCs) in the presence of DMSO or of the indicated doses of ABT-737 (right panel). The percentage of growth inhibition is expressed as the mean and SD of values calculated at day 13 of culture as described in “Study design.” Mann-Whitney nonparametric test showed statistical significance (*P = .028 for 5, 10, and 20 nM between normal and PV samples). (B) Number of BFU-E colonies obtained from the semisolid culture of normal (N) and JAK2V617F-high PV CD34+ cells (PV) plated in the presence of 500 nM ABT-737 or of an equivalent volume of DMSO (−). Data were analyzed with Wilcoxon matched pairs test and showed a statistical significance (**P = .007 between untreated and ABT-737–treated PV samples), whereas the difference between untreated and ABT-737–treated normal samples was not statistically significant. (C) Number of endogenous erythroid colonies (EECs) produced by normal (N) and PV peripheral blood mononuclear cells plated in the presence of 500 nM ABT-737 or of an equivalent volume of DMSO (−). **Mann-Whitney nonparametric test showed a statistical significance of P = .008 between untreated and ABT-737–treated PV samples. (D) GATA-1 expression levels in normal (N) and PV erythroblasts cultured with 3 U/mL erythropoietin or 0.3 U/mL erythropoietin, respectively and treated for 4 days with 20 nM ABT-737 or with an equivalent volume of DMSO (−). The right panel shows the quantification (mean ± SD) of bands shown on the left normalized to beta-actin. *Mann-Whitney nonparametric test showed a statistical significance of P < .05 between GATA-1 protein levels of ABT-737–treated normal and PV samples.
ABT-737 inhibits PV erythroblast proliferation and endogenous erythroid colony production. (A) ABT-737 inhibits the proliferation of PV erythroblasts. Erythroblasts at day 4 of liquid culture derived from CD34+ cells of 3 healthy donors (N) or of 3 JAK2V617F-high patients (PV) were plated in medium containing 3 U/mL erythropoietin (for normal HPCs) or 0.3 U/mL erythropoietin (for PV HPCs) in the presence of 20 nM ABT-737 (ABT-737) or an equivalent volume of DMSO (−). Cell growth is expressed as the mean and SEM of samples counted at different days of culture (left panel). Statistical analysis performed by means of 2-way ANOVA with Bonferroni post-tests showed statistical significances (*P < .05 at day 10; **P < .01 at day 13; and **P < .01 at day 15 between ABT-737–treated normal and PV samples). The percentage of growth inhibition exerted by increasing doses of ABT-737 was assessed by cultivating day-4 erythroblasts from 5 JAK2V617F-positive patients (PV) or 5 healthy donors (N) in medium containing 3 U/mL erythropoietin (for normal HPCs) or 0.3 U/mL erythropoietin (for PV HPCs) in the presence of DMSO or of the indicated doses of ABT-737 (right panel). The percentage of growth inhibition is expressed as the mean and SD of values calculated at day 13 of culture as described in “Study design.” Mann-Whitney nonparametric test showed statistical significance (*P = .028 for 5, 10, and 20 nM between normal and PV samples). (B) Number of BFU-E colonies obtained from the semisolid culture of normal (N) and JAK2V617F-high PV CD34+ cells (PV) plated in the presence of 500 nM ABT-737 or of an equivalent volume of DMSO (−). Data were analyzed with Wilcoxon matched pairs test and showed a statistical significance (**P = .007 between untreated and ABT-737–treated PV samples), whereas the difference between untreated and ABT-737–treated normal samples was not statistically significant. (C) Number of endogenous erythroid colonies (EECs) produced by normal (N) and PV peripheral blood mononuclear cells plated in the presence of 500 nM ABT-737 or of an equivalent volume of DMSO (−). **Mann-Whitney nonparametric test showed a statistical significance of P = .008 between untreated and ABT-737–treated PV samples. (D) GATA-1 expression levels in normal (N) and PV erythroblasts cultured with 3 U/mL erythropoietin or 0.3 U/mL erythropoietin, respectively and treated for 4 days with 20 nM ABT-737 or with an equivalent volume of DMSO (−). The right panel shows the quantification (mean ± SD) of bands shown on the left normalized to beta-actin. *Mann-Whitney nonparametric test showed a statistical significance of P < .05 between GATA-1 protein levels of ABT-737–treated normal and PV samples.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Stefano Guida for technical assistance and Mauro Biffoni for flow cytometry analysis.
This work was supported by the Italian Association for Cancer Research. M.S. was supported by an Italy-USA fellowship of the Italian Ministry of Health.
Authorship
Contribution: A.Z. designed the study, performed research, and wrote the paper in collaboration with A.T.; F.P. performed experiments together with F.F. and prepared the figures; M.S. performed experiments and statistical analysis; G.G. provided blood samples; and R.D.M. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ann Zeuner, Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy; e-mail: a.zeuner@iss.it.

![Figure 1. PV erythroblasts overexpress Bcl-2 and Bcl-XL and are sensitive to ABT-737–induced apoptosis. CD34+ hematopoietic progenitors isolated from healthy donors and patients with PV were grown in liquid culture for 7 days in medium containing 3 U/mL erythropoietin (for normal HPCs) or 0.3 U/mL erythropoietin (for PV HPCs) as described in “Study design.” The numbers assigned to PV samples correspond to patients described in Table S1. (A) Western blot analysis of Bcl-2, Bcl-XL, and Mcl-1 levels in erythroblasts of healthy controls (N) and PV patients (PV). The right panel shows the quantification of protein levels normalized to beta-actin. (B) Levels of Bcl-2 and Bcl-XL expression in erythroblasts derived from 15 healthy donors or 18 patients with PV with different JAK2V617F allele burdens. Values correspond to the quantification of Western blot bands normalized to beta-actin. Data were analyzed by means of nonparametric Kruskal-Wallis test and the Dunn post-test to compare each group (***P < .001 [Bcl-2]; *P < .05 [Bcl-XL] between normal and JAK2V617 high erythroblasts). (C) Cell death induced by treatment of normal and PV erythroblasts for 16 hours with 75 μM cytosine arabinoside (AraC) or 150 μM hydroxyurea as evaluated by ethidium bromide/acridine orange staining and fluorescence microscopy. Mann-Whitney nonparametric test showed statistical significance (**P = .006 [AraC]; **P = .002 [hydroxyurea] between normal and JAK2V617F-medium/high erythroblasts). (D) Annexin V/7-AAD staining of erythroblasts derived from 5 healthy donors (N), 5 patients with PV with low JAK2V617F allele burden (PV Low/Null), and 5 patients with PV with high JAK2V617F allele burden (PV High). Bars represent the mean and SD of values obtained by treating normal and PV erythroblasts with ABT-737 at the indicated doses or with an equivalent volume of DMSO (−) for 48 hours. The left panel shows a representative experiment from each of the categories analyzed, with numbers indicating the total percentage of Annexin V+ cells. Data were analyzed by means of 2-way analysis of variance (ANOVA) with Bonferroni post-tests and showed a statistically significant difference (*P < .05 at doses of 500 nM and 1 μM between normal and JAK2V617F-high PV samples). Experiments shown in panels C and D were conducted in erythroid medium containing 0.3 U/mL Epo for both normal and PV erythroblasts.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/7/10.1182_blood-2008-03-143321/4/m_zh80090931510001.jpeg?Expires=1767902192&Signature=Qe0XSkbt6~k8hw3FcetL9xvhwWLclArZpEDWDyuB~r0NBoQHegyUZ8FZhbeWj1KYPl~vva4BULhbUPb8USfsLZCN0GWeFJdpYrjq0QztFTNp1zv2rMy3YzeQXidHtE76L2x2fnk8EZd3K3lfmV1EvLRZa83DeZbYplVp0jvtn3yTaWC681RKrGO3if7-Rs3k3KRvYLFH8n0xP7NwnNiaAbSvEv1LKZM2Nfti4GeI~UBpd0jfft64xojzVgcDjIVbVu5CBdPbqV7~iMTF9Jhp-zSufKo1XIqt9~HLHm4OSQa8-kVSIMJCKpl7EtFUxFwx~IcH-rfVmFU8EbXBdaSLtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)