In this issue of Blood, Romange and colleagues describe 1-7F9, a human monoclonal antibody targeting KIRs on NK cells.1 This antibody activates NK cells by blocking the interaction between inhibitory KIRs and target cell HLA class I molecules. This effect is demonstrated by the authors in vitro and in a mouse model of AML.
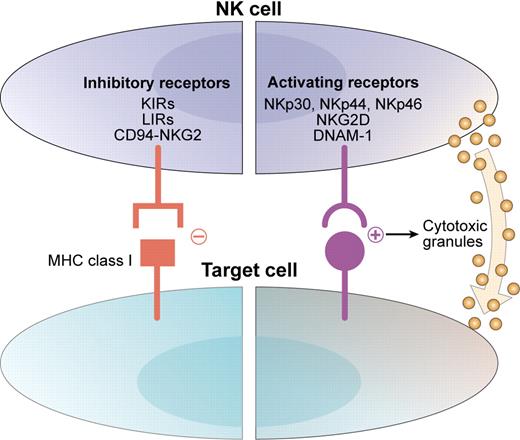
Natural killer (NK) cells are important members of the innate immune system and can exert antitumor effects. Their potential for lysis of malignant cells is a function of a dynamic balance between activation and inhibition (see figure).2 Interactions between activating receptors on NK cells and ligands on infected, transformed, or stressed cells results in enhanced NK-cell activity.3 Simultaneously, inhibitory receptors (such as inhibitory killer immunoglobulin-like receptors [KIRs]) interact with human leukocyte antigen (HLA) class I molecules on target cells to suppress autoreactivity. The disruption of this interaction by loss of appropriate class I molecules (as occurs in tumor or virally infected cells) is thought to disinhibit NK cells and forms the basis for the “missing-self” hypothesis of NK-cell tumor immunity.4
NK-cell reactivity is determined by the balance between activating and inhibitory signals. Activating receptors such as the natural cytotoxicity receptors (NKp30, NKp44, and NKp46), NKG2D, and DNAM-1 interact with ligands on target cells and direct the NK cell toward cytotoxicity. Inhibitory receptors such as inhibitory KIRs, leukocyte Ig-like receptor (LIR), and CD94/NKG2A interact with MHC class I molecules and protect autologous targets from NK cytotoxicity. Adapted from Stagg et al. Drug News Perspect. 2007;20(3):155-163. Copyright © 2007 Prous Science, SA. All rights reserved.
NK-cell reactivity is determined by the balance between activating and inhibitory signals. Activating receptors such as the natural cytotoxicity receptors (NKp30, NKp44, and NKp46), NKG2D, and DNAM-1 interact with ligands on target cells and direct the NK cell toward cytotoxicity. Inhibitory receptors such as inhibitory KIRs, leukocyte Ig-like receptor (LIR), and CD94/NKG2A interact with MHC class I molecules and protect autologous targets from NK cytotoxicity. Adapted from Stagg et al. Drug News Perspect. 2007;20(3):155-163. Copyright © 2007 Prous Science, SA. All rights reserved.
Exploiting NK-cell reactivity for tumor killing is well supported by preclinical and clinical data. Improvements in relapse rate and graft-versus-host disease (GVHD) have been reported among acute myeloid leukemia (AML) patients undergoing hematopoietic stem cell transplantation with donor cells whose KIRs are mismatched against recipients' HLA class I ligands.5 However, prospectively finding appropriately alloreactive NK cells (based on KIR and HLA class I typing) in conjunction with an adequately matched stem cell allograft is difficult. The current study of antibody 1-7F9 targeting inhibitory KIRs therefore demonstrates a promising approach to exploiting NK-cell antitumor therapy, without the need to identify an appropriate KIR-HLA mismatch, by manipulating the NK cell itself toward autoreactivity rather than acquiring an alloreactive NK pool.
The 1-7F9 antibody binds the inhibitory KIRs 2DL1, -2 and -3 on NK cells and subsequently blocks their interaction with the HLA class I molecules on target cells. This theoretically “disinhibits” the NK cells, rendering them cytotoxic to target tumor cells. The authors' hypothesis is supported by findings in vitro and in 2 in vivo models. Strikingly, pretreatment with 1-7F9 and HLA-C–matched NK cells prevents development of AML and prolongs survival after infusion of human AML blasts in nonobese diabetic/severe combined immunodeficient (NOD-SCID) mice.
The potential for clinical application of this antibody is substantial as KIRs 2DL1, -2, and -3 are each expressed in more than 80% of humans and in a variety of ethnic populations.6 In addition, the antibody is an IgG4 isotype, which does not readily bind complement and minimally binds CD64 (FcγRI) on monocytes, limiting the potential for antibody-mediated NK-cell depletion. Finally, the binding of 1-7F9 appears to successfully block the KIR-class I interaction while maintaining the integrity of the NK–target cell interaction.
On a deeper level, the positive findings in this study suggest a therapeutic window—a superbly delicate balance between activating and inhibitory signals of NK cells that may be exploited to target tumor cells exclusively. That is, tumor cells (in this case, AML) are on the brink of eliciting reaction from NK cells and are being protected by that delicate but specific bond between the KIR and the class I molecule. Furthermore, the data in this study support the “missing-self hypothesis”: NK-mediated tumor immunity is, at least in part, a function of the mismatch between KIR and HLA. The clinical relevance of this concept has been clouded by the variability of receptors within each patient's NK-cell repertoire and the inconsistent data linking clinical outcome and KIR-HLA mismatch.7 The success of this antibody suggests that KIR-HLA mismatch is a necessary mechanism of NK-cell immunosurveillance: the 1-7F9 antibody is so specific that any increase in cytotoxicity is likely due to the disruption of the KIR-HLA class I interaction.
There are some questions raised by this study. How do the disinhibiting effects of this antibody compare with the cytotoxicity of KIR-HLA–mismatched NK cells in an allotransplantation setting? Will autoreactivity of NK cells activated by 1-7F9 be a clinical problem? Finally, the idea of using autologous NK cells for this therapy may be limited purely by the number of available NK cells in the patient after chemotherapy for a hematologic malignancy.
The 1-7F9 antibody presents a unique opportunity to shift the paradigm from searching for alloreactive NK cells to activating autoreactive NK cells. From the perspective of cost-effectiveness and ease, this is an attractive strategy. From the perspective of maximizing cytotoxicity, however, this therapy may not open the therapeutic window wide enough. Further manipulations could include ex vivo expansion, activation, and addition of NK-modulating therapies such as lenalidomide. Nevertheless, this article provides exciting new possibilities for the field of NK-cell therapy.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■