Dendritic cells (DCs) are known to secrete exosomes that transfer membrane proteins, like major histocompatibility complex class II, to other DCs. Intercellular transfer of membrane proteins is also observed during cognate interactions between DCs and CD4+ T cells. The acquired proteins are functional and play a role in regulation of immune responses. How membrane protein transfer is achieved and regulated is unclear. Here we show that T cells can recruit major histocompatibility complex class II–containing DC exosomes secreted in the extracellular milieu during cognate DC–T-cell interactions. Recruitment of these exosomes required T-cell activation and was dependent on leukocyte function–associated antigen-1 (LFA-1) rather than on T-cell receptor specificity. Indeed, inducing a high-affinity state of LFA-1 on resting T cells was sufficient to provoke exosome binding. These results imply that DC exosomes secreted in the extracellular milieu during cognate T-cell–DC interactions are targeted to T cells activated in that microenvironment.
Introduction
During cognate T cell–dendritic cell (DC) interactions, several proteins, including major histocompatibility complex (MHC) and costimulatory molecules, are transferred from DCs to T cells (reviewed by Davis1 ). Transfer of MHC class II/peptide complexes from DCs to CD4+ T cells occurs in vivo and can play an important role in down-regulation of immune responses via T cell to T-cell presentation of antigen.2,–4 How these proteins are transferred and what determines their secretion and targeting are unclear. It has been proposed that DC exosomes, approximately 100-nm vesicles formed within multivesicular bodies,5 could be unique vectors for intercellular communication. Depending on the activation status of the DCs, they contain a narrow spectrum of molecules involved in immune responses and signal transduction.6,–8 Here we determine how T cells bind DC exosomes secreted in the extracellular milieu during cognate interactions.
Methods
Cell cultures
The splenic C57BL/6 immature DC-line D19 and D1 transduced with I-Akβ10 were cultured as described.11 Bovine exosomes were removed from fetal calf serum by ultracentrifugation. The p53-specific CD4+ T-cell clone (p53 T cells), generated in a C57BL/6 p53−/− mouse and provided by Prof C. Melief (Leiden University Medical Center, Leiden, The Netherlands),12 recognizes the murine p53 77-96 peptide (p53p). A novel CD4+ T-cell line recognizing the ovalbumin 323-339 peptide (OVAp) was generated from draining lymph nodes of OVAp/CFA-immunized C57BL/6 mice. T cells were cyclically restimulated with peptide-pulsed irradiated splenocytes and expanded with IL-2. Experiments were performed at standard tissue culture conditions10 and were approved by the institutional ethical animal committees at Utrecht University (Utrecht, The Netherlands).
Exosome-binding experiments
DC–T-cell coculture supernatant was cleared from cells and potentially contaminating membranes by sequential centrifugation steps up to 30 minutes at 10 000g.13 T cells (106) were incubated with 3 mL of this exosome-containing supernatant for 5 or 24 hours on plates coated or not with 10 μg/mL anti-CD3 and 5 μg/mL anti-CD28 antibody (provided by Dr L. Boon, Bioceros, Utrecht, The Netherlands). Where indicated, T cells were incubated with exosomes in the presence of anti–leukocyte function-associated antigen-1 (LFA-1; M17/4), provided by Prof C. Figdor (Nijmegen, The Netherlands), and/or manganese chloride (Sigma-Aldrich, St Louis, MO).
Flow cytometry
Cells were harvested and immunolabeled for 30 minutes on ice in phosphate-buffered saline/1% bovine serum albumin. Phycoerythrin and allophycocyanin-conjugated anti–I-Ab (M5.114), anti-CD9 (KMC8), anti-CD3 (145-2C11), anti-CD69 (H1.2F3), anti–interferon-γ (IFN-γ; XMG1.2), anti–I-Ak (Ox6), and isotype control antibodies were from BD PharMingen (San Diego, CA). Where indicated, T cells were labeled with 0.5 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA) for 15 minutes at 37°C or with 2 μM PKH26-GL (Sigma-Aldrich) for 5 minutes at room temperature. Paramagnetic epoxy beads (Dynal Biotech, Lake Success, NY) were coupled to anti-MHC class II (M5/114) or anti–ICAM-1 antibodies (BD PharMingen) according to the manufacturer's instructions. Exosomes from 1.0 mL 10 000g culture supernatant were adsorbed onto 6 × 104 antibody-coated beads during 24 hours at room temperature by end-over-end rotation and immunolabeled for I-Ab or CD9. Beads and cells were analyzed by flow cytometry using a FACSCalibur and CellQuest (BD Biosciences, San Jose, CA) or FCS Express software.
Results and discussion
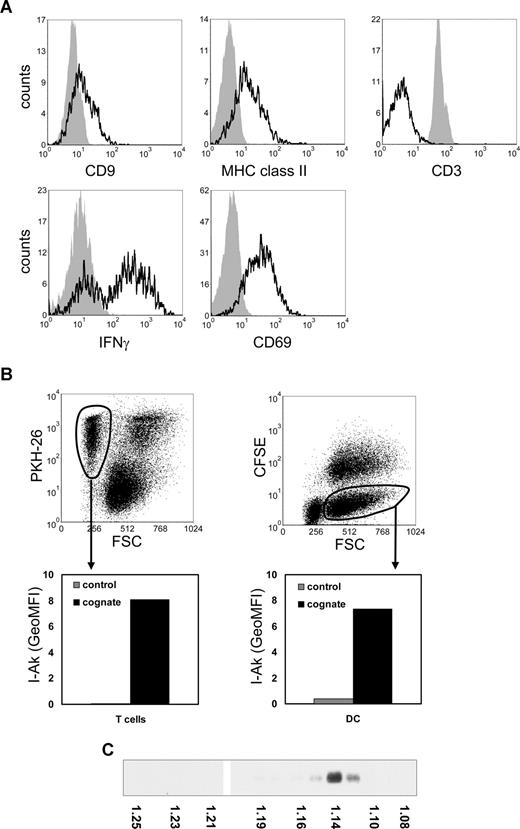
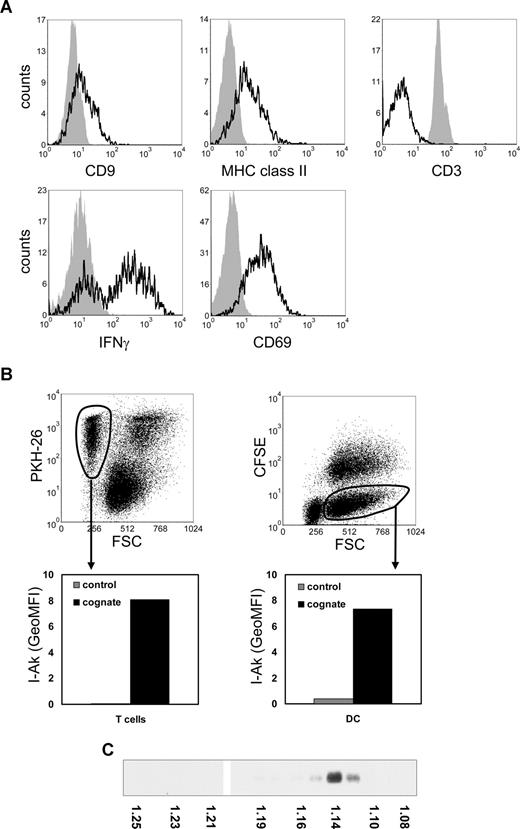
Consistent with reported findings on MHC class II acquisition by murine T cells,3,14 we found that mouse T cells, which do not synthesize MHC class II,15 acquired MHC class II during cognate DC–T-cell cocultures (Figure 1A). CD9, a tetraspanin enriched on DC exosomes,6 was also acquired by cocultured T cells (Figure 1A), suggesting that acquisition of these molecules was mediated by DC-derived exosomes. Previously, it was shown that DC exosomes associated to bystander DCs.16,–18 We analyzed whether MHC class II released by DCs during cognate interaction with T cells could be acquired by both T cells and bystander DCs. In a 3-cell culture system, containing nontransduced DC expressing only endogenous I-Ab, DCs also expressing transduced I-Akβ,11 p53-specific T cells, and p53p, we observed transfer of I-Akβ to both nontransduced DC and T cells (Figure 1B). These data imply that, besides DC, T cells are targets for DC exosomes. Indeed, our recent electron microscopy data confirm that MHC class II and CD9 containing exosomes are present on the T-cell plasma membrane and that the majority of these exosomes neither fuse with the plasma membrane nor are efficiently internalized (S.I.B., E.N.M.N-‘t.H., Guillaume van Niel, Maaike S. Pols, Toine ten Broeke, Marjolein Lauwen, Ferry Ossendorp, Cornelis J.M. Melief, Graça Raposo, Richard Wubbolts, M.H.M.W., and W.S., manuscript submitted, August 14, 2008).
Transfer of DC proteins to T cells and bystander DCs during cognate T cell–DC interactions. (A) DCs were pulsed for 2 hours with 5 μM p53p before coculture with T cells. Pulsed DCs (5 × 106) were cultured for 24 hours with CFSE-labeled p53 T cells (107). Cells were stained for MHC class II, CD9, CD3, CD69, or IFN-γ and analyzed by flow cytometry. Histograms indicate staining of gated CFSE-positive T cells derived from either T-cell cultures (solid histograms) or from cognate cocultures with DCs (open histograms) and were corrected for nonspecific staining as determined by isotype control antibodies. Reduction of CD3 and up-regulation of CD69 expression and IFN-γ production are indicative of T-cell activation. (B) Nonlabeled nontransduced (bystander) DCs (8 × 105) were cultured for 24 hours in the presence of CFSE-labeled DCs transduced with I-Akβ (8 × 105), PKH26-labeled p53 T cells (2 × 106), and 5 μM p53p in 4 mL of culture medium. Cocultured p53 T cells (left) and CFSE-negative bystander DCs (right) were gated on FL2 or FL1 and FSC levels as indicated (top panels), to exclude T-cell–DC clusters. The indicated gated populations were analyzed for I-Akβ (OX6) staining (bottom panels). Indicated are geometric mean fluorescence intensity (GeoMFI) values of cells derived from the cognate 3-cell culture system (cognate) or control DCs and T-cell cultures. The data shown are representative for 3 independent experiments. (C) Exosomes produced during 24-hour cognate DC–T-cell cocultures were collected by centrifuging the 10 000g supernatant for 1 hour at 100 000g, resuspended in 2.5 M sucrose, 20 mM Tris-HCl, pH 7.2, and floated by centrifugation to equilibrium into a 2.0- to 0.4-M sucrose gradient.19 The presence of MHC class II-β in gradient fractions was analyzed by Western blotting using rabbit polyclonal anti–MHCII-β obtained from Dr N. Barois (University of Oslo, Oslo, Norway). The density of the gradient fractions is indicated at the bottom of the blot.
Transfer of DC proteins to T cells and bystander DCs during cognate T cell–DC interactions. (A) DCs were pulsed for 2 hours with 5 μM p53p before coculture with T cells. Pulsed DCs (5 × 106) were cultured for 24 hours with CFSE-labeled p53 T cells (107). Cells were stained for MHC class II, CD9, CD3, CD69, or IFN-γ and analyzed by flow cytometry. Histograms indicate staining of gated CFSE-positive T cells derived from either T-cell cultures (solid histograms) or from cognate cocultures with DCs (open histograms) and were corrected for nonspecific staining as determined by isotype control antibodies. Reduction of CD3 and up-regulation of CD69 expression and IFN-γ production are indicative of T-cell activation. (B) Nonlabeled nontransduced (bystander) DCs (8 × 105) were cultured for 24 hours in the presence of CFSE-labeled DCs transduced with I-Akβ (8 × 105), PKH26-labeled p53 T cells (2 × 106), and 5 μM p53p in 4 mL of culture medium. Cocultured p53 T cells (left) and CFSE-negative bystander DCs (right) were gated on FL2 or FL1 and FSC levels as indicated (top panels), to exclude T-cell–DC clusters. The indicated gated populations were analyzed for I-Akβ (OX6) staining (bottom panels). Indicated are geometric mean fluorescence intensity (GeoMFI) values of cells derived from the cognate 3-cell culture system (cognate) or control DCs and T-cell cultures. The data shown are representative for 3 independent experiments. (C) Exosomes produced during 24-hour cognate DC–T-cell cocultures were collected by centrifuging the 10 000g supernatant for 1 hour at 100 000g, resuspended in 2.5 M sucrose, 20 mM Tris-HCl, pH 7.2, and floated by centrifugation to equilibrium into a 2.0- to 0.4-M sucrose gradient.19 The presence of MHC class II-β in gradient fractions was analyzed by Western blotting using rabbit polyclonal anti–MHCII-β obtained from Dr N. Barois (University of Oslo, Oslo, Norway). The density of the gradient fractions is indicated at the bottom of the blot.
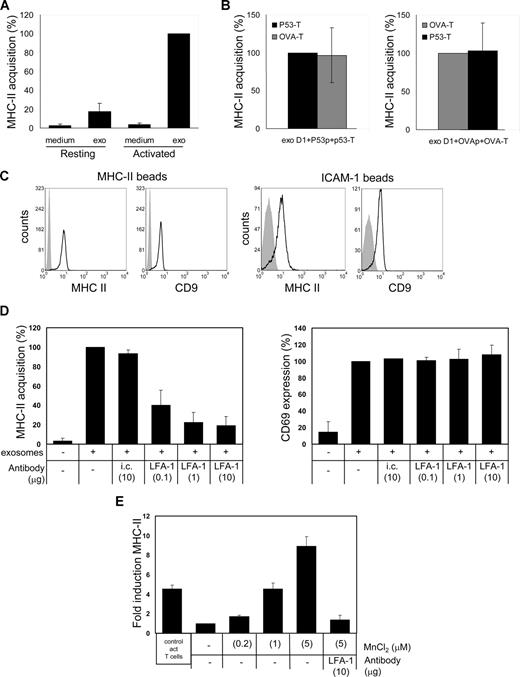
Here we determined the requirements for T-cell recruitment of DC exosomes secreted during cognate T-cell–DC interactions. To uncouple exosome secretion from recruitment, T cells were incubated with exosome containing 10 000g supernatants13 prepared from DC–T-cell cocultures. In this supernatant, MHC class II was exclusively associated to exosomes, as confirmed by its characteristic buoyant density (1.14 g/mL) in a sucrose density gradient19,20 (Figure 1C), and by electron microscopy analysis (S.I.B., E.N.M.N-‘tH., Guillaume van Niel, Maaike S. Pols, Toine ten Broeke, Marjolein Lauwen, Ferry Ossendorp, Cornelis J.M. Melief, Graça Raposo, Richard Wubbolts, M.H.M.W., and W.S., manuscript submitted, August 14, 2008). We found that only during activation, and not in the resting state, T cells efficiently recruited these exosomes (Figure 2A). This suggests that DC exosomes secreted in the extracellular milieu during cognate T-cell–DC interactions can be efficiently targeted to activated T cells present in that microenvironment.
DC exosome recruitment by T cells requires cellular activation and is dependent on active LFA-1. (A) p53 T cells (106) were incubated for 24 hours with 2.5 mL control medium (medium) or exosome containing 10 000g supernatant derived from cocultures of p53p-pulsed DC and p53 T cells (exo), in the absence (resting) or presence (activated) of anti-CD3/anti-CD28. T cells were labeled for MHC class II and analyzed by flow cytometry. GeoMFI values are expressed as percentages of the values for activated T cells incubated with exosomes (mean ± SD of 3 independent experiments). (B) p53 T cells (■, 106) or OVA T cells ( , 106) were cultured for 24 hours in 2.5 mL 10 000g supernatant from cognate DC-p53 T-cell cocultures (left) or cognate DC-OVA T-cell cocultures (right) during anti-CD3/anti-CD28 activation. T cells were labeled for MHC class II and analyzed by flow cytometry. GeoMFI values are expressed either as percentages of the values for p53 T cells incubated with exosomes from cognate DC-p53 T-cell cocultures (left) or as the percentages of the values for OVA T cells incubated with exosomes from cognate DC-OVA T-cell cocultures (right; mean ± SD of 3 independent experiments). (C) Exosomes present in 10 000g supernatant from 24-hour cognate DC-p53 T-cell cocultures were adsorbed onto anti-MHC class II or anti–ICAM-1–coated beads, stained for CD9 and MHC class II, and analyzed by flow cytometry. Histograms indicate staining with specific antibodies (open histograms) or isotype control antibodies (filled histograms). (D) p53 T cells (106) were cultured for 5 hours with anti-CD3/anti-CD28 either in 2.5 mL control medium or in 10 000g supernatant from 24-hour cognate DC- p53 T-cell cocultures in the absence or presence of indicated amounts (μg/2.5 mL) of anti–LFA-1 or isotype control (i.c.) antibody. Cells were stained for acquired MHC class II (left) or the activation marker CD69 (right) and analyzed by flow cytometry. GeoMFI values are expressed as percentage of signal in the absence of anti–LFA-1 (mean ± SD of 3 independent experiments). (E) Resting p53 T cells were cultured with medium or exosomes as in panel D in the absence or presence of MnCl2 and/or anti–LFA-1 as indicated. Cells were stained for MHC class II and analyzed by flow cytometry. For comparison, exosome binding by activated T cells is included (left bar in graph). The GeoMFI value for T cells incubated with exosomes in the absence of MnCl2 was set to 1, and data are expressed as fold increase over this value (mean ± SD of 3 independent experiments).
, 106) were cultured for 24 hours in 2.5 mL 10 000g supernatant from cognate DC-p53 T-cell cocultures (left) or cognate DC-OVA T-cell cocultures (right) during anti-CD3/anti-CD28 activation. T cells were labeled for MHC class II and analyzed by flow cytometry. GeoMFI values are expressed either as percentages of the values for p53 T cells incubated with exosomes from cognate DC-p53 T-cell cocultures (left) or as the percentages of the values for OVA T cells incubated with exosomes from cognate DC-OVA T-cell cocultures (right; mean ± SD of 3 independent experiments). (C) Exosomes present in 10 000g supernatant from 24-hour cognate DC-p53 T-cell cocultures were adsorbed onto anti-MHC class II or anti–ICAM-1–coated beads, stained for CD9 and MHC class II, and analyzed by flow cytometry. Histograms indicate staining with specific antibodies (open histograms) or isotype control antibodies (filled histograms). (D) p53 T cells (106) were cultured for 5 hours with anti-CD3/anti-CD28 either in 2.5 mL control medium or in 10 000g supernatant from 24-hour cognate DC- p53 T-cell cocultures in the absence or presence of indicated amounts (μg/2.5 mL) of anti–LFA-1 or isotype control (i.c.) antibody. Cells were stained for acquired MHC class II (left) or the activation marker CD69 (right) and analyzed by flow cytometry. GeoMFI values are expressed as percentage of signal in the absence of anti–LFA-1 (mean ± SD of 3 independent experiments). (E) Resting p53 T cells were cultured with medium or exosomes as in panel D in the absence or presence of MnCl2 and/or anti–LFA-1 as indicated. Cells were stained for MHC class II and analyzed by flow cytometry. For comparison, exosome binding by activated T cells is included (left bar in graph). The GeoMFI value for T cells incubated with exosomes in the absence of MnCl2 was set to 1, and data are expressed as fold increase over this value (mean ± SD of 3 independent experiments).
DC exosome recruitment by T cells requires cellular activation and is dependent on active LFA-1. (A) p53 T cells (106) were incubated for 24 hours with 2.5 mL control medium (medium) or exosome containing 10 000g supernatant derived from cocultures of p53p-pulsed DC and p53 T cells (exo), in the absence (resting) or presence (activated) of anti-CD3/anti-CD28. T cells were labeled for MHC class II and analyzed by flow cytometry. GeoMFI values are expressed as percentages of the values for activated T cells incubated with exosomes (mean ± SD of 3 independent experiments). (B) p53 T cells (■, 106) or OVA T cells ( , 106) were cultured for 24 hours in 2.5 mL 10 000g supernatant from cognate DC-p53 T-cell cocultures (left) or cognate DC-OVA T-cell cocultures (right) during anti-CD3/anti-CD28 activation. T cells were labeled for MHC class II and analyzed by flow cytometry. GeoMFI values are expressed either as percentages of the values for p53 T cells incubated with exosomes from cognate DC-p53 T-cell cocultures (left) or as the percentages of the values for OVA T cells incubated with exosomes from cognate DC-OVA T-cell cocultures (right; mean ± SD of 3 independent experiments). (C) Exosomes present in 10 000g supernatant from 24-hour cognate DC-p53 T-cell cocultures were adsorbed onto anti-MHC class II or anti–ICAM-1–coated beads, stained for CD9 and MHC class II, and analyzed by flow cytometry. Histograms indicate staining with specific antibodies (open histograms) or isotype control antibodies (filled histograms). (D) p53 T cells (106) were cultured for 5 hours with anti-CD3/anti-CD28 either in 2.5 mL control medium or in 10 000g supernatant from 24-hour cognate DC- p53 T-cell cocultures in the absence or presence of indicated amounts (μg/2.5 mL) of anti–LFA-1 or isotype control (i.c.) antibody. Cells were stained for acquired MHC class II (left) or the activation marker CD69 (right) and analyzed by flow cytometry. GeoMFI values are expressed as percentage of signal in the absence of anti–LFA-1 (mean ± SD of 3 independent experiments). (E) Resting p53 T cells were cultured with medium or exosomes as in panel D in the absence or presence of MnCl2 and/or anti–LFA-1 as indicated. Cells were stained for MHC class II and analyzed by flow cytometry. For comparison, exosome binding by activated T cells is included (left bar in graph). The GeoMFI value for T cells incubated with exosomes in the absence of MnCl2 was set to 1, and data are expressed as fold increase over this value (mean ± SD of 3 independent experiments).
, 106) were cultured for 24 hours in 2.5 mL 10 000g supernatant from cognate DC-p53 T-cell cocultures (left) or cognate DC-OVA T-cell cocultures (right) during anti-CD3/anti-CD28 activation. T cells were labeled for MHC class II and analyzed by flow cytometry. GeoMFI values are expressed either as percentages of the values for p53 T cells incubated with exosomes from cognate DC-p53 T-cell cocultures (left) or as the percentages of the values for OVA T cells incubated with exosomes from cognate DC-OVA T-cell cocultures (right; mean ± SD of 3 independent experiments). (C) Exosomes present in 10 000g supernatant from 24-hour cognate DC-p53 T-cell cocultures were adsorbed onto anti-MHC class II or anti–ICAM-1–coated beads, stained for CD9 and MHC class II, and analyzed by flow cytometry. Histograms indicate staining with specific antibodies (open histograms) or isotype control antibodies (filled histograms). (D) p53 T cells (106) were cultured for 5 hours with anti-CD3/anti-CD28 either in 2.5 mL control medium or in 10 000g supernatant from 24-hour cognate DC- p53 T-cell cocultures in the absence or presence of indicated amounts (μg/2.5 mL) of anti–LFA-1 or isotype control (i.c.) antibody. Cells were stained for acquired MHC class II (left) or the activation marker CD69 (right) and analyzed by flow cytometry. GeoMFI values are expressed as percentage of signal in the absence of anti–LFA-1 (mean ± SD of 3 independent experiments). (E) Resting p53 T cells were cultured with medium or exosomes as in panel D in the absence or presence of MnCl2 and/or anti–LFA-1 as indicated. Cells were stained for MHC class II and analyzed by flow cytometry. For comparison, exosome binding by activated T cells is included (left bar in graph). The GeoMFI value for T cells incubated with exosomes in the absence of MnCl2 was set to 1, and data are expressed as fold increase over this value (mean ± SD of 3 independent experiments).
Next, we asked which T-cell proteins were responsible for recruiting DC exosomes. We first tested whether T-cell receptor (TCR)–mediated recognition of MHC class II/peptide complexes on exosomes was essential for T-cell binding. We found that exosomes produced during cognate cultures of DCs with ovalbumin-specific T cells or p53-specific T cells could be recruited efficiently by both p53-specific and ovalbumin-specific T cells (Figure 2B). Although we do not exclude a role for specific TCR-MHC class II/peptide interactions in intercellular protein transfer during cognate T-cell–DC interactions, we conclude that DC exosomes secreted in the extracellular milieu are efficiently transferred to activated T cells irrespective of their TCR specificity. During T-cell activation, TCR signaling induces a transient conformational change in LFA-1, therewith highly increasing the affinity for ICAM-1 (reviewed by Kinashi21 ). Because only activated T cells recruited DC exosomes, binding to activated T cells could involve high-affinity LFA-1/ICAM-1 interactions. Exosomes secreted by lipopolysaccharide-activated DCs were shown to contain ICAM-1, which was required for exosome binding to bystander DCs.17 We found that MHC class II and CD9 bearing exosomes were efficiently captured from DC–T-cell coculture 10 000g supernatant by both anti–MHC class II– and anti–ICAM-1–coated beads (Figure 2C). Thus, DC exosomes produced during cognate interaction with T cells contained ICAM-1. To test whether LFA-1 on T cells mediated exosome binding, DC exosome recruitment assays were performed in the presence of an LFA-1 blocking antibody. Indeed, anti–LFA-1 antibody interfered dose-dependently with the recruitment of DC exosomes by activated T cells without affecting T-cell activation (Figure 2D), suggesting that LFA-1 on activated T cells is involved in DC exosome binding. Furthermore, these data support the exosomal origin of transferred MHC class II and argue against a major contribution of soluble nonexosomal MHC class II.22 If active LFA-1 was the only determining factor in DC exosome binding, induction of a high-affinity conformation of LFA-1 on resting T cells should allow these cells to recruit exosomes. Because the divalent cation Mn2+ induces and stabilizes the high-affinity state of LFA-1,23 we measured the exosome binding capacity of resting T cells in the presence of MnCl2. Indeed, MnCl2 rendered resting T cells capable of binding DC exosomes in a dose-dependent fashion (Figure 2E). The specificity of this process was indicated by complete blocking of exosome binding to MnCl2-treated resting T cells with anti–LFA-1 (Figure 2E). Thus, the affinity status of LFA-1 determined the efficacy of exosome acquisition by recipient T cells.
Previously, DC exosomes were shown to associate with bystander DCs, and it was proposed that the exosomal transfer of MHC-peptide complexes to recipient antigen presenting cells could be involved in spreading of antigen and amplification of immune responses.16 We here show that also T cells recruit MHC class II–bearing DC exosomes. Presentation of acquired MHC class II/peptide complexes by T cells can inhibit other activated/memory T cells.4 Our finding that only activated T cells efficiently recruited DC exosomes secreted during cognate T-cell–DC interactions could be a way to tightly regulate T cell–mediated down-regulation of immune responses.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs R. Wubbolts, T. ten Broeke, M. Nolte, and L. Taams for helpful discussions and critical reading of the manuscript; Prof C. Melief (Leiden University Medical Center, Leiden, The Netherlands) for the kind gift of the p53 T-cell clone; and Dr L. Boon (Bioceros, Utrecht, The Netherlands) and Prof C. Figdor (Nijmegen, The Netherlands) for the kind gifts of antibodies.
Authorship
Contribution: E.N.M.N.-‘t.H. designed and performed research and wrote the paper; S.I.B. performed research; S.M.A. contributed a vital new T-cell line; W.S. designed research; and M.H.M.W. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marca H. M. Wauben, Faculty of Veterinary Medicine, Department of Biochemistry & Cell Biology, Utrecht University, Yalelaan 2, 3584CM Utrecht, The Netherlands; e-mail: M.H.M.Wauben@uu.nl.



 , 106) were cultured for 24 hours in 2.5 mL 10 000g supernatant from cognate DC-p53 T-cell cocultures (left) or cognate DC-OVA T-cell cocultures (right) during anti-CD3/anti-CD28 activation. T cells were labeled for MHC class II and analyzed by flow cytometry. GeoMFI values are expressed either as percentages of the values for p53 T cells incubated with exosomes from cognate DC-p53 T-cell cocultures (left) or as the percentages of the values for OVA T cells incubated with exosomes from cognate DC-OVA T-cell cocultures (right; mean ± SD of 3 independent experiments). (C) Exosomes present in 10 000g supernatant from 24-hour cognate DC-p53 T-cell cocultures were adsorbed onto anti-MHC class II or anti–ICAM-1–coated beads, stained for CD9 and MHC class II, and analyzed by flow cytometry. Histograms indicate staining with specific antibodies (open histograms) or isotype control antibodies (filled histograms). (D) p53 T cells (106) were cultured for 5 hours with anti-CD3/anti-CD28 either in 2.5 mL control medium or in 10 000g supernatant from 24-hour cognate DC- p53 T-cell cocultures in the absence or presence of indicated amounts (μg/2.5 mL) of anti–LFA-1 or isotype control (i.c.) antibody. Cells were stained for acquired MHC class II (left) or the activation marker CD69 (right) and analyzed by flow cytometry. GeoMFI values are expressed as percentage of signal in the absence of anti–LFA-1 (mean ± SD of 3 independent experiments). (E) Resting p53 T cells were cultured with medium or exosomes as in panel D in the absence or presence of MnCl2 and/or anti–LFA-1 as indicated. Cells were stained for MHC class II and analyzed by flow cytometry. For comparison, exosome binding by activated T cells is included (left bar in graph). The GeoMFI value for T cells incubated with exosomes in the absence of MnCl2 was set to 1, and data are expressed as fold increase over this value (mean ± SD of 3 independent experiments).
, 106) were cultured for 24 hours in 2.5 mL 10 000g supernatant from cognate DC-p53 T-cell cocultures (left) or cognate DC-OVA T-cell cocultures (right) during anti-CD3/anti-CD28 activation. T cells were labeled for MHC class II and analyzed by flow cytometry. GeoMFI values are expressed either as percentages of the values for p53 T cells incubated with exosomes from cognate DC-p53 T-cell cocultures (left) or as the percentages of the values for OVA T cells incubated with exosomes from cognate DC-OVA T-cell cocultures (right; mean ± SD of 3 independent experiments). (C) Exosomes present in 10 000g supernatant from 24-hour cognate DC-p53 T-cell cocultures were adsorbed onto anti-MHC class II or anti–ICAM-1–coated beads, stained for CD9 and MHC class II, and analyzed by flow cytometry. Histograms indicate staining with specific antibodies (open histograms) or isotype control antibodies (filled histograms). (D) p53 T cells (106) were cultured for 5 hours with anti-CD3/anti-CD28 either in 2.5 mL control medium or in 10 000g supernatant from 24-hour cognate DC- p53 T-cell cocultures in the absence or presence of indicated amounts (μg/2.5 mL) of anti–LFA-1 or isotype control (i.c.) antibody. Cells were stained for acquired MHC class II (left) or the activation marker CD69 (right) and analyzed by flow cytometry. GeoMFI values are expressed as percentage of signal in the absence of anti–LFA-1 (mean ± SD of 3 independent experiments). (E) Resting p53 T cells were cultured with medium or exosomes as in panel D in the absence or presence of MnCl2 and/or anti–LFA-1 as indicated. Cells were stained for MHC class II and analyzed by flow cytometry. For comparison, exosome binding by activated T cells is included (left bar in graph). The GeoMFI value for T cells incubated with exosomes in the absence of MnCl2 was set to 1, and data are expressed as fold increase over this value (mean ± SD of 3 independent experiments).