Abstract
The ability of the adaptive immune system to respond rapidly and robustly upon repeated antigen exposure is known as immunologic memory, and it is thought that acquisition of memory T-cell function is an irreversible differentiation event. In this study, we report that many phenotypic and functional characteristics of antigen-specific CD8 memory T cells are lost when they are deprived of contact with dendritic cells. Under these circumstances, memory T cells reverted from G1 to the G0 cell-cycle state and responded to stimulation like naive T cells, as assessed by proliferation, dependence upon costimulation, and interferon-γ production, without losing cell surface markers associated with memory. The memory state was maintained by signaling via members of the tumor necrosis factor receptor superfamily, CD27 and 4-1BB. Foxo1, a transcription factor involved in T-cell quiescence, was reduced in memory cells, and stimulation of naive CD8 cells via CD27 caused Foxo1 to be phosphorylated and emigrate from the nucleus in a phosphatidylinositol-3 kinase–dependent manner. Consistent with these results, maintenance of G1 in vivo was compromised in antigen-specific memory T cells in vesicular stomatitis virus-infected CD27-deficient mice. Therefore, sustaining the functional phenotype of T memory cells requires active signaling and maintenance.
Introduction
Upon primary exposure to antigen, naive T cells bearing complementary antigen receptors (TCRs) undergo rapid activation and clonal expansion, leading to the generation of effector T cells.1 The majority of antigen-specific effector T cells undergo apoptosis after antigen clearance.2 A small subset survives and gives rise to long-lived memory T cells. Upon antigen re-encounter, memory T cells respond swiftly and robustly to eliminate the pathogen. Based on their tissue distribution, cell surface markers, and effector functions, memory T cells have been divided into 2 major subsets.1 Memory T cells expressing receptors such as CD62L and C-C chemokine receptor 7, which allow efficient homing to lymph nodes (LNs), are termed central memory (TCM) cells; memory T cells lacking LN-homing receptors and preferentially residing in nonlymphoid tissues are termed effector memory (TEM) cells. Both memory subsets display high levels of CD44.1
How T memory cells develop remains a matter of debate. Among the possibilities is a sequential model suggesting that TCM and TEM cells are part of a continuum in a linear naive-effector–TEM-TCM differentiation pathway.1 In contrast, the divergent differentiation model proposes that naive T cells can develop directly into either effector or memory T-cell subsets.3 A recently formulated variation of this is the “1 cell, 2 fates” model, in which a single T cell can give rise to both an effector and a memory daughter cell through asymmetric division.4 In any case, CD4 and CD8 memory T-cell populations can be maintained for many years in vivo.1 Although earlier studies suggested that continued antigenic stimulation is required for maintaining T-cell memory,5 neither cognate antigen nor major histocompatibility complex (MHC)–encoded molecules are required for the long-term survival of memory T cells.6
Members of the tumor necrosis factor receptor (TNFR) family are involved in T-cell costimulation and play a role in T-cell survival.7 Mice deficient in OX40, CD27, or 4-1BB show greater defects in secondary compared with primary responses when infected with pathogens such as lymphocytic choriomeningitis virus (LCMV), vesicular stomatitis virus (VSV), and influenza.8,9 Some TNFR family members, such as CD27, are constitutively displayed on the T-cell surface, whereas others such as OX40 and 4-1BB are induced by T-cell activation.10 Interaction of CD27 with its ligand, CD70, influences both CD4 and CD8 memory T-cell responses.7 Although the interaction of 4-1BB and OX40 with their ligands also affects memory T-cell generation of both subsets, for some viruses such as LCMV, 4-1BB appears to be more important for CD8 memory.7 Stimulation through costimulatory TNFR family members can enhance antiviral and anticancer responses in vivo, and blockade of these costimulatory pathways can be beneficial in the treatment of autoimmunity7 and graft rejection,11 making them potential targets for therapeutic interventions.
The transition from a naive to a functional memory state is thought to be a progressive, unidirectional process.12 In this study, we show that several important aspects of the T-cell memory phenotype are actively maintained by signaling through members of the TNFR superfamily, and that these features revert to a naive-like phenotype when such signaling is disrupted.
Methods
Mice
C57BL/6 (B6) mice were obtained from Frederick Cancer Research Facility, and B6.SJL-Ptprca Pepcb/BoyJ (45.1) from The Jackson Laboratory, and were bred and maintained in a National Cancer Institute pathogen-free animal facility. CD27−/− mice were originally obtained from Jannie Borst (The Netherlands Cancer Institute)13 and maintained in a pathogen-free facility at Rush University Medical Center. Study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the National Cancer Institute/National Institutes of Health.
Reagents and antibodies
Hoechst 33342, Percoll, and pyronin Y were purchased from Molecular Probes, Amersham Biosciences, and Polysciences, respectively. Purified anti–mouse CD3 (145-2C11), and directly conjugated monoclonal antibodies against CD4, CD8α, TCR-β, CD25, CD16/CD32, CD69, CD45.1, CD27, interferon (IFN)-γ, immunoglobulin G1, CD45.1, and Ly6C were from BD Pharmingen or BD Biosciences. Phorbol 12-myristate 13-acetate (PMA), ionomycin (Iono), monensin, wortmannin, LY294002, anti-tubulin, and anti–lamin A were purchased from Sigma-Aldrich; carboxyfluorescein diacetate succinimidyl ester (CFSE) from Invitrogen; and antibodies against CD62L, CD44, and OX40L from eBiosciences. Anti-CD70 (FR70) and anti–4-1BBL (TKS-1) have been described.14 Anti-Foxo1, anti-Akt, and anti–phospho-Ser473-Akt were obtained from Cell Signaling Technology. Mouse T-cell purification columns (Cellect) were obtained from Cedarlane Laboratories. CD11c+ dendritic cells (DCs) were isolated with magnetic beads (Miltenyi Biotec). HL-1 medium supplement was obtained from Lonza Walkersville. LCMV-Armstrong strain was a gift from Rafi Ahmed (Emory University School of Medicine). MHC class I tetramers containing VSV N-peptide (RGYVYQGL) were obtained from the National Institutes of Health Tetramer Facility. The H-2Db/LCMV glycoprotein (gp)33-41 (KAVYNFATC) tetramer was purchased from Beckman Coulter. Both were labeled with allophycocyanin.
Cell preparation, culture, and T-cell stimulation
LNs, bone marrow (BM), and spleens were obtained from 4- to 10-month-old mice. T cells were purified by negative selection with T Cellect columns to a purity of at least 90%. All cultures were performed with RPMI 1640 (Biofluids) containing 10% heat-inactivated fetal calf serum, 4 mM glutamine, 5 μM 2-mercaptoethanol, and the serum-free medium supplement HL-1 (complete medium). Cell viability after 48 hours of culture was typically 50% or greater. To assess the effect of culture on cell phenotype and function, 5 × 105 T cells were cultured in 24-well plates with or without other cell populations, as indicated. In some experiments, anti-CD28 (10 μg/mL) or anti-CD27 (5 μg/mL) was coated on the plastic. For activation with antibodies, 96-well plates were coated overnight at 4°C with anti-CD3 in phosphate-buffered saline (PBS; 5 μg/mL), and 105 purified T cells were cultured in the presence of anti-CD28 (2 μg/mL) in a volume of 200 μL. For DC isolation, anti-mouse immunoglobulin G micro beads (Miltenyi Biotec) were used to positively select CD11c+ cells. In some experiments, naive and memory cells were obtained by sorting for CD8+CD44low and CD8+CD44high cells with a FACSAria (BD Biosciences), respectively. The purity of the populations was more than 99%.
Percoll gradient fractionation
Percoll gradient separation was performed as described.15 Briefly, purified T cells were layered on top of a discontinuous Percoll gradient (53%:65%:72%), and after centrifugation at 1900g for 12 minutes at 4°C, cells were retrieved from 3 interfaces as follows: low density (< 53%), intermediate density (53%-65% interface), and high density (65%-72%).
Intracellular IFN-γ production
In most experiments, T cells were stimulated with or without PMA (10 ng/mL) and Iono (500 ng/mL) for 6 or 18 hours. Monensin (3 μM) was added to the cultures for the final 3 hours. The cells were surface stained with labeled anti-CD8 and gp33-41 tetramer (in LCMV experiments) at room temperature for 30 minutes, and intracellular IFN-γ was assessed using the BD Cytofix/Cytoperm and Perm/Wash Buffer kit (BD Biosciences). For mice previously infected with VSV, splenocytes were stimulated for 5 hours in vitro with VSV N-peptide RGYVYQGL (1 μg/mL) in the presence of GolgiStop (BD Biosciences; 1 μg/mL) and then stained.
DNA and RNA staining
Cells were incubated with Hoechst 33342 and pyronin Y, respectively, as described.15 Cytofluorimetric evaluation was performed with a LSRII using DiVa software (BD Biosciences), with bandpass filters of 440 plus or minus 40 for Hoechst 33342 and 585 plus or minus 26 for pyronin Y. All data were analyzed with FlowJo software (TreeStar) using doublet discrimination.
Cell-cycle analysis
Purified T cells (5 × 105 per well) were labeled with 5 μM CFSE for 10 minutes at 37°C and stimulated with anti-CD3, and CFSE staining was analyzed at the indicated times by flow cytometry.
Adoptive transfer
Splenic CD8 T cells from CD45.2 B6 mice were purified and separated on Percoll. The intermediate-density cells were cultured for 48 hours, and then they or freshly isolated intermediate-density CD8 T cells were adoptively transferred intravenously into congenic CD45.1 recipients (5 × 106 cells/mouse). Two weeks later, splenocytes were harvested and purified CD8 T cells were stained for CD45.1, CD45.2, CD44, CD8, and gp33-41 tetramers.
Western blotting
Immunoblotting and resolution by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) were performed, as described,15 and proteins were detected by chemiluminescence (Thermo Scientific).
Preparation of cytosolic and nuclear extracts
Cells (5 × 106) were washed in cold PBS, resuspended in 100 μL of buffer A (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 1.5 mM MgCl2, 10 mM KCl, and 0.5 mM dithiothreitol, pH 7.9) with protease inhibitors and incubated on ice for 15 minutes. Nonidet P-40 was added to a final concentration of 0.5%, and the cells were vortexed for 10 seconds. Nuclei were pelleted by centrifugation at 6500g for 15 seconds, resuspended in 100 μL of buffer C (20 mM HEPES, 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM ethylenediaminetetraacetic acid [EDTA], and 25% vol/vol glycerol, pH 7.9) with protease inhibitors, and incubated on ice for 30 minutes. The samples were centrifuged at 13 000g for 10 minutes to remove DNA, and aliquots of nuclear extract were frozen.
Viral infection
For LCMV, B6 mice were infected by intraperitoneal injection of 2 × 105 plaque-forming units of the Armstrong strain. For VSV, mice were immunized intravenously with 106 plaque-forming units of VSV (Indiana strain) in 200 μL of PBS.
Antibody blockade
DC were incubated with 2 μg/mL of the indicated antibodies for 30 minutes at 37°C and then washed. A half million (5 × 105) intermediate-density T cells were cultured with 12.5 × 105 DC for 48 hours in 24-well plates in 1 mL complete medium.
Results
Memory CD8 T cells are of intermediate density and in G1
Memory T cells have higher basal phosphorylation of activation-promoting kinases, lower levels of inhibitory signaling proteins,16,17 and a gene expression pattern intermediate between naive (resting) and effector (active) T cells.18,19 They also have increased expression of genes that facilitate entry into the cell cycle, increased total RNA levels, and exist in a more advanced G1 phase than naive T cells.20,21 We have reported that a subset of resting T cells activated via the TCR for a time insufficient to cause proliferation move from G0 to G1, and even after several days of rest, responds rapidly to restimulation in a manner similar to memory cells.15 To characterize the cell-cycle state and its possible relevance to T-cell memory, we examined 2 sources: naturally arising polyclonal CD8 memory T cells and LCMV gp33-specific, tetramer-positive CD8 memory T cells.
T-cell density decreases as cells advance through the cell cycle.22 One can separate cells residing in G1 from those in G0 and those actively in cycle with discontinuous Percoll density gradients.15 CD8 T cells from spleens of unmanipulated mice were separated on a Percoll gradient. There were relatively few viable cells in the most buoyant first layer, and they were not analyzed further. The intermediate- and high-density fractions were characterized (Figure 1). Cell density was reflected in cell size, the more buoyant cells being slightly larger as measured by forward scatter (Figure 1A). Cell-cycle state was addressed by simultaneous measurement of RNA and DNA. The high-density CD8 cells were in G0, and the intermediate-density cells were mostly in G1. As shown in Figure 1B, high-density CD8 cells expressed low levels of CD44 and high levels of CD62L, a naive T-cell phenotype, whereas intermediate-density CD8 cells expressed high levels of CD44 and CD62L, the phenotype of TCM cells.1 The intermediate-density fraction was also enriched in cells bearing high levels of Ly6C, a marker of CD8 memory cells.23 Similar results were obtained with purified T cells from LN and bone marrow (data not shown).
Memory CD8 T cells are of intermediate density and in G1. Splenic T cells were purified and fractionated on a Percoll density gradient. (A) Cell size (forward scatter [FSC]) and cell-cycle analysis of gated CD8 T cells in the indicated fractions were determined by flow cytometry. The horizontal bar in the right panels represents the amount of background fluorescence emitted from Hoechst-stained cells in the pyronin Y channel. The percentages of cells in the regions corresponding to G0, G1, and S/G2 plus M are indicated. (B) Cell surface expression of CD44, CD62L, and Ly6C on CD8 T cells in the indicated fractions. (C) IFN-γ production and CD69 expression by intermediate- and high-density CD8 T cells stimulated with (solid line) or without (shaded histogram) PMA/Iono (left panel). (D) T cells were enriched from LCMV-immunized mice and fractionated on a Percoll density gradient. The distribution of gp33-41 tetramer-positive CD8 T cells in the intermediate- and high-density fractions is shown in the left panel, and the cells in the gated areas were analyzed for size (middle panel) and cell-cycle state (right panels). (E) Cell surface expression of the indicated markers on intermediate-density gp33-41 tetramer-positive CD8 T cells versus high-density CD8 cells. (F) IFN-γ production and CD69 expression by intermediate-density gp33-41 tetramer-positive and high-density CD8 T cells.
Memory CD8 T cells are of intermediate density and in G1. Splenic T cells were purified and fractionated on a Percoll density gradient. (A) Cell size (forward scatter [FSC]) and cell-cycle analysis of gated CD8 T cells in the indicated fractions were determined by flow cytometry. The horizontal bar in the right panels represents the amount of background fluorescence emitted from Hoechst-stained cells in the pyronin Y channel. The percentages of cells in the regions corresponding to G0, G1, and S/G2 plus M are indicated. (B) Cell surface expression of CD44, CD62L, and Ly6C on CD8 T cells in the indicated fractions. (C) IFN-γ production and CD69 expression by intermediate- and high-density CD8 T cells stimulated with (solid line) or without (shaded histogram) PMA/Iono (left panel). (D) T cells were enriched from LCMV-immunized mice and fractionated on a Percoll density gradient. The distribution of gp33-41 tetramer-positive CD8 T cells in the intermediate- and high-density fractions is shown in the left panel, and the cells in the gated areas were analyzed for size (middle panel) and cell-cycle state (right panels). (E) Cell surface expression of the indicated markers on intermediate-density gp33-41 tetramer-positive CD8 T cells versus high-density CD8 cells. (F) IFN-γ production and CD69 expression by intermediate-density gp33-41 tetramer-positive and high-density CD8 T cells.
A similar analysis was performed with memory CD8 T cells from mice previously infected with the LCMV Armstrong strain. This induces a potent CD8 T-cell response that results in viral clearance within 8 to 10 days.24 LCMV-specific CD8 T cells can be followed by staining with anti-CD8 and MHC class I Db tetramers containing the viral peptide gp33-41.25 Two or more months after LCMV infection, the majority of splenic tetramer-positive CD8 T cells were found of intermediate density (Figure 1D first column). These cells were larger than the high-density cells and had elevated RNA levels. The tetramer-positive CD8 T cells were uniformly CD44high, Ly6Chigh, and were biphasic for CD62L, suggesting the presence of both TCM and TEM cells (Figure 1E). In contrast, the high-density fraction was mostly composed of CD44lowCD62Lhigh cells that had biphasic levels of Ly6C, as expected of naive T cells.
A characteristic of T-cell memory is their rapid effector response upon stimulation. The intermediate and high-density fractions were incubated with or without PMA/Iono, and IFN-γ production was measured by intracellular staining (Figure 1C). Although both populations responded by up-regulating CD69, the intermediate-density, but not the high-density, cells from unmanipulated mice produced large amounts of IFN-γ. Similar results were obtained with LCMV gp33 tetramer-positive CD8 cells of intermediate density compared with CD8 cells of high density (Figure 1F). We conclude that both naturally arising and LCMV-specific memory CD8 T cells are of intermediate density with elevated RNA levels.
Cultured memory CD8 cells revert to a naive-like state
Whereas T-cell memory is thought to be an irreversible state of differentiation, residency in G1 is not. Persistence of T memory cells in G1 might reflect changes in their genetic program. In contrast, T-cell memory phenotype and function could be a reflection of their G1 state. If so, and if it were possible to revert the intermediate-density T cells to G0, one would expect their functional characteristics to change. To discriminate between these, intermediate- (memory) and high- (naive) density purified splenic T cells from unmanipulated mice were deprived of any external stimuli by culturing them alone for 48 hours. The initial size of intermediate CD8 T cells was slightly greater than that of high-density cells, but after 48 hours the size was the same (Figure 2A top row). A similar result was obtained when RNA content was quantitated (Figure 2A bottom row). The levels of the memory marker CD44 were still high after 48 hours of in vitro culture (data not shown). It is important to note that the loss of cell size and RNA was not due to selective survival of a small number of contaminating naive cells, because cell viability of naturally arising memory CD8 cells was 50% at 48 hours (a typical result) and the cells expressed high levels of the memory marker CD44. To determine whether these changes were reflected in function, intermediate-density T cells were incubated for 48 hours (reverted) and then activated. Similar to the results shown in Figure 1, freshly isolated intermediate-density CD8 T cells produced much more IFN-γ than naive CD8 cells (Figure 2B). Importantly, the reverted CD8 cells responded similarly to naive CD8 cells. Memory T cells are less dependent on CD28 costimulation than naive T cells.26,27 We measured the proliferation of intermediate-density, high-density, and reverted CD8 T cells after stimulation with anti-CD3 and no costimulation (Figure 2C). Intermediate-density memory CD8 T cells underwent 1 cycle of division after 24 hours, whereas naive and reverted CD8 T cells remained undivided. By 48 hours, the large majority of CD8 memory T cells had divided 2 to 3 times and most naive CD8 T cells were undivided, a difference that was even more striking at 72 hours. Entry into cell cycle followed the same pattern: fresh intermediate-density CD8 T cells progressed into the cell cycle more rapidly than high-density cells, and the reverted cells were indistinguishable from the naive (Figure 2D).
CD8 T-cell memory characteristics are reversible. (A) Cell size (forward scatter [FSC]) and pyronin Y RNA) staining of intermediate and high-density CD8 T cells before and after in vitro incubation for 24 and 48 hours. (B) IFN-γ production by intermediate and high-density CD8 T cells before and after in vitro incubation for 48 hours. Shaded histograms represent IFN-γ production by unstimulated cells. (C) Intermediate, high, and intermediate-density CD8 T cells that had been cultured in medium for 48 hours were labeled with CFSE. Cells were stimulated with plate-bound anti-CD3 for the indicated times, at which point CFSE dilution was determined by flow cytometry. (D) Cell-cycle analysis of intermediate-density, high-density, and 48-hour in vitro incubated intermediate-density (48-hour–intermediate) CD8 T cells stimulated with or without anti-CD3/CD28 for the indicated periods of time. (E) Cell cycle of high-density, intermediate-density gp33-41 tetramer-positive, and intermediate-density 48-hour cultured gp33-41 tetramer-positive CD8 T cells from mice previously infected with LCMV. (F) IFN-γ production by stimulated intermediate-density gp33-41 tetramer-positive and high-density CD8 T cells before and after incubation in medium for 48 hours. Shaded histograms represent isotype control. (G) The cultured intermediate-density cells shown in (E) were adoptively transferred into naive B6 recipients. Two weeks later, tetramer-positive CD8 T cells were analyzed for cell-cycle state (right panel). Freshly isolated high-density CD8 T cells are shown as a control (left panel).
CD8 T-cell memory characteristics are reversible. (A) Cell size (forward scatter [FSC]) and pyronin Y RNA) staining of intermediate and high-density CD8 T cells before and after in vitro incubation for 24 and 48 hours. (B) IFN-γ production by intermediate and high-density CD8 T cells before and after in vitro incubation for 48 hours. Shaded histograms represent IFN-γ production by unstimulated cells. (C) Intermediate, high, and intermediate-density CD8 T cells that had been cultured in medium for 48 hours were labeled with CFSE. Cells were stimulated with plate-bound anti-CD3 for the indicated times, at which point CFSE dilution was determined by flow cytometry. (D) Cell-cycle analysis of intermediate-density, high-density, and 48-hour in vitro incubated intermediate-density (48-hour–intermediate) CD8 T cells stimulated with or without anti-CD3/CD28 for the indicated periods of time. (E) Cell cycle of high-density, intermediate-density gp33-41 tetramer-positive, and intermediate-density 48-hour cultured gp33-41 tetramer-positive CD8 T cells from mice previously infected with LCMV. (F) IFN-γ production by stimulated intermediate-density gp33-41 tetramer-positive and high-density CD8 T cells before and after incubation in medium for 48 hours. Shaded histograms represent isotype control. (G) The cultured intermediate-density cells shown in (E) were adoptively transferred into naive B6 recipients. Two weeks later, tetramer-positive CD8 T cells were analyzed for cell-cycle state (right panel). Freshly isolated high-density CD8 T cells are shown as a control (left panel).
Cell-cycle state and function of antigen-specific memory cells were assessed after culturing intermediate-density LCMV-specific memory CD8 T cells for 48 hours. There was no loss of antigen-specific T cells during this time, as assessed by enumerating the numbers of tetramer-positive CD8 cells before and after culture. During this period, RNA levels declined, and memory T cells reverted from G1 to G0 (Figure 2E) while maintaining a high level of CD44 (data not shown). After 48 hours of culture, the intermediate-density CD8 tetramer-positive T cells behaved like the high-density cells, failing to produce IFN-γ (Figure 2F). To test whether the reverted gp33-41–specific memory T cells maintain their G0 state or return to G1 if restored to a normal environment, the reverted memory CD8 cells shown in Figure 2D were adoptively transferred into congenic naive recipients. After 2 weeks, the tetramer-positive CD8 memory T cells in the spleen were found to still be small and CD44high (data not shown) and in G0 (Figure 2G). Therefore, in the absence of overt stimulation, the G0 state of reverted memory T cells appears to be stable.
G1 and the rapid activation response are maintained by interaction with costimulatory ligands on DCs
If the characteristic memory T-cell cycle state and ability to respond rapidly to stimulation are reversible, how are they maintained in vivo? We asked whether interactions with accessory cells are important. Intermediate-density splenic CD8 T cells from unmanipulated mice were cultured for 48 hours with DCs. Whereas the cells cultured in medium alone had much reduced RNA content, those cocultured with DCs remained largely in G1 (Figure 3A). This required cell-cell contact, because T cells separated from the DC by a semipermeable membrane failed to maintain their G1 state (data not shown). One candidate for a receptor-ligand pair is CD27, a TNFR superfamily member that is required for the normal memory T-cell responses, and its ligand CD70.13 The possible contribution of CD27 was assessed in 2 ways. First, whereas intermediate-density purified CD8 T cells reverted to a naive state when cultured alone, most of the cells maintained their size (data not shown) and G1 state when cultured with agonistic anti-CD27 antibodies (Figure 3A). Second, inclusion of a blocking antibody to CD70 reduced the ability of DC to preserve the G1 state. OX40 and 4-1BB are also members of the TNFR superfamily that have been implicated in the maintenance of T-cell memory.7,10 When intermediate-density CD8 T cells were incubated with DC, anti-4-1BBL partially prevented the rescue of the G1 state, whereas anti-OX40L did not (Figure 3B).
CD8 T-cell memory is maintained by DCs. (A) Cell-cycle analysis of intermediate-density CD8 T cells from unmanipulated mice before and after incubation under the indicated conditions for 48 hours. The numbers represent the percentage of cells in the different regions. (B) Cell-cycle analysis of intermediate-density CD8 T cells from unmanipulated mice before and after incubation under the indicated conditions for 48 hours. (C-D) RNA levels of high-density (blue line) and intermediate-density (red line) gp33-41 tetramer-positive CD8 T cells from mice previously infected with LCMV, before and after incubation under the indicated conditions for 48 hours. The dashed blue line in the 48-hour panels represents the solid blue line for high-density T cells shown at time 0, and is shown to facilitate comparison with intermediate-density RNA levels. (E) IFN-γ production by high-density or intermediate-density tetramer-positive or CD8 T cells from mice previously infected with LCMV in response to PMA/Iono before and after 48 hours of incubation under the indicated conditions. Shaded histograms represent IFN-γ production by unstimulated cells.
CD8 T-cell memory is maintained by DCs. (A) Cell-cycle analysis of intermediate-density CD8 T cells from unmanipulated mice before and after incubation under the indicated conditions for 48 hours. The numbers represent the percentage of cells in the different regions. (B) Cell-cycle analysis of intermediate-density CD8 T cells from unmanipulated mice before and after incubation under the indicated conditions for 48 hours. (C-D) RNA levels of high-density (blue line) and intermediate-density (red line) gp33-41 tetramer-positive CD8 T cells from mice previously infected with LCMV, before and after incubation under the indicated conditions for 48 hours. The dashed blue line in the 48-hour panels represents the solid blue line for high-density T cells shown at time 0, and is shown to facilitate comparison with intermediate-density RNA levels. (E) IFN-γ production by high-density or intermediate-density tetramer-positive or CD8 T cells from mice previously infected with LCMV in response to PMA/Iono before and after 48 hours of incubation under the indicated conditions. Shaded histograms represent IFN-γ production by unstimulated cells.
An analysis of intermediate-density CD8 memory cells from mice previously infected with LCMV was performed. RNA levels decreased in tetramer-positive cells incubated in medium alone, but were maintained when they were cocultured with DCs (Figure 3C). RNA levels decreased to levels found in freshly isolated naive cells when CD27-CD70 interactions were blocked. The maintenance of RNA levels in memory T cells cultured with DCs was diminished by anti-4-1BBL, but not anti-OX40L (Figure 3D). Cellular responses to activation agreed with these observations. Stimulation of freshly isolated tetramer-positive CD8 T cells resulted in IFN-γ production by the majority of intermediate-density cells, with only a small number of high-density cells producing low amounts (Figure 3E). After 48 hours of incubation in medium, only a small number of the tetramer-positive CD8 T cells produced IFN-γ. Cells that were incubated with DCs retained the ability to produce IFN-γ, although the amount produced per cell was not as high as for fresh cells. Incubation in the presence of either anti-CD70 or anti–4-1BBL prevented maintenance of effector T-cell function, whereas incubation with anti-OX40L did not affect the DC “rescue.” Therefore, CD8 memory T cells reverted to a naive-like phenotype upon culture, which was prevented by signaling via CD27 and 4-1BB.
CD27 signaling maintains G1 and inactivates Foxo1 via phosphatidylinositol 3-kinase–Akt
Phosphatidylinositol 3-kinase (PI3K) and its downstream mediator Akt play important roles in cyclin D3 expression, the G0/G1 transition, and increases in lymphocyte size and metabolic activity in response to activation.28 Little phospho-Akt was detected in unstimulated CD8 cells, but it was easily detectable at 10 minutes and continued to rise 30 minutes after activation with plate-bound anti-CD27 (Figure 4A). Akt phosphorylation was prevented by inhibition of PI3K. Fresh naive and memory CD8 cells expressed similar amounts of Akt, but phospho-Akt was detected only in the memory subset (Figure 4B). Furthermore, Akt phosphorylation was lost when the memory T cells were cultured for 48 hours alone, but not when the cells were cultured with plate-bound anti-CD27. This maintenance of Akt phosphorylation was prevented by inhibition of PI3K. Therefore, spontaneous Akt activation is a characteristic of memory T cells and can be induced and maintained by stimulation via CD27.
CD27 signaling maintains G1 and inactivates Foxo1 via PI3K and Akt. (A) Purified splenic CD8 T cells were incubated with or without anti-CD27 for the indicated times. Cells were pretreated with wortmannin (100 nM) or LY294002 (10 μM) 1 hour before stimulation. (B) Akt phosphorylation in the high-density naive or intermediate-density memory splenic CD8 T cells before and after 48 hours of culture under the indicated conditions with or without wortmannin or LY294002. Cell lysates were prepared, and phospho-Ser473 Akt and total Akt were detected by immunoblotting. (C) Splenocytes were sorted for CD8+CD44high and CD8+CD44low, and lysates of equal numbers of cells were loaded on SDS-PAGE and immunoblotted for Foxo1 and β-actin. (D) Purified splenic CD8 T cells were stimulated with plate-bound anti-CD27 for the indicated times, after which nuclear and cytosolic fractions were prepared. Tubulin and lamin A were used as controls for the efficacy of the fractionation. (E) Purified splenic CD8 T cells were fractionated on Percoll, and the intermediate-density cells were analyzed at time 0 or after 48 hours of culture under the indicated conditions. Wortmannin or LY294002 was added during culture with either DCs or plate-bound anti-CD27. Cell cycle was analyzed as in Figure 1. Cell viability after 48 hours was 58% to 73%. (F) The intermediate-density CD8 T cells were lysed immediately or after 48 hours of culture under the indicated conditions. Equal protein concentrations were loaded on SDS-PAGE gels and immunoblotted for cyclin D3 or β-actin. (G) Cyclin D3 levels in intermediate-density CD8 T cells before and after incubation under the indicated conditions. Cell lysates were prepared, and cyclin D3 and β-actin levels were determined by immunoblotting.
CD27 signaling maintains G1 and inactivates Foxo1 via PI3K and Akt. (A) Purified splenic CD8 T cells were incubated with or without anti-CD27 for the indicated times. Cells were pretreated with wortmannin (100 nM) or LY294002 (10 μM) 1 hour before stimulation. (B) Akt phosphorylation in the high-density naive or intermediate-density memory splenic CD8 T cells before and after 48 hours of culture under the indicated conditions with or without wortmannin or LY294002. Cell lysates were prepared, and phospho-Ser473 Akt and total Akt were detected by immunoblotting. (C) Splenocytes were sorted for CD8+CD44high and CD8+CD44low, and lysates of equal numbers of cells were loaded on SDS-PAGE and immunoblotted for Foxo1 and β-actin. (D) Purified splenic CD8 T cells were stimulated with plate-bound anti-CD27 for the indicated times, after which nuclear and cytosolic fractions were prepared. Tubulin and lamin A were used as controls for the efficacy of the fractionation. (E) Purified splenic CD8 T cells were fractionated on Percoll, and the intermediate-density cells were analyzed at time 0 or after 48 hours of culture under the indicated conditions. Wortmannin or LY294002 was added during culture with either DCs or plate-bound anti-CD27. Cell cycle was analyzed as in Figure 1. Cell viability after 48 hours was 58% to 73%. (F) The intermediate-density CD8 T cells were lysed immediately or after 48 hours of culture under the indicated conditions. Equal protein concentrations were loaded on SDS-PAGE gels and immunoblotted for cyclin D3 or β-actin. (G) Cyclin D3 levels in intermediate-density CD8 T cells before and after incubation under the indicated conditions. Cell lysates were prepared, and cyclin D3 and β-actin levels were determined by immunoblotting.
Foxo1, a member of the forkhead box subgroup O transcription factor family, is preferentially expressed in lymphoid cells in which it up-regulates a variety of genes, including those involved in cellular quiescence and apoptosis.28,29 Activated PI3K-Akt phosphorylates Foxo1, leading to its release from DNA, association with 14-3-3 proteins, and movement to the cytoplasm, resulting in increases in cellular metabolism and cell-cycle progression.30,31 The expression of Foxo1 was much higher in naive than in memory CD8 cells (Figure 4C). In CD8 T cells, Foxo1 was found only in the nucleus and in the unphosphorylated state (Figure 4D). Within 10 minutes of stimulation with anti-CD27, Foxo1 migrated to the cytosol as a phosphorylated species. Therefore, CD27-mediated activation of PI3K-Akt results in inactivation of the cell cycle and metabolic inhibitor Foxo1.
The role of CD27-induced PI3K-Akt activation in maintaining various aspects of the memory cell phenotype was addressed by culturing memory CD8 cells for 48 hours alone, with DCs in the presence or absence of anti-CD70, or with plate-bound agonistic anti-CD27 antibodies. Fresh memory CD8 T cells were largely in G1, returned to G0 when cultured alone, but remained in G1 when cultured with DC or plate-bound anti-CD27 (Figure 4E). Inhibition of PI3K abrogated maintenance of G1 seen in the presence of anti-CD27 or DCs, in the latter case similar to the results obtained with anti-CD70. Cyclin D3 is elevated in memory T cells and is thought to be important for their rapid proliferative response to activation.15,20,21 Cyclin D3 was barely detectable in the high-density cells (data not shown), but readily detectable in fresh intermediate-density CD8 T cells (Figure 4F). After culture for 48 hours, cyclin D3 fell to almost undetectable levels. Notably, coculture with DCs prevented the loss of cyclin D3, and inclusion of blocking antibodies against CD70 (Figure 4F) and 4-1BBL, but not OX40L (Figure 4G), inhibited the ability of DCs to maintain cyclin D3 levels. Consistent with this, stimulation via CD27 maintained cyclin D3 levels. Furthermore, maintenance of cyclin D3 by DCs or anti-CD27 was prevented by inhibition of PI3K. Therefore, as with cell size, cell cycle, and function, the maintenance of intermediate-density CD8 cyclin D3 is dependent on PI3K-Akt activation downstream of CD27.
Reduced maintenance of antigen-specific memory cells in G1 in CD27-deficient mice
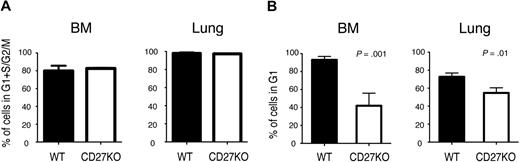
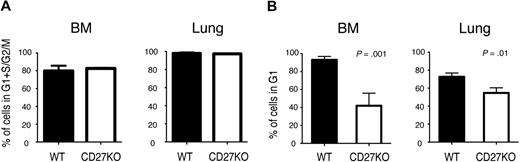
Despite nearly normal numbers of tetramer-positive virus-specific CD8 T cells, CD27-deficient animals have an impaired secondary response to viral pathogens.9,13 We asked whether CD27-deficient antigen-specific T cells could maintain the normal memory G1 state. Wild-type (WT) and CD27-deficient mice were infected with VSV, and the RNA/DNA content of CD8 T cells specific for the VSV N-peptide in the BM, where most CD8 TCM reside,32 was assessed over time. On day 7 after infection, N-tetramer–positive CD8 T cells were actively cycling, and there was no difference between the number of WT and CD27-deficient tetramer-positive cells in G1 plus S plus G2/M (Figure 5A), indicating that the lack of CD27 had no apparent effect on this aspect of the primary response. One to 2 months after infection, however, differences between WT and CD27−/− mice were apparent. The frequency and number of N-tetramer–positive CD8 T cells were modestly reduced (approximately 2-fold) in the absence of CD27 (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), which is often described in memory T cells of TNFR family genetically targeted mice.7 Notably, whereas almost all WT N-tetramer memory CD8 T cells in the BM were in G1, on average one-third of the CD8 tetramer-positive CD27-deficient cells were in G1, the rest being in G0 (Figure 5B). The reduction in the percentage of CD8 tetramer-positive cells in G1 in the lung was not as great, although it was still statistically significant. When cells from mice 2 to 6 months post-VSV infection were stimulated with VSV N-peptide in vitro, CD27-deficient cells showed a 10% to 15% reduction in the number of tetramer-positive BM and lung CD8 cells that produced IFN-γ (n = 5). These results indicate that although there is variability between tissues, CD27 contributes to the maintenance of antigen-specific memory T cells in G1 in vivo.
Cell-cycle state of antigen-specific memory CD8 T cells in CD27−/− mice. WT or CD27−/− mice were infected intravenously with VSV. (A) After 7 days, BM and lung VSV-N tetramer-positive CD8 T cells were analyzed for RNA/DNA content, and the number of cells not in G0 is shown. The results represent the mean ± SEM of 3 mice per group. (B) After 35 to 65 days, BM and lung VSV-N tetramer–positive CD8 memory T cells were analyzed for RNA/DNA content. The data are the combined results of 2 independent experiments, showing the mean ± SEM of 8 mice total per group. The number of cells in S plus G2/M was less than 1%.
Cell-cycle state of antigen-specific memory CD8 T cells in CD27−/− mice. WT or CD27−/− mice were infected intravenously with VSV. (A) After 7 days, BM and lung VSV-N tetramer-positive CD8 T cells were analyzed for RNA/DNA content, and the number of cells not in G0 is shown. The results represent the mean ± SEM of 3 mice per group. (B) After 35 to 65 days, BM and lung VSV-N tetramer–positive CD8 memory T cells were analyzed for RNA/DNA content. The data are the combined results of 2 independent experiments, showing the mean ± SEM of 8 mice total per group. The number of cells in S plus G2/M was less than 1%.
Discussion
The hyperresponsiveness of memory T cells after re-encounter with antigen is best exemplified by rapid progression into the cell cycle, cytokine production, and activation of effector functions.18,21,33 In this work, we have been able to distinguish memory from naive CD8 T cells based on their size, density, and cell-cycle status, and found that stimulation via TNFR superfamily members is required to maintain memory cell-cycle state and hyperresponsiveness. Although it is possible that maintenance of the memory-like functional state is the consequence of some as yet uncharacterized molecular events, the simplest interpretation is that it is being in G1 itself that is a major contributor. Memory (G1), but not naive T cells express mRNA transcripts for effector genes, such as IFN-γ, and cytotoxic molecules, such as perforin and granzyme B, although they are not translated until re-encounter with antigen.33 It is worth noting that the higher RNA level in G1 cells also reflects increases in ribosomal RNA.34 Thus, the rapid and heightened memory response can be explained because these cells do not need to generate mRNA or the ribosomal machinery for protein synthesis de novo. The correlation between cell-cycle state and function is strengthened by the finding that CD27 signaling activates PI3K-Akt. Activated PI3K and Akt enhance cellular metabolism by a variety of mechanisms, including derepression of mammalian target of rapamycin (mTOR), resulting in increased protein translation, and up-regulation of the glucose transporter GLUT1, yielding increased glycolytic capacity.35 Another result is the phosphorylation, translocation, and inactivation of Foxo1, thus derepressing genes involved in preventing cell-cycle progression and apoptosis. Recently, mice in which expression of Foxo1 was specifically disrupted in T cells were found to have a shift in T-cell homeostasis, with a loss of naive cells and an expansion of CD44high cells, the CD8 T cells having a TCM (CD62Lhigh) phenotype.29 This is consistent with our finding that memory CD8 T cells express low levels of Foxo1 that are maintained by signaling through CD27. We favor the notion, therefore, that TNFR superfamily member–dependent residency in G1 is a major component of the state of readiness that results in accelerated memory responses.
Because antigenic memory persists in vivo for many years,1 the finding that CD8 T-cell memory responses can revert to resemble those of naive cells was surprising. Unlike isolated memory CD8 T cells, cells cultured in the presence of DC maintained their cell-cycle status and rapid functional recall to activation due to occupancy of the TNFR superfamily members CD27 and 4-1BB with their corresponding ligands. Previous studies have documented a reversion from the CD45RO (memory) phenotype to higher molecular weight (naive) CD45 species on memory CD4 T cells in vivo, which in a rat adoptive transfer model could be prevented by exposure to antigen.36,37 CD4 cells that acquired the naive CD45 isoform were still able to provide antigen-specific B-cell help, arguing that CD45 isoform expression is not a reliable marker of memory function.36 The present work, in contrast, deals with the physical and functional characteristics of the memory state, and its dependence on signaling via TNFR family members in vitro and in vivo.
CD27 has roles in both primary and secondary immune responses. Infection of CD27-deficient mice with influenza virus resulted in reduced numbers of T cells in infected tissues and the spleen during the primary response, at least in part because signaling via CD27 enhanced survival of TCR-stimulated cells.13 The effect on the secondary response was even more profound, the recruitment of T cells in CD27-deficient mice being similar to that in the primary response of WT animals. The difference between primary and secondary responses was even greater when CD27-deficient mice were infected with LCMV.9 In this case, the primary response was normal, as measured by the expansion of tetramer-positive CD8 cells and control of virus. Notably, despite equivalent numbers of antigen-specific tetramer-positive and CD44high memory T cells, upon viral rechallenge the CD27-deficient mice cleared the virus much less well, and the antigen-specific cells expanded only half as well, as the WT. These observations are consistent with our finding that infection of CD27-deficient mice with VSV stimulated a normal early response, but that antigen-specific CD8 memory T cells had difficulty maintaining their G1 status. Mice in which B cells express a CD70 transgene exhibit the complementary phenotype, with gradual MHC-dependent conversion of naive T cells to TEM cells, enhanced TEM cell generation after influenza infection, and survival when challenged with lethal doses of a poorly immunogenic tumor.38,39
The 4-1BB is expressed at low levels on memory CD8, but not naive T cells.10 Consistent with this, the initial expansion of antigen-specific T cells in influenza-infected 4-1BBL–deficient mice was normal,40 but there was a long-term decrease in the antigen-specific CD8 T memory pool size and the secondary response was no better than the primary. In another study, in vitro secondary responses of CD8 T cells from influenza-infected 4-1BBL–deficient mice were diminished.7 Moreover, despite a normal rate of homeostatic proliferation, fewer in vitro generated memory CD8 cells were recovered after being adoptively transferred into 4-1BBL−/− compared with WT recipients.41 As opposed to these loss-of-function approaches, stimulation via 4-1BB has been found to enhance CD8 T-cell immune responses and the generation of long-term memory.42 Our finding that blocking the interaction of 4-1BB with its ligand partially prevented maintenance of memory CD8 cells in G1 suggests at least one mechanism by which it functions to enhance secondary responses.
It is unlikely that programming of naive T cells to assume a memory phenotype is dependent on TNFR superfamily members, because memory T-cell development was unaffected by the absence or antibody blockade of these molecules.7,13,43 Therefore, the effect of these molecules on memory T cells seems to occur after the effector stage. We suspect that there are other signals in vivo that can contribute to the maintenance of the memory state. One such example may be interleukin-15 (IL-15), which is a critical mediator of CD8 T-cell survival.44 In fact, we have found that the addition of IL-15 to cultured memory T cells also prevents reversion to the G0 state, and causes some CD8 T cells to enter S phase (A.A. and J.D.A., unpublished observation). It seems likely that there are a variety of in vivo cues that promote maintenance of the G1 state, and thus, rapid recall to reactivation. It is intriguing that once memory T cells reverted, they maintained their G0 state when introduced into a naive host. This raises the possibility that causing memory cells to revert might moderate aggressive secondary responses while leaving responses by the reverted naive T cells intact, providing a possible alternative to immunosuppression to treat disorders involving chronic ongoing immune responses. In any case, the results in this study indicate that the functional characteristics that define the state of memory T cells are not simply the result of differentiation, but require active signaling to be maintained.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Rafi Ahmed, Donald R. Latner, and Koichi Araki (Emory University) for providing us with the LCMV Armstrong strain and advice.
This work was supported by the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute.
National Institutes of Health
Authorship
Contribution: A.A. and J.D.A. designed the study; A.A., D.B.C., M.L.G.T., and R.T.S. performed the research; A.A., D.B.C., M.L.G.T., I.M., R.T.S., A.L.M., and J.D.A. analyzed the data; H.Y. generated and purified critical antibodies; and A.A. and J.D.A. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan D. Ashwell, National Cancer Institute, National Institutes of Health, Bldg 37, Rm 3002, Bethesda, MD 20892; e-mail: jda@pop.nci.nih.gov.

![Figure 1. Memory CD8 T cells are of intermediate density and in G1. Splenic T cells were purified and fractionated on a Percoll density gradient. (A) Cell size (forward scatter [FSC]) and cell-cycle analysis of gated CD8 T cells in the indicated fractions were determined by flow cytometry. The horizontal bar in the right panels represents the amount of background fluorescence emitted from Hoechst-stained cells in the pyronin Y channel. The percentages of cells in the regions corresponding to G0, G1, and S/G2 plus M are indicated. (B) Cell surface expression of CD44, CD62L, and Ly6C on CD8 T cells in the indicated fractions. (C) IFN-γ production and CD69 expression by intermediate- and high-density CD8 T cells stimulated with (solid line) or without (shaded histogram) PMA/Iono (left panel). (D) T cells were enriched from LCMV-immunized mice and fractionated on a Percoll density gradient. The distribution of gp33-41 tetramer-positive CD8 T cells in the intermediate- and high-density fractions is shown in the left panel, and the cells in the gated areas were analyzed for size (middle panel) and cell-cycle state (right panels). (E) Cell surface expression of the indicated markers on intermediate-density gp33-41 tetramer-positive CD8 T cells versus high-density CD8 cells. (F) IFN-γ production and CD69 expression by intermediate-density gp33-41 tetramer-positive and high-density CD8 T cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/10/10.1182_blood-2009-05-220087/4/m_zh89990941690001.jpeg?Expires=1766081320&Signature=xfGJkVRCfmjk3C4pgQcbMDp6JpRgeO2FkXgWsMO83aNcbbscXBZ~8GiUEDRt8JY2MJR6hyWNphnmTKlW3lkJJF3~QjJkt1MU-Tfai5f24ajnl1wGGHP~huR42iVt5n~Woewhv1TFPxzdqBbcOEHW8QjJRkEHsz3I4tkUUjH2~RU5wzVRz4kCx-2KrriCD5OXCq71XTY24u5epsUFMfGp8mWXFh7K3ng~dmIU35U81bFB~bVqks0I5B7WSM2w~pYNIeahe5GJbtUgnUh9hTcYzyx7hIDoTA01dqRwIjcfi1ThKrOlgl8kjl23rR-vcJvuHtg5RIUatkYy1nhH5Oj-qQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. CD8 T-cell memory characteristics are reversible. (A) Cell size (forward scatter [FSC]) and pyronin Y RNA) staining of intermediate and high-density CD8 T cells before and after in vitro incubation for 24 and 48 hours. (B) IFN-γ production by intermediate and high-density CD8 T cells before and after in vitro incubation for 48 hours. Shaded histograms represent IFN-γ production by unstimulated cells. (C) Intermediate, high, and intermediate-density CD8 T cells that had been cultured in medium for 48 hours were labeled with CFSE. Cells were stimulated with plate-bound anti-CD3 for the indicated times, at which point CFSE dilution was determined by flow cytometry. (D) Cell-cycle analysis of intermediate-density, high-density, and 48-hour in vitro incubated intermediate-density (48-hour–intermediate) CD8 T cells stimulated with or without anti-CD3/CD28 for the indicated periods of time. (E) Cell cycle of high-density, intermediate-density gp33-41 tetramer-positive, and intermediate-density 48-hour cultured gp33-41 tetramer-positive CD8 T cells from mice previously infected with LCMV. (F) IFN-γ production by stimulated intermediate-density gp33-41 tetramer-positive and high-density CD8 T cells before and after incubation in medium for 48 hours. Shaded histograms represent isotype control. (G) The cultured intermediate-density cells shown in (E) were adoptively transferred into naive B6 recipients. Two weeks later, tetramer-positive CD8 T cells were analyzed for cell-cycle state (right panel). Freshly isolated high-density CD8 T cells are shown as a control (left panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/10/10.1182_blood-2009-05-220087/4/m_zh89990941690002.jpeg?Expires=1766081320&Signature=xJx0fTglMaKu0oKP5ndo3Gi8lCTD0M5TnIRM1uoxJpxlBzuONQ06HiyvEKcdTtpraUdQF2O8E5ZxF69B7kd4kBFDF-BzJbpON2vQbI86eL56X4pqup9dMN7Er2B-kt6-o~RWtoXzKfvGNBF56vDyg2FqF0pb3Mdzie~4tjWQjUfQwslzygmMnltjnXviOmjRAlA2DH52wum3dwp45Vy7lu7WpOw5KhgsA-nf8PbCjOT3lXDi1ovi6Fqmqx-v-96EB2DOjsANNNMu7x0wlhOls243M1L9A0DO2nxOeh8Jmk3uL0JwD7Bm0Smyqbj2a91rE3Hyxxg~eq8tfyeEQrEY1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 1. Memory CD8 T cells are of intermediate density and in G1. Splenic T cells were purified and fractionated on a Percoll density gradient. (A) Cell size (forward scatter [FSC]) and cell-cycle analysis of gated CD8 T cells in the indicated fractions were determined by flow cytometry. The horizontal bar in the right panels represents the amount of background fluorescence emitted from Hoechst-stained cells in the pyronin Y channel. The percentages of cells in the regions corresponding to G0, G1, and S/G2 plus M are indicated. (B) Cell surface expression of CD44, CD62L, and Ly6C on CD8 T cells in the indicated fractions. (C) IFN-γ production and CD69 expression by intermediate- and high-density CD8 T cells stimulated with (solid line) or without (shaded histogram) PMA/Iono (left panel). (D) T cells were enriched from LCMV-immunized mice and fractionated on a Percoll density gradient. The distribution of gp33-41 tetramer-positive CD8 T cells in the intermediate- and high-density fractions is shown in the left panel, and the cells in the gated areas were analyzed for size (middle panel) and cell-cycle state (right panels). (E) Cell surface expression of the indicated markers on intermediate-density gp33-41 tetramer-positive CD8 T cells versus high-density CD8 cells. (F) IFN-γ production and CD69 expression by intermediate-density gp33-41 tetramer-positive and high-density CD8 T cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/10/10.1182_blood-2009-05-220087/4/m_zh89990941690001.jpeg?Expires=1766115274&Signature=py-YaCgx6sgchpHfPTZrQ0B5i0uCAas6ZhSI2iW2XubFVRGWGmfWpqxGJQuc95OWncz-zvnco7mcIE2yR8Zk16kNIIv31lLvtj3eC~OlhMwVSOLSVkHl4GWNjBtmnRNesDzMsmrKIdnmPpx6Yg6VHkJS9yQdnj-AFDI026aQBeZvWWS30YgygDylJ-wyL76Z-7He2sjis38v0VF-K8rR7wsocxsZI5Stnq8e96FH1flkyEIQTfY4FVoA4A3J2T7uVrM-0SzBpdlocnk4jcGGYXt069aVAERy1anYe9YC~cpFNwRcfJVVxCCDnAteXOWwQP5JB1T34Lcs~1aKv4E6cA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)