Abstract
Chronic granulomatous disease (CGD) patients have impaired nicotinamide adenine dinucleotide phosphate (NADPH) oxidase function, resulting in poor antimicrobial activity of neutrophils, including the inability to generate neutrophil extracellular traps (NETs). Invasive aspergillosis is the leading cause of death in patients with CGD; it is unclear how neutrophils control Aspergillus species in healthy persons. The aim of this study was to determine whether gene therapy restores NET formation in CGD by complementation of NADPH oxidase function, and whether NETs have antimicrobial activity against Aspergillus nidulans. Here we show that reconstitution of NET formation by gene therapy in a patient with CGD restores neutrophil elimination of A nidulans conidia and hyphae and is associated with rapid cure of preexisting therapy refractory invasive pulmonary aspergillosis, underlining the role of functional NADPH oxidase in NET formation and antifungal activity.
Introduction
Activated neutrophils kill microbes intracellularly after phagocytosis and by extracellular mechanisms, including neutrophil extracellular traps (NETs), which are composed of chromatin decorated with granular proteins.1 NETs bind bacteria1 and fungi2 and expose antimicrobial molecules. Generation of NETs requires reactive oxygen species produced by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.3
Chronic granulomatous disease (CGD) is caused by mutations in genes encoding NADPH oxidase subunits. CGD patients do not produce reactive oxygen species, kill microbes poorly, and are susceptible to recurrent life-threatening infections.4 Aspergillus spp infections cause pneumonia and disseminated disease and are the leading cause of death in these patients.4-6
It is unclear how Aspergillus infections are controlled in healthy persons.7-13 In CGD patients, these infections are frequently refractory to antifungal therapy, treatment with interferon-γ, or granulocyte transfusions.5 Here we show that the recently discovered NADPH oxidase-dependent microbicidal pathway through NETs1-3 is efficient against Aspergillus nidulans conidia and hyphae in vitro and that restoration of NET formation by GT of X-CGD aided clearing severe invasive A nidulans infection in vivo.
Methods
Gene therapy
We treated an 8.5-year-old boy with X-linked gp91phox-deficient CGD and therapy refractory A nidulans lung infection with a monocistronic long terminal repeat-driven gamma-retroviral SF71gp91phox vector (see supplemental data, available on the Blood website; see the Supplemental Materials link at the top of the online article). The protocol for the patient's treatment was approved by the ethics review board of the University Children's Hospital Zurich and the Swiss Expert Committee for Bio-Safety, after written informed consent from his parents in accordance with the Declaration of Helsinki. For follow-up monitoring, gp91phox expression was measured by fluorescence-activated cell sorter (FACS) on peripheral neutrophils after 30 minutes of staining at room temperature with 10 μg/mL gp91phox-fluorescein isothiocyanate (FITC) antibody (Anti-Flavocytochrome b558, clone 7D5, MBL). NADPH oxidase activity was measured by standard dihydrorhodamine and nitroblue tetrazolium tests (supplemental materials). Bone marrow colony assays and determination of proviral gp91phox sequences in genomic DNA were performed as described.14
NET induction
NET formation was visualized as described (supplemental materials) and quantified after stimulation of 5 × 104 neutrophils for 3 hours with 40 nM phorbol 12-myristate 13-acetate (PMA) and staining the NET-DNA with 1 μM Sytox green (Invitrogen) in a black 96-well plate (BD Biosciences). The plates were read in a fluorescence microplate reader (Victor,3 PerkinElmer Life and Analytical Sciences) with a filter setting of 485 nm/535 nm (excitation/emission).
NET antifungal activity
The A nidulans strain used was isolated from bronchoalveolar lavage fluid of the patient; conidia were grown and collected as described.7 Neutrophils after GT were stained with gp91phox-FITC antibody and sorted by FACS (FACSAria, BD Biosciences) into gp91phox-negative (gp91phox−) and -positive (gp91phox+) populations. A total of 105 neutrophils were activated with PMA (40 nM) at 37°C for 4 hours in a 96-well plate, and then infected with conidia (multiplicity of infection A nidulans/neutrophils = 0.5) plus or minus prior digestion of NETs with 10 U/mL of Micrococcal Nuclease (MNase, Worthington Biochemical) for 30 minutes. Afterward the plates were centrifuged for 5 minutes at 400g and incubated for 16 hours at 37°C, allowing germination and hyphal outgrowth. Alternatively, 5 × 104 conidia were incubated for 12 hours at 37°C to allow hyphal outgrowth; then 105 neutrophils were added, centrifuged for 5 minutes at 400g, and incubated for 2 and 5 hours with PMA (40 nM) to induce NET formation, plus or minus 10 U/mL MNase. Fungal growth was quantified with XTT (Invitrogen) as described.15
Results and discussion
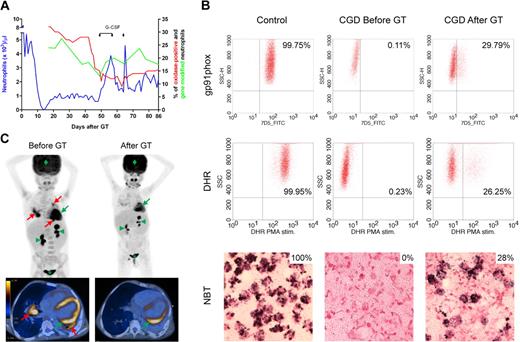
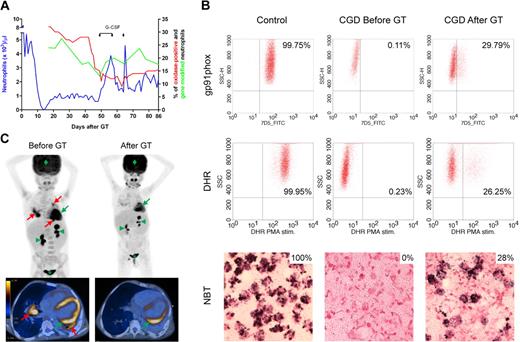
In healthy subjects, NETs might be essential to eliminate fungi because hyphae are too large to be phagocytosed.7,9,12,13,16-21 CGD patients are unable to make NETs.3 Indeed, neutrophils of our X-CGD patient could only make NETs and inhibit growth of A nidulans after genetic complementation by GT. Neutrophils expressing functional gp91phox increased from 0% to 26% to 29% 6 weeks after GT (Figure 1A-B), then decreased and leveled at approximately 16% for up to 3 months. The A nidulans infection completely cleared 6 weeks after GT (Figure 1C), correlating with the rise in neutrophils with NADPH oxidase activity.
Restoration of NADPH oxidase function. (A) Hematopoietic reconstitution and gene marking after GT. Absolute neutrophil counts (left y-axis), quantification of gene-modified cells in peripheral neutrophils by quantitative polymerase chain reaction and quantification of neutrophils with NADPH oxidase activity by DHR test (right y-axis) are shown. When the percentage of transduced neutrophils decreased, granulocyte colony-stimulating factor (5 μg/kg per day subcutaneously) was administered on days 49 to 57 and on day 64. (B) Reconstitution of NADPH oxidase activity. Before and 6 weeks after GT, gp91phox protein expression was measured by FACS analysis after 30 minutes of staining with 10 μg/mL gp91phox-FITC antibody. Superoxide production was assessed by oxidation of DHR on stimulation with PMA and by reduction of nitroblue tetrazolium to formazan (dark precipitate) after stimulation with opsonized zymosan. The thresholds were determined using unstained (FACS) or unstimulated (DHR) cells for each experiment. (C) PET-CT scan. Before GT, PET-CT scan showed several active infectious foci with fluorine-18-fluoro-2-deoxy-D-glucose uptake in both lungs of the patient (red arrows); the infection cleared 6 weeks after administration of gene-corrected cells. In green, physiologic FDG uptake in heart (arrow), kidneys (arrowheads), bladder (*), and brain (diamond) are indicated for reference.
Restoration of NADPH oxidase function. (A) Hematopoietic reconstitution and gene marking after GT. Absolute neutrophil counts (left y-axis), quantification of gene-modified cells in peripheral neutrophils by quantitative polymerase chain reaction and quantification of neutrophils with NADPH oxidase activity by DHR test (right y-axis) are shown. When the percentage of transduced neutrophils decreased, granulocyte colony-stimulating factor (5 μg/kg per day subcutaneously) was administered on days 49 to 57 and on day 64. (B) Reconstitution of NADPH oxidase activity. Before and 6 weeks after GT, gp91phox protein expression was measured by FACS analysis after 30 minutes of staining with 10 μg/mL gp91phox-FITC antibody. Superoxide production was assessed by oxidation of DHR on stimulation with PMA and by reduction of nitroblue tetrazolium to formazan (dark precipitate) after stimulation with opsonized zymosan. The thresholds were determined using unstained (FACS) or unstimulated (DHR) cells for each experiment. (C) PET-CT scan. Before GT, PET-CT scan showed several active infectious foci with fluorine-18-fluoro-2-deoxy-D-glucose uptake in both lungs of the patient (red arrows); the infection cleared 6 weeks after administration of gene-corrected cells. In green, physiologic FDG uptake in heart (arrow), kidneys (arrowheads), bladder (*), and brain (diamond) are indicated for reference.
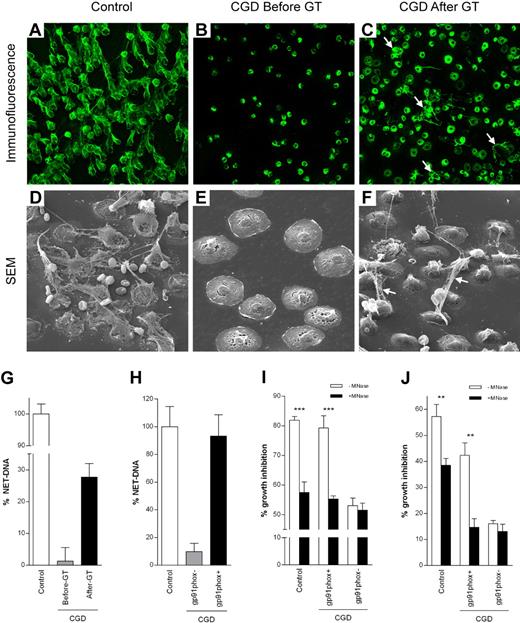
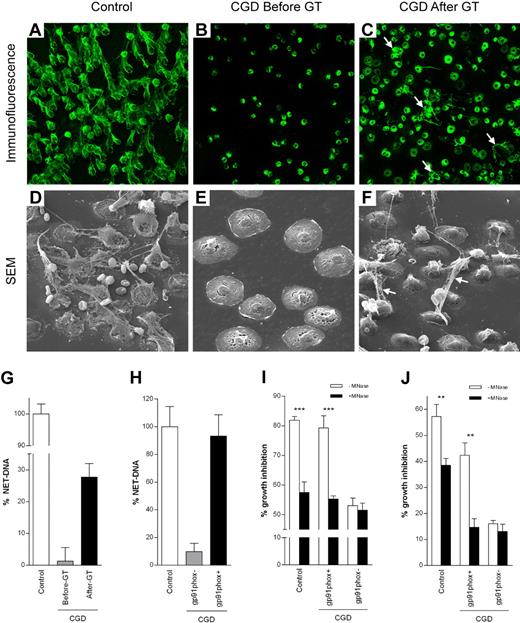
The patient's neutrophils did not make NETs before GT as analyzed by fluorescence (not shown), immunofluorescence, and scanning electron microscopy (Figure 2A-B, D-E).3 After GT, the patient's neutrophils made NETs (Figure 2C,F), the percentage of cells releasing NET-DNA (28%; Figure 2G) correlating with the level of oxidase chimerism (Figure 1B). Activation of sorted neutrophils showed that reconstitution with functional NADPH oxidase allowed corrected CGD neutrophils to make NETs (Figure 2H).
NET formation and inhibition of A nidulans growth. Control (A,D), but not CGD (B,E), neutrophils made NETs on 3-hour PMA stimulation. For immunofluorescence, NETs were stained with an antibody that recognizes neutrophil elastase (green; A-C). NETs were clearly visible also by scanning electron microscopy (SEM; D-F). Neutrophils isolated from the CGD patient before GT could be activated because they flattened out (E) but did not make NETs. The ability to form NETs was partially restored by GT 6 weeks after GT (C,F white arrows). (G) Quantification of NET-DNA released after 3 hours of PMA stimulation of control neutrophils, CGD neutrophils before and 6 weeks after GT or (H) after stimulation of CGD gp91phox+ and CGD gp91phox− FACS-sorted neutrophils. CGD gp91phox+ neutrophils showed normal NET formation, whereas CGD gp91phox− neutrophils showed only residual NET formation. FACS-sorting efficiency was 90% to 92% for CGD gp91phox− and 95% to 96% for CGD gp91phox+ cells. (I-J) NET inhibition of A nidulans conidia and hyphae. (I) Conidia were plated on FACS-sorted neutrophils prestimulated with PMA plus or minus MNAse (ie, when NET formation was complete, cells were dead and therefore incapable of phagocytosis). Hyphal outgrowth was measured after 16 hours. (J) Hyphae were coincubated with FACS-sorted neutrophils, and PMA plus or minus MNAse and hyphal viability was assessed after 5 hours. (G-J) Data are mean ± SD of a representative triplicate experiment. Inhibition of fungal growth is expressed as percentage of control values (A nidulans conidia or hyphae incubated in media). The differences between −MNase and +MNase were significant (for control and CGD gp91phox+ cells) by Student t test: **P < .01; ***P < .001.
NET formation and inhibition of A nidulans growth. Control (A,D), but not CGD (B,E), neutrophils made NETs on 3-hour PMA stimulation. For immunofluorescence, NETs were stained with an antibody that recognizes neutrophil elastase (green; A-C). NETs were clearly visible also by scanning electron microscopy (SEM; D-F). Neutrophils isolated from the CGD patient before GT could be activated because they flattened out (E) but did not make NETs. The ability to form NETs was partially restored by GT 6 weeks after GT (C,F white arrows). (G) Quantification of NET-DNA released after 3 hours of PMA stimulation of control neutrophils, CGD neutrophils before and 6 weeks after GT or (H) after stimulation of CGD gp91phox+ and CGD gp91phox− FACS-sorted neutrophils. CGD gp91phox+ neutrophils showed normal NET formation, whereas CGD gp91phox− neutrophils showed only residual NET formation. FACS-sorting efficiency was 90% to 92% for CGD gp91phox− and 95% to 96% for CGD gp91phox+ cells. (I-J) NET inhibition of A nidulans conidia and hyphae. (I) Conidia were plated on FACS-sorted neutrophils prestimulated with PMA plus or minus MNAse (ie, when NET formation was complete, cells were dead and therefore incapable of phagocytosis). Hyphal outgrowth was measured after 16 hours. (J) Hyphae were coincubated with FACS-sorted neutrophils, and PMA plus or minus MNAse and hyphal viability was assessed after 5 hours. (G-J) Data are mean ± SD of a representative triplicate experiment. Inhibition of fungal growth is expressed as percentage of control values (A nidulans conidia or hyphae incubated in media). The differences between −MNase and +MNase were significant (for control and CGD gp91phox+ cells) by Student t test: **P < .01; ***P < .001.
To test whether efficient eradication of the patient's infection was the result of the recovered ability to make NETs, neutrophils were infected with the A nidulans strain isolated from the patient. Approximately 80% of conidia germination (Figure 2I) and 45% of hyphal growth (Figure 2J) were inhibited by CGD gp91phox+ neutrophils, comparable with the antimicrobial activity of control neutrophils. CGD gp91phox− neutrophils were inefficient in controlling fungal growth (Figure 2I-J, supplemental Figure 1). When NETs were dismantled with MNase before infection, fungicidal activity was abrogated to that of CGD gp91phox− neutrophils.
In the absence of NETs, control of hyphal growth was independent of NADPH oxidase activity: when neutrophils were infected after 2-hour PMA stimulation, before NETs had been made, CGD gp91phox+, CGD gp91phox−, and neutrophils from healthy donor controlled growth of A nidulans with similar modest efficiency (Figure S2) in a NET-independent fashion because antimicrobial activity was not affected by MNase. This limited NET-independent antimicrobial activity, presumably by conidia phagocytosis, degranulation, or unknown mechanisms, suggests an NADPH oxidase-independent antifungal mechanism, clinically ineffective before GT. These data propose that the patient's clearance of fungal infection after GT was controlled by NETs. Definitive in vivo proof is obviously technically impossible.
Alveolar macrophages probably constitute the first line of defense to conidia that escape mucociliary clearance in healthy persons.13,16 Whether reconstituted NADPH oxidase function in alveolar macrophages also contributed to microbial killing in the patient presented is difficult to assess. In neutrophils, however, conidia resist intracellular killing because of their relative tolerance to reactive oxygen species.7,9,12,19-22 Our results suggest that conidia are killed mainly extracellularly rather than after phagocytosis. Both conidia and hyphae get ensnared by neutrophils and probably killed within NETs by concentrated antimicrobials. Cooperation of gp91phox− and gp91phox+ neutrophils in NET antifungal activity is doubtful because we showed that gp91phox− neutrophils do not make NETs when coincubated with gp91phox+ neutrophils (unsorted cells in Figure 2F-G),10 suggesting that the amount of H2O2 released by gp91phox+ neutrophils is insufficient to induce NETs in gp91phox− cells.3
A GT approach to treat CGD may be used to overcome recalcitrant, life-threatening infections but is currently limited as salvage therapy to experimental studies in selected patients with very poor performance status and lacking an human leukocyte antigen-identical hematopoietic stem cell donor.4 GT was rapidly beneficial to our CGD patient who had suffered from an otherwise incurable fungal infection. Until day 86 after GT, there was no clonal dominance in bone marrow culture-derived CD34+ cells (not shown) or expansion of gene-corrected cells in blood (Figure 1A). There is a risk, however, of insertional mutagenesis by transactivating retroviral vector insertions into proto-oncogenes, as shown in a recent GT trial with 2 adult CGD patients who developed monosomy 7 and myelodysplastic syndrome (M. Grez, Institute of Biomedical Research, Georg-Speyer-Haus, Frankfurt, Germany, oral communication, April 2009) using the same gamma-retroviral SF71gp91phox vector.14 In addition, 5 patients developed leukemia in 2 GT trials in children with severe combined immunodeficiency.23,24 These experiences mandate the careful follow-up of patients.
In conclusion, we show that the severe immunodeficient phenotype and the high susceptibility to Aspergillus infection of CGD patients might be linked to the absence of NETs, and that restoration of NADPH oxidase function and NET formation by GT leads to rapid cure of refractory invasive aspergillosis in X-linked CGD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patient and his family for their trust; the medical and nursing staff of the bone marrow transplantation unit of University Children's Hospital Zurich; Manuel Grez and Klaus Kühlke for developing and providing the SF71gp91phox vector, respectively; Maja Rutishauser, Corinne Wenk, and Oralea Büchi for technical assistance; Ursula Lüthi and Klaus Marquardt for electron microscopy; Britta Laube for help with immunofluorescence imaging; Alex Imhof for isolating A nidulans conidia; and Hans Steinert for carrying out PET-CT scans.
This work was supported by a grant of the Chronic Granulomatous Disorder Research Trust, United Kingdom (J.R., M.B.), a Forschungskredit der Universität Zürich 2006 grant (J.R., M.B.), and a grant from the Stiftung für wissenschaftliche Forschung an der Universität Zürich/Baugarten Stiftung (R.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: M.B. and A.H. performed the experiments, analyzed data, and contributed to the writing of the manuscript; V.B. did the immunofluorescence image acquisition and contributed to data analysis; U.S. did the bone marrow cultures and contributed to data analysis; R.A.S. designed the clinical gene therapy protocol, attended the patient together with J.R., and contributed to writing of the manuscript; and A.Z. and J.R. designed and directed the study and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janine Reichenbach, Division of Immunology/Haematology/BMT, University Children's Hospital Zurich, Steinwiesstrasse 75, 8032 Zurich, Switzerland; e-mail: janine.reichenbach@kispi.uzh.ch; or Arturo Zychlinsky, Department of Cellular Microbiology, Max Planck Institute for Infection Biology, Charitéplatz 1, Berlin 10117, Germany; e-mail: zychlinsky@mpiib-berlin.mpg.de.
References
Author notes
*M.B. and A.H. contributed equally to this study.
†A.Z. and J.R. contributed equally to this study.