Abstract
In this single-center, cross-sectional study, we evaluated 44 very long-term survivors with a median follow-up of 17.5 years (range, 11-26 years) after hematopoietic stem cell transplantation. We assessed the telomere length difference in human leukocyte antigen-identical donor and recipient sibling pairs and searched for its relationship with clinical factors. The telomere length (in kb, mean ± SD) was significantly shorter in all recipient blood cells compared with their donors' blood cells (P < .01): granulocytes (6.5 ± 0.9 vs 7.1 ± 0.9), naive/memory T cells (5.7 ± 1.2 vs 6.6 ± 1.2; 5.2 ± 1.0 vs 5.7 ± 0.9), B cells (7.1 ± 1.1 vs 7.8 ± 1.1), and natural killer/natural killer T cells (4.8 ± 1.0 vs 5.6 ± 1.3). Chronic graft-versus-host disease (P < .04) and a female donor (P < .04) were associated with a greater difference in telomere length between donor and recipient. Critically short telomeres have been described in degenerative diseases and secondary malignancies. If this hypothesis can be confirmed, identification of recipients at risk for cellular senescence could become part of monitoring long-term survivors after hematopoietic stem cell transplantation.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative treatment option for a variety of malignant and nonmalignant disorders.1 Overall survival has improved during the past decades, and the number of long-term survivors is continuously increasing. Still, HSCT remains associated with considerable early- and late-treatment–related morbidity and mortality.2 Many late complications, such as secondary cancer, cataract, infertility, endocrine dysfunctions, or bone and joint complications, have been well described. For other organ dysfunctions, an unequivocal relationship with the transplantation procedure is more difficult to demonstrate.3 There is no good evidence for a functional defect of the lymphohematopoietic system. However, limitations in the proliferative capacity of HSC and premature cellular senescence of the donor-derived hematopoiesis have been incriminated in late marrow failure syndrome or malignant transformation. More information from the very long-term survivors is needed. Allogeneic HSCT provides an ideal setting to evaluate the fate of the same HSC in 2 different environments, those of the original donor and the recipient.
Telomeres are specialized DNA-protein structures at the end of linear chromosomes.4 With each cell replication, telomere repeats are lost, and this mechanism serves as a mitotic clock that induces cellular senescence when telomeres become critically short.5 Telomere shortening can therefore be used as a marker of the replicative history and has been described after HSCT.6,7 Shortened telomere lengths have been found in various hematopoietic cell subsets after allogeneic HSCT8-11 and have been implicated in late graft failure.12 Telomere attrition has mainly been observed during the first year after allogeneic HSCT; thereafter, telomere length dynamics of recipients appear not to differ from their donors,13-15 although follow-up in these studies was limited. The aim of our study was to evaluate the difference between telomere length in leukocyte subsets of very long-term survivors after HSCT and their respective sibling donors with the same time follow-up since HSCT.
Methods
We conducted a single-center, cross-sectional study comparing 44 very long-term survivors (> 10 years) after HSCT and their respective human leukocyte antigen-identical sibling donors in pairs on the same day by performing a complete clinical and biologic examination. The comparison of the telomere length between recipient and donor and its relationship with clinical factors was the main focus of this report. Leukocytes were obtained from peripheral blood after informed consent was obtained in accordance with the Declaration of Helsinki and according to institutional guidelines. The study was approved by the local ethics committee at University Hospital Basel in Basel, Switzerland.
The characteristics of the 44 recipients and their respective sibling donors are summarized in Table 1. Twenty of the recipients (45.5%) and 24 of the donors (54.5%) were female. The median age at HSCT of donors and recipients was 25.8 years (range, 2-46 years) and 26.8 years (range, 5-50 years) respectively. The median follow-up since HSCT was 17.5 years (range, 11-26 years). Four recipients received HSCT for aplastic anemia and 40 recipients for hematologic malignancy. All patients received bone marrow with a median number of 4.2 × 108/kg body weight nucleated cells. Total body irradiation (TBI) was part of the conditioning in 39 (89%) of the recipients. Acute graft-versus-host disease (GVHD; grade 2 or greater) at any time after HSCT was observed in 26 (59%) and chronic GVHD in 22 (50%) patients. Chronic GVHD was still present at time of examination in 9 recipients (20.4%).
Leukocytes were separated by ammonium chloride lysis and cryopreserved until further analysis. Automated multicolor flow fluorescence in situ hybridization was used to measure the telomere length in granulocytes, naive and memory T cells, B cells, and natural killer (NK)/NKT cells of the patients and their donors as has been described previously.16,17 All samples were blinded for this analysis.
The telomere length difference between recipients and donors was analyzed by pairs for total leukocytes and subsets by the use of the nonparametric Wilcoxon rank test. The interactions between risk factors and the telomere length differences were evaluated by the use of linear regression analysis. A stepwise multiple regression with forward selection was performed to investigate the influence on telomere length differences (dependent variable) of acute/chronic GVHD, sex, age and age disparity of donor/recipient, TBI, and time of follow-up since HSCT (independent variables). Differences between the results of comparative tests were considered significant if the 2-sided P value was less than .05. Statistical analysis was performed by the use of SPSS statistical software (SPSS for Windows, Release 15.0.1, SPSS Inc).
Results and discussion
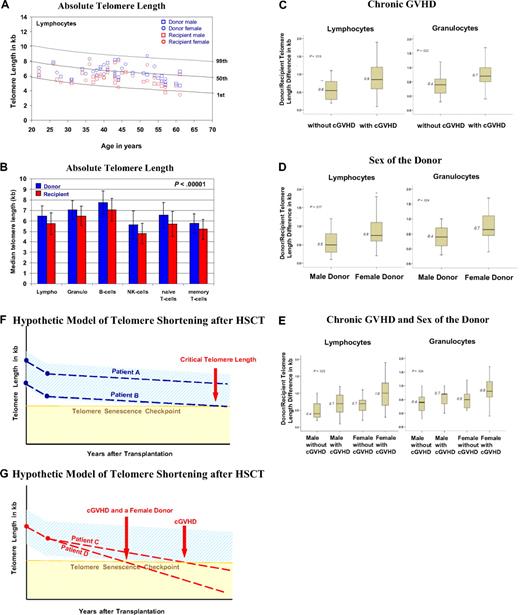
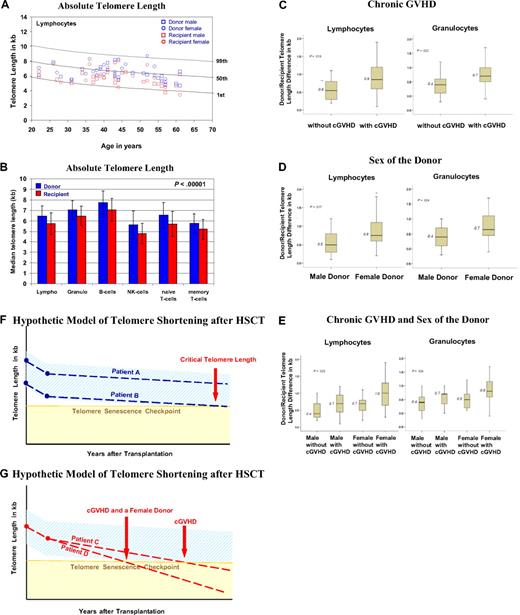
The age-matched and cell-type–specific absolute telomere length values for practically all recipients and donors were between the 1st and 99th percentiles of the normal distribution (Figure 1A).18 The telomere length (in kb, mean ± 1 SD) was significantly shorter in all peripheral blood leukocytes of recipients compared with donors (P < .01), including granulocytes (6.5 ± 0.9 vs 7.1 ± 0.9), naive and memory T cells (5.7 ± 1.2 vs 6.6 ± 1.2; 5.2 ± 1.0 vs 5.7 ± 0.9), B cells (7.1 ± 1.1 vs 7.8 ± 1.1), and NK/NKT cells (4.8 ± 1.0 vs 5.6 ± 1.3; Figure 1B). In all cases, the telomeres were on average 0.6 to 0.9 kb shorter in recipients, enough of a difference to permit a blinded investigator unequivocally to distinguish recipients' telomeres from those of their respective donors. Furthermore, we found significantly greater telomere length differences in the granulocytes and lymphocytes of recipients with chronic GVHD (P < .04; Figure 1C) and recipients undergoing transplantation with a female donor (P < .04; Figure 1D) irrespective of recipient's sex. In multiple regression analysis, there was a significant disparity in telomere length difference for recipients with a female donor and chronic GVHD (Figure 1E). The difference in telomere length is more than twice for this situation compared with a male donor without chronic GVHD, corresponding to approximately 30 to 60 years of cell aging. Age of the donor and recipient, age disparity between donor/recipient, sex of the recipient, TBI, number of transplanted nucleated cells, peripheral blood counts in donors and recipients, and acute GVHD had no significant impact on telomere length in recipients. Donor/recipient age differences did not influence our results; however, the use of new transplantation modalities with an increased age donor/recipient disparity might affect telomere length. Furthermore, because 89% of the recipients received TBI, we cannot definitively exclude TBI as a factor contributing to shorter telomeres. One clear limitation of this study is its design, which does not allow us to directly evaluate the telomere length changes over time.
Results on telomere length analysis. (A) Age-dependent telomere length percentiles (1%, 50%, and 99%) of lymphocytes based on telomere length measurements from 400 healthy persons.18 The age-related telomere length values of the male and female donors and recipients are represented for each donor/recipient pair. (B) The mean ± SD of telomere length (in kilobase pairs) for the diverse subsets of leukocytes from the 44 donors (blue) and recipients (red) is shown. (C-E) Factors influencing telomere length: box plots for the telomere length difference in kilobytes (telomere length value of the donor minus that of the recipient) of granulocytes and lymphocytes are shown. A greater value indicates a greater telomere length difference. (C) Telomere length difference in lymphocytes (P = .013) and granulocytes (P = .022) of recipients with or without chronic GVHD. (D) Telomere length difference in lymphocytes (P = .017) and granulocytes (P = .024) of recipients with a female or male donor. (E) Telomere length differences in lymphocytes (P = .025) and granulocytes (P = .024) of recipients with a male or female donor and with or without chronic GVHD and a male or female donor. (F-G) Simplified hypothetical model of telomere shortening after HSCT. The decrease in telomere length over a period of years after transplantation is shown. The light blue area indicates the expected range of telomere length, and the yellow area indicates where telomere length becomes critically short. The broken lines indicate the telomere length decrease. (F) Patient A, who received donor cells with longer telomeres, and patient B, who received donor cells with shorter telomeres. (G) Patient C, who experienced chronic GVHD, and patient D, who experienced chronic GVHD and had a female donor. Note the different time points at which the broken line of the telomere decline crosses with the telomere senescence checkpoint line (horizontal light brown line).
Results on telomere length analysis. (A) Age-dependent telomere length percentiles (1%, 50%, and 99%) of lymphocytes based on telomere length measurements from 400 healthy persons.18 The age-related telomere length values of the male and female donors and recipients are represented for each donor/recipient pair. (B) The mean ± SD of telomere length (in kilobase pairs) for the diverse subsets of leukocytes from the 44 donors (blue) and recipients (red) is shown. (C-E) Factors influencing telomere length: box plots for the telomere length difference in kilobytes (telomere length value of the donor minus that of the recipient) of granulocytes and lymphocytes are shown. A greater value indicates a greater telomere length difference. (C) Telomere length difference in lymphocytes (P = .013) and granulocytes (P = .022) of recipients with or without chronic GVHD. (D) Telomere length difference in lymphocytes (P = .017) and granulocytes (P = .024) of recipients with a female or male donor. (E) Telomere length differences in lymphocytes (P = .025) and granulocytes (P = .024) of recipients with a male or female donor and with or without chronic GVHD and a male or female donor. (F-G) Simplified hypothetical model of telomere shortening after HSCT. The decrease in telomere length over a period of years after transplantation is shown. The light blue area indicates the expected range of telomere length, and the yellow area indicates where telomere length becomes critically short. The broken lines indicate the telomere length decrease. (F) Patient A, who received donor cells with longer telomeres, and patient B, who received donor cells with shorter telomeres. (G) Patient C, who experienced chronic GVHD, and patient D, who experienced chronic GVHD and had a female donor. Note the different time points at which the broken line of the telomere decline crosses with the telomere senescence checkpoint line (horizontal light brown line).
Chronic inflammation and oxidative stress have been associated with telomere shortening.19 In humans, this relationship has been shown in atherosclerosis, chronic inflammatory disease, obesity, and diabetes. The finding of a greater telomere length difference in patients with chronic GVHD, a chronic inflammatory disease, fits with these reports. The effect of the donor sex on the telomere length difference needs more explanation. In a general population, men have shorter telomeres in leukocytes and a slightly greater rate of telomeric loss with age.20 At birth,21 there is no sex difference; therefore, environmental factors, such as cardiovascular risk factors, inflammation, or hormonal state, must play a role. This telomere length advantage in women is lost after HSCT independently from the recipient sex. Estrogen has been shown to protect telomeres from shortening in vitro22,23 and in vivo.24,25 After HSCT, the protective hormonal background is no longer present in a male recipient and may also be absent in female recipients because of their posttransplant estrogen deficiency.
Previous studies have shown a significant telomere shortening early after allogeneic HSCT in the period of marrow reconstitution without further loss over time.13-15 In such a model, recipients with shorter telomeres at time of transplantation would keep shorter telomeres after HSCT (Figure 1F).8 According to our results, we believe that this concept needs to be revised. Telomere shortening in long-term survivors may be determined by the initial telomere loss during the repopulation period as well as by host-related factors such as chronic GVHD or insufficient levels of estrogen (Figure 1G). Critically short telomeres might have an impact on very long-term survivors, leading to an additional risk for degenerative diseases and secondary malignancies. If this hypothesis can be confirmed in larger studies, the identification of recipients at risk for cellular senescence could become part of monitoring long-term survivors after HSCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Diagnostic and Therapeutic Hematology for their valuable contribution in this study. We also thank Katie Perret, one of the study nurses, for her efficient support in the organization of the outpatient controls of the recipients and their respective donors.
This work was supported by a grant from Novartis (Basel, Switzerland; to G.M.B.), the Swiss National Research Foundation (Nr 3200B0-118176), the Horten Foundation, Lugano, Switzerland; and the Werner Geissberger Foundation (to the Basel Stem Cell Transplantation Team).
Authorship
Contributions: G.M.B., A.R., A.G., and A.T. designed the research; G.M.B., A.R., A.M., S. M., J.H. D.H., and J.R. performed the experiments and collected the data; G.M.B., A.R., A.M., S.M., M.S., A.G., and A.T. analyzed and interpreted the data; G.M.B., A.R., A.G., and A.T. wrote the paper; and A.M., S.M., M.S., J.H., D.H, and J.R. reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gabriela Baerlocher, MD, Department of Hematology, University Hospital Bern, Freiburgstrasse 4, CH-3010 Bern, Switzerland and Department of Clinical Research, University of Bern, Murtenstrasse 35, CH-3010 Bern, Switzerland; e-mail: Gabriela.Baerlocher@insel.ch.
References
Author notes
*G.M.B. and A.R. contributed equally to this work.