Abstract
Hereditary hemochromatosis is an iron overload disorder that can lead to the impairment of multiple organs and is caused by mutations in one or more different genes. Type 1 hemochromatosis is the most common form of the disease and results from mutations in the HFE gene. Juvenile hemochromatosis (JH) is the most severe form, usually caused by mutations in hemojuvelin (HJV) or hepcidin (HAMP). The autosomal dominant form of the disease, type 4, is due to mutations in the SLC40A1 gene, which encodes for ferroportin (FPN). Hereditary hemochromatosis is commonly found in populations of European origin. By contrast, hemochromatosis in Asia is rare and less well understood and can be masked by the presence of iron deficiency and secondary iron overload from thalassemia. Here, we provide a comprehensive report of hemochromatosis in a group of patients of Asian origin. We have identified novel mutations in HJV, HAMP, and SLC40A1 in countries not normally associated with hereditary hemochromatosis (Pakistan, Bangladesh, Sri Lanka, and Thailand). Our family studies show a high degree of consanguinity, highlighting the increased risk of iron overload in many countries of the developing world and in countries in which there are large immigrant populations from these regions.
Introduction
Hemochromatosis is a disorder characterized by excessive iron absorption from the diet leading to iron overload in multiple organs and eventual toxicity. It is a genetically heterogeneous disease caused by mutations in one or more different genes. The most common form is due to mutations in the HFE gene (type 1), whereas juvenile hemochromatosis (JH), although less common, is the most severe form of the disease, with clinical symptoms that include cardiomyopathy and hypogonadotrophic hypogonadism. JH is usually a result of mutations in hemojuvelin (HJV) or, more rarely, in hepcidin (HAMP).1,2 An autosomal dominant form of hemochromatosis (type 4) is due to mutations in the SLC40A1 gene. SLC40A1 encodes ferroportin (FPN), a transmembrane iron exporter that can be inhibited by the peptide hormone hepcidin, thereby restricting iron uptake and release.3 Further evidence shows that hepcidin itself is up-regulated by hemojuvelin by the bone morphogenetic protein (BMP) signaling pathway.4
HFE-hemochromatosis is mostly found in populations of European origin and almost always results from 2 mutations, H63D and C282Y.5 In addition, a steadily increasing number of causative non-HFE hemochromatosis mutations are being identified and, like type 1 hemochromatosis, they have been most frequently described in patients originating from Europe, including Greece, Italy, and the United Kingdom.1,2,6 Commonly recurring mutations include G320V in hemojuvelin and V162del in ferroportin.
In contrast, reports of hemochromatosis in regions of Asia have been relatively rare, and the condition is less well defined. In these particular regions, the association of HFE mutations with iron overload is minimal and varied.7-15 There have only been a few brief reports of non-HFE hemochromatosis in Asia, which appear to be isolated mutations with most cases around Eastern Asia.16-18 In the Indian subcontinent, reports are even fewer with only 2 cases of type 4 hemochromatosis identified and no cases of JH.19,20 At present, a difficulty with recognizing hereditary hemochromatosis in some parts of Asia is the presence of hemoglobin disorders such as thalassemia and the resulting secondary iron overload.21 Furthermore, the presentation of the disease may also be masked by iron deficiency in these developing countries.22
To gain a better understanding of the genetic background of hemochromatosis in Asia, we studied a series of patients with unexplained iron overload originating from different regions. Here we present, for the first time, details of a group of patients with juvenile hemochromatosis from the Indian subcontinent and type 4 hemochromatosis from Southeast Asia. Several families from the Indian subcontinent were found to carry novel HJV and HAMP mutations and a mutation in ferroportin was identified in Thailand, where non-HFE hemochromatosis has never previously been characterized. These findings suggest that primary iron overload may not be as rare as was previously thought in these geographic areas.
Methods
Patients
Informed consent for molecular studies was obtained from all persons described here in accordance with the Declaration of Helsinki. We received hemochromatosis-gene testing requests for a total of 32 unrelated patients originating from the Indian subcontinent and Thailand with unexplained iron overload. All cases were unrelated to HFE hemochromatosis and have not been transfused. Eight of these cases, from 8 different families totaling 42 members, form the basis of this study. Healthy subjects of similar ethnic origin were used as controls. Institutional review board approval for this study was obtained from all participating institutions.
Genetic analysis
Patients' genomic DNA was extracted from whole-blood samples using the QIAamp DNA Blood Midi Kit (QIAGEN, Dorking, United Kingdom) according to the manufacturer's protocol. The coding regions and splice sites of the hemojuvelin, hepcidin, transferrin receptor 2, and ferroportin genes were amplified for screening. Polymerase chain reactions (PCRs) were carried out with the use of AmpliTaq or AmpliTaq Gold DNA polymerase (ABI Applied Biosystems, Foster City, CA) and in the presence of Pwo SuperYield proofreading enzyme (Roche Diagnostics, Mannheim, Germany), according to manufacturer's instructions. Denaturing high-pressure liquid chromatography (DHPLC) was used to screen for mutations in all amplicons with the use of standard conditions. DHPLC was carried out on a WAVE Nucleic Acid Fragment Analysis System (Transgenomic, Omaha, NE) and analyzed using the Navigator software (Transgenomic Inc.) according to the manufacturer's instructions. Amplicons with elution profiles containing a heteroduplex peak indicating a sequence variation were sequenced directly with the use of the ABI PRISM BigDye Terminator Sequencing Kit version 3.1 (ABI Applied Biosystems) and purified with the use of the DyeEx 2.0 Spin Kit (QIAGEN). Automated fluorescent sequencing was performed on an ABI PRISM 3100 Capillary Sequencer (Hitachi; ABI Applied Biosystems). Primer pairs and specific conditions for amplifying and screening of the genes are provided in the supplemental data (supplemental Tables 1-5, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results
The country of origin of the probands together with the mutations identified are summarized in Table 1. Clinical, genetic, and laboratory findings are summarized in Tables 2 and 3. The sequence analysis of the mutations is provided in the supplementary data (supplemental Figure 1). All the mutations causing JH in families A to G were absent in 100 South Asian control chromosomes. There was no evidence of cardiac complications in any of the patients.
Family A
The proband first presented at the age of 19 years with diabetes and raised iron parameters (Table 2). Endocrine tests were also abnormal. DHPLC and sequence analysis showed that the proband is a compound heterozygote for 2 single nucleotide missense mutations in HJV, a novel cysteine-to-tyrosine mutation at position 80 (C80Y) and the common glycine-to-valine mutation at position 320 (G320V; Tables 1 and 2). Her father is of Bangladeshi origin, and her mother is British. Neither were available for genetic testing. The G320V mutation is frequently described in families of European origin and has not previously been reported in Asians. For this reason, it is likely that the patient inherited the G320V mutation from her British mother and the novel C80Y mutation from her father. This C80Y mutation is the first HJV mutation to be found in the Bangladeshi population.
Family B
The proband IV-6 (Figure 1), from Pakistan, was first diagnosed with diabetes and secondary infertility at the age of 26 years (Table 2). He was also diagnosed as having osteoporosis. He is married to his cousin once removed (V-2), and his parents are multiply consanguineous (Figure 1). Mutation detection showed that he is homozygous for a single nucleotide mutation, causing a change from glycine to arginine at position 99 of hemojuvelin (G99R; Table 1). This mutation has previously been reported in the Albanian population.6 The proband's 3 siblings and aunt (III-8), who are unaffected, are heterozygous for G99R.
Pedigree of family B with the HJV G99R mutation. The proband (IV-6) is married to his cousin once removed (V-2), who is wild type. Only the proband, his wife, siblings, and aunt (III-8) were available for testing. Arrow indicates proband. Age at diagnosis is shown in parentheses.
Pedigree of family B with the HJV G99R mutation. The proband (IV-6) is married to his cousin once removed (V-2), who is wild type. Only the proband, his wife, siblings, and aunt (III-8) were available for testing. Arrow indicates proband. Age at diagnosis is shown in parentheses.
Family C
A second patient from Pakistan, unrelated to family B, was also found to be homozygous for the HJV G99R mutation (Table 2). There was no family history of liver disease. She was identified after routine screening for general malaise and recurrent urinary tract infections, although we cannot confirm whether this is a direct consequence of the genetic hemochromatosis.
Family D
The proband, from Pakistan, presented at age 23 years with secondary infertility (Table 2). He also experienced pain in his ankles and knees. He was found to be homozygous for a novel missense mutation in HJV, the substitution of proline 192 by leucine (P192L; Table 1). His parents and 6 of his 10 siblings are heterozygous for this mutation, whereas another 3 siblings are homozygotes. A sister, who is a homozygote aged 31 years, has a raised transferrin saturation and ferritin. One brother has biopsy-proven cirrhosis, and a further brother is awaiting a liver biopsy, but tests to date suggest that he has cirrhosis. Four of the siblings have married their paternal first cousins. Only one sibling is wild-type.
Family E
The proband, also from Pakistan, first presented with abnormal liver function tests at age 32 years. There was no evidence of diabetes (Table 2). Mutation analysis showed that he is homozygous for a novel single nucleotide substitution, resulting in a change from leucine to proline at position 194 (L194P; Table 1). This patient, in comparison to the other patients with JH described in this study, appears to have a slightly later presentation of hemochromatosis.
Family F
The proband, a Sri Lankan male and the son of a non–consanguineous marriage, first presented at age 17 years with rapid weight loss, polyuria, and hyperpigmentation (Table 2). Liver function tests were also abnormal. There were no features of arthritis. Subsequent mutation detection found the proband to be homozygous for a novel single nucleotide T deletion in exon 4 of HJV (Table 1), resulting in a frameshift. If the transcripts were to be translated we predict a novel 23 amino acid extension after residue 342 before a premature stop codon results in a truncated protein with a total length of 365 residues. His father was heterozygous for this mutation. His mother was unavailable for study.
Family G
The proband, from Pakistan and son of multiply consanguineous parents, first presented with hypogonadotrophic hypogonadism, gynecomastia, and increased liver enzyme activity at age 21 years (Table 2). He has now been venesected 50 times with normalization of liver enzymes. He was found to be homozygous for a 2-nucleotide AG deletion in exon 2 of HAMP (Table 1). This novel deletion alters the reading frame after the first 41 amino acid residues of the protein and extends beyond the stop codon of the normal transcript. This completely disrupts the expression of the active peptide, which is entirely encoded by exon 3. The proband's parents, who were first cousins, are heterozygotes for the deletion and have normal iron indices. The brother of the proband is also a heterozygote with normal iron levels, but a sister is an affected homozygote aged 24 years.
Family H
The proband, a 38-year-old Thai, was first detected as having abnormal liver function tests (Table 3). He had no evidence of arthritis, diabetes, or an inherited hemoglobin disorder. The proband's older brother (47 years old), who had a history of hyperpigmentation, impotence (aged 41), and diabetes (aged 43), was observed to have similar iron and liver function profiles to the proband. Two sisters who were clinically well at presentation also had raised iron parameters (Table 3). The proband tolerated venesection treatment well. Mutation analysis found him to be heterozygous for a cysteine-to-tyrosine change at position 326 (C326Y) in FPN (Table 1). The older brother and 2 sisters were also found to be heterozygous for this FPN mutation, whereas a further 3 siblings were wild type. Seven common α-thalassemias caused by deletions (–SEA, –FIL, –THAI, –MED, -α20.5, -α3.7, and -α4.2) were excluded by Gap-PCR analysis using standard conditions, and hemoglobin analysis for all members of the family showed normal HbA and A2 levels, therefore excluding secondary iron overload because of thalassemia. PCR RFLP using SfaNI digestion was used to confirm the mutation, which was not detected in 600 other Thai and 200 Vietnamese chromosomes from healthy controls screened, confirming that C326Y is a causative mutation.
Discussion
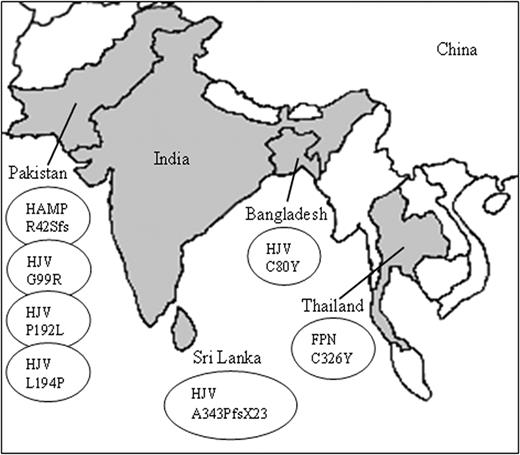
We report several novel mutations in HJV, HAMP, and SLC40A1 that result in different presentations of iron overload, originating from countries not normally associated with hemochromatosis (Figure 2). To date, data on iron overload in the Indian subcontinent and Southeast Asia have been minimal. We and others have previously searched for the common HFE alleles in those regions and found them to be rare.10,23,24 The only reports of HFE-associated hemochromatosis have been an HFE splice-site mutation of Vietnamese origin and a case of C282Y homozygosity in Japan.25,26 We have also shown that the H63D and C282Y mutations arose independently in Sri Lanka.27 Until now, only a few isolated cases of non-HFE hemochromatosis have been reported, and most cases of iron overload are due to secondary hemosiderosis. Nevertheless, the remarkable variation in the degree of iron loading in patients with non–transfusion-dependent forms of α- and β-thalassemia intermedia11,21,28-31 from similar Asian environments suggest that hereditary hemochromatosis may be more common than previously thought, although to date no causal mutations have been identified.
All the mutations identified here are located in highly conserved regions of the proteins. There has been a previous report of a HJV C80R mutation in whites,32 whereby the cysteine is replaced by arginine rather than the tyrosine mutation of Bangladeshi origin that we describe here. This suggests that C80 is an important residue of the protein. The first report of the HJV G99R mutation was of Albanian origin,6 and our discovery of this mutation in 2 independent cases from the Pakistani population indicates that it is a recurrent mutation not restricted to Europe and may be the common mutation in this region. The G99 residue is located in the highly conserved RGD (Arg-Gly-Asp) motif of the protein; the first mutation reported to affect this position was G99V.1 In other proteins the RGD motif has been shown to be a general integrin binding motif.33 It has been shown that HJV N-terminal mutants, including those at positions C80 and G99, are able to reach the plasma membrane but not able to activate hepcidin, suggesting that N-terminal mutants are unable to interact correctly with signaling molecules such as the BMP receptors.34 The HJV C-terminal mutants, conversely, appear to be retained in the endoplasmic reticulum and fail to reach the plasma membrane.35
HJV is a member of the repulsive guidance molecule (RGM) family and is highly conserved among different species from mouse to zebrafish. For all the residues affected by the HJV mutations, an equivalent identical residue can be found across different species and the other RGM family proteins, with the exception of L194 (Figure 3). Both the P192 and L194 residues lie in the partial von Willebrand factor D domain of the protein. However, leucine 194 is only found in HJV from mammals. This might explain the later onset of the disease in the proband in family E, because the semiconservative nature of the residue may have a less adverse affect on protein function. As judged by their early age of onset and high frequency of hypogonadism, with one exception, these Asian patients with JH have a very similar clinical phenotype to that described in this condition in developed countries. The lack of any evidence of cardiac involvement is surprising and could reflect the occurrence of the disease in a different environment or genetic background. This important issue remains to be explored further.
Multiple sequence alignment of HJV. Alignment of regions of the human hemojuvelin protein sequence with the mutations, against sequences of the mouse, rat, and zebrafish hemojuvelin, and human, mouse, and zebrafish RGMs. Sequence alignment between the different species shows high sequence similarity. Leucine 194 is only found in mammalian HJV. Mutations are indicated by the arrows. * indicates identical residues in the alignment; colon (:), conserved substitutions; and period (.), semiconserved substitutions of residues. Alignments were performed with ClustalW version 2.0.10 (European Bioinformatics Institute, Cambridge, United Kingdom).
Multiple sequence alignment of HJV. Alignment of regions of the human hemojuvelin protein sequence with the mutations, against sequences of the mouse, rat, and zebrafish hemojuvelin, and human, mouse, and zebrafish RGMs. Sequence alignment between the different species shows high sequence similarity. Leucine 194 is only found in mammalian HJV. Mutations are indicated by the arrows. * indicates identical residues in the alignment; colon (:), conserved substitutions; and period (.), semiconserved substitutions of residues. Alignments were performed with ClustalW version 2.0.10 (European Bioinformatics Institute, Cambridge, United Kingdom).
The FPN C326Y mutation in our patients is also at a highly conserved residue among different species. According to proposed structural models for FPN, C326 is located in a peptide loop on the extracellular side of the membrane between 2 transmembrane domains.36-39 It has been shown that the extracellular loop containing C326 is the hepcidin binding domain that is evolutionarily conserved, and amino acid substitutions in this region prevent hepcidin binding and the subsequent internalization of FPN.40 Our patients with the C326Y mutation all have a raised transferrin saturation. This is due to iron release by the C326Y mutant that is resistant to hepcidin inhibition.41,42 They also have a significantly higher mean corpuscular volume (average ± SD, 101 ± 1.63 fL) compared with their nonaffected siblings (87.3 ± 2.52 fL; P < .05). This has previously been observed in patients with type 1 hemochromatosis assessed before or after iron depletion by venesection.43
We are receiving an increasing number of cases of Asian origin with iron overload. The patients with JH who we have identified here describe other relatives with similar disorders who were not available for analysis. We have yet to find a mutation in any of the known hemochromatosis genes for the remaining 24 families of the 32 with iron overload, suggesting that there are other unrecognized genes responsible for hemochromatosis. HFE hemochromatosis has been rarely encountered in Asians, and the few reported cases of non-HFE hemochromatosis have failed to describe the underlying mutations.15,44-47 As evident here, this may well be because these reports were based on screening for known mutations commonly found in Europe rather than a comprehensive analysis of each candidate gene for new mutations.
The finding of such a diverse set of new mutations in patients with non-HFE hemochromatosis from different Asian countries raises the important question of how common such conditions are in these populations. As emphasized in the present study the high frequency of consanguineous marriages in many Asian populations, together with the complete penetrance of non-HFE hemochromatosis, would tend to increase the frequency of these disorders. Although hitherto the extremely high prevalence of iron deficiency in Asian populations22 might have prevented or delayed their clinical expression, as these countries go through the demographic transition associated with public health measures and better standards of living and diet, these conditions might start to pose an important health burden. Similarly, as the large Asian populations in the developed countries are exposed to Western diets and increased iron, hemochromatosis may become more prevalent. It is equally important to determine the frequency of these mutations because of their potential for explaining the variability in iron loading that occurs in Asian patients with extremely common intermediate forms of thalassemia who do not have regular transfusions, hemoglobin E/β-thalassemia for example.
Clearly, although much remains to be learned about the frequency of non-HFE hemochromatosis in Asian populations in different environments, it is important to recognize the existence of these conditions and to initiate appropriate screening for persons who present early in life with diabetes, particularly if hypogonadism coexists.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all the families who agreed to participate in this study without whom it would not have been possible. We thank Dr Indra van Mourik for referring a family to us, and Dr Sarah Ball and all the physicians and nurses who have cared for the patients in this study. Dr Neil Morgan kindly provided the South Asian DNA control samples. We thank Kevin Clark for performing the sequencing.
K.J.H.R. acknowledges the support of The Haemochromatosis Society of Great Britain. V.V. and C.L. are principal investigators of the Siriraj-Thalassemia Research Program, Thailand. D.J.W. and A.P. thank the Wellcome Trust for support.
Authorship
Contribution: C.Y.L. performed the experiments and wrote the paper; A.T.M.-C. and V.V. performed experiments; Y.C., S.S., C.L., D.O., A.J.R., J.H., P.W., A.P., H.J.d.S., A.D., C.M., M.J., R.G., N.K., W.N., G.B., A.C., and I.W.-M. were responsible for the referral, care, and biochemical studies of patients; D.J.W. referred patients and wrote the paper; and K.J.H.R. coordinated the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chun Yu Lok, MRC Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, Headington, Oxford OX3 9DS, United Kingdom; e-mail: chunyu.lok@ccc.ox.ac.uk.