Abstract
Sickle cell disease (SCD) is associated with a high incidence of ischemic stroke. SCD is characterized by hemolytic anemia, resulting in reduced nitric oxide-bioavailability, and by impaired cerebrovascular hemodynamics. Cerebrovascular CO2 responsiveness is nitric oxide dependent and has been related to an increased stroke risk in microvascular diseases. We questioned whether cerebrovascular CO2 responsiveness is impaired in SCD and related to hemolytic anemia. Transcranial Doppler-determined mean cerebral blood flow velocity (Vmean), near-infrared spectroscopy-determined cerebral oxygenation, and end-tidal CO2 tension were monitored during normocapnia and hypercapnia in 23 patients and 16 control subjects. Cerebrovascular CO2 responsiveness was quantified as Δ% Vmean and Δμmol/L cerebral oxyhemoglobin, deoxyhemoglobin, and total hemoglobin per mm Hg change in end-tidal CO2 tension. Both ways of measurements revealed lower cerebrovascular CO2 responsiveness in SCD patients versus controls (Vmean, 3.7, 3.1-4.7 vs 5.9, 4.6-6.7 Δ% Vmean per mm Hg, P < .001; oxyhemoglobin, 0.36, 0.14-0.82 vs 0.78, 0.61-1.22 Δμmol/L per mm Hg, P = .025; deoxyhemoglobin, 0.35, 0.14-0.67 vs 0.58, 0.41-0.86 Δμmol/L per mm Hg, P = .033; total-hemoglobin, 0.13, 0.02-0.18 vs 0.23, 0.13-0.38 Δμmol/L per mm Hg, P = .038). Cerebrovascular CO2 responsiveness was not related to markers of hemolytic anemia. In SCD patients, impaired cerebrovascular CO2 responsiveness reflects reduced cerebrovascular reserve capacity, which may play a role in pathophysiology of stroke.
Introduction
Cerebral infarction is one of the most devastating complications of sickle cell disease (SCD), occurring in approximately 10% of patients in the first 2 decades of life.1-3 Furthermore, silent cerebral infarctions occur in approximately 17% of pediatric patients4,5 and are associated with poor educational and cognitive functioning.6 SCD is characterized by chronic hemolytic anemia and ongoing vaso-occlusion with exacerbations often requiring medical care.7-9 The vaso-occlusive process in SCD is of a complex nature mediated by red cell and leukocyte adhesion, inflammation, oxidative stress, and a hypercoagulable state, all resulting in endothelial injury and dysfunction.8 In addition, by reducing the nitric oxide (NO) bioavailability and by damaging the endothelium through the catalyzation of oxidative reactions in endothelial cells, chronic hemolysis leads to vascular complications.10-12
Elevated cerebral blood flow (CBF) velocity (≥ 200 cm/s), measured by transcranial Doppler (TCD), has been identified as a risk factor for stroke in SCD.13 However, little is known about the mechanism of (ischemic) stroke in SCD patients, and it has not been elucidated whether the increased CBF velocity plays a causative role in stroke or whether it is a result of SCD-related hemodynamic disturbances.
CBF is tightly regulated to maintain constancy of cerebral perfusion in the face of various systemic blood pressures by both local mechanisms and autonomic control (cerebral autoregulation).14-16 Recently, we demonstrated that dynamic cerebral autoregulation is impaired in SCD.17 Independent from cerebral autoregulation, the influence of the partial arterial carbon dioxide (CO2) tension on CBF is of importance. Under normal conditions, increments and decrements of the partial arterial and thus end-tidal CO2 tension (PETCO2) increase and decrease CBF by cerebral vasodilatation and vasoconstriction, respectively.18 This phenomenon is known as the cerebrovascular CO2 responsiveness and reflects the vasodilatatory capacity of the cerebral vasculature or cerebrovascular reserve capacity.19-22 A reduced cerebrovascular reserve capacity has been demonstrated to be an independent predictor of cerebrovascular ischemic events.23-25
Cerebrovascular CO2 responsiveness has been demonstrated to be impaired in patients with endothelial dysfunction and cerebral microangiopathy, possibly the result of a reduced NO bioavailability.26-28 Because SCD is associated with increased risk of stroke and characterized by low NO bioavailability resulting from its decreased generation (endothelial dysfunction)29 and increased scavenging by cell-free heme (chronic hemolysis),10,11 we tested the hypothesis that the cerebrovascular CO2 responsiveness is impaired in SCD and may be related to the degree of hemolysis.
The cerebrovascular CO2 responsiveness can be quantified by relating changes in TCD-determined middle cerebral artery (MCA) mean CBF velocity (Vmean) or near-infrared spectroscopy (NIRS) determined changes in frontal cortical hemoglobin concentrations to artificially increased PETCO2 as an estimate of arterial CO2 tension.30,31 In this study, we evaluated cerebrovascular CO2 responsiveness in SCD patients by simultaneously recording changes in both MCA Vmean and frontal cortical concentrations of oxygenated (oxyhemoglobin, [O2Hb]), deoxygenated (deoxyhemoglobin, [dHb]), and total hemoglobin ([t-Hb]) in response to hypercapnia.28,30
Methods
Study population
Consecutive SCD patients of the outpatient clinic of the department of Clinical Hematology, Academic Medical Center, Amsterdam and age-, gender-, and ethnicity-matched healthy normal hemoglobin AA controls were recruited for the study. Inclusion criteria for SCD patients were age more than or equal to 18 years and homozygous sickle cell anemia (HbSS) confirmed by high performance liquid chromatography. Exclusion criteria were history of stroke, hypertension (systolic > 140 mm Hg and/or diastolic > 90 mm Hg), use of cardiovascular medication, blood transfusion in the preceding 4 months, and acute vaso-occlusive events (painful crises, acute chest syndrome, sequestration crises, or priapism) in the preceding 4 weeks. All participants received verbal and written explanation of the objectives and procedure of the study and subsequently provided written informed consent. The study was approved by the AMC Medical Ethical Commission, and experiments were performed in accordance with the Declaration of Helsinki.
Recordings
The TCD (DWL Multidop X4)–derived CBF velocity was measured in the proximal segment of the MCA and insonated through the posterior temporal window. Once the optimal signal-to-noise ratio was obtained, the probe was secured with a headband (Marc 600; Spencer Technologies). MCA Vmean was used for evaluation of changes in CBF assuming that changes in MCA Vmean are representative of those in CBF. TCD monitors blood flow velocity rather than blood flow, and changes in the diameter of the insonated vessel could modulate velocity independently of flow. However, the MCA is a conductance rather than a resistance vessel, and changes in mean arterial pressure and CO2 have negligible effects on its luminal diameter.32,33
Changes in cerebral oxygenation were monitored using NIRS (Oxymon; Artinis Medical Systems BV) which, based on the transparency of tissue to light in the near-infrared region, detects changes in tissue chromophores, that is, mainly O2Hb and dHb. With the use of a modified Lambert-Beer law, changes in light absorption at different wavelengths are measured, and tissue oxygenation is monitored. To estimate changes in [O2Hb] and [dHb], a differential path length factor of 6.0 was applied to account for the scattering of light in the tissue. Changes in regional cerebral tissue oxygenation were followed by NIRS at wavelengths of 775 and 850 nm. [O2Hb] and [dHb] were recorded at 10 Hz with the light source and sensing optodes positioned on the ipsilateral side of the TCD insonation above the supraorbital ridge below the hairline, with an interoptode distance of 5.0 cm, and secured with a lightproof holder attached to a headband. NIRS determined brain capillary oxygenation is functionally related to the balance between arterial and jugular venous oxygen saturation. Changes in cerebral blood volume are reflected by changes in total hemoglobin concentration [t-Hb] expressed as the sum of [O2Hb] and [dHb]. Changes in [O2Hb] and [dHb] in micromolar are calculated with baseline (normocapnic resting) values as reference set at 0 μM.
PETCO2 was measured by a sampling infrared capnograph (Tonocap, Datex-Ohmeda). Brachial arterial blood pressure (BP) was measured with an automated noninvasive device (HEM-705CP; Omron). Peripheral transcutaneous O2 saturation was measured using a pulse oximeter (Novametrix 515A).
Protocol and measurements
Measurements were performed in supine position in a quiet environment with an ambient temperature of 22°C. All participants were asked to abstain from caffeinated beverages for at least 12 hours before measurement. After instrumentation, volunteers started breathing through a standard spirometry mouthpiece, with the lips sealed tightly around its edge to prevent air leakage. A nose peg was applied during the measurements to prevent nasal breathing. After 5 minutes of baseline normocapnic measurements, the mouthpiece was attached to a gas mixture of 5% CO2/95% O2. Changes in PETCO2 provided a continuous quality check of breathing through the mouthpiece. The participants continued breathing this hypercapnic gas mixture until the rising CBF velocity and cerebral [O2Hb], [dHb], and [t-Hb] signals reached a steady state.
Blood samples
Blood samples were drawn via venipuncture. Standard blood counts (Hb, hematocrit [Ht], leucocytes, platelets, and reticulocyte percentage) were performed in ethylenediaminetetraacetic acid blood (Cell-Dyn 4000; Abbott). Lactate dehydrogenase (LDH) and total bilirubin levels were measured in heparinized plasma with spectrophotometry (P800 Modular; Roche).
Data analysis
Signals of MCA blood flow velocity and PETCO2 were collected and analog-to-digital–converted with a sampling frequency of 100 Hz. NIRS data were analog-to-digital–converted with a sampling frequency of 10 Hz. All data were stored on hard disc for off-line analysis. Beat-to-beat values for MCA Vmean were derived as the integral over one beat divided by the corresponding beat interval.
Cerebrovascular CO2 responsiveness was expressed as percentage change of MCA Vmean per millimeter of mercury change in PETCO2 and as changes in [O2Hb], [dHb], and [t-Hb] per millimeter of mercury change in PETCO2 between normocapnia and hypercapnia.
Statistics
Based on the study of Lavi et al27 (with a calculated SD of 0.4, a difference in population means of 0.4 and type 1 error of 0.05), 19 patients and 14 controls had to be included in the study to attain 80% power for the difference in cerebrovascular CO2 responsiveness as determined by the TCD measured Vmean. Data are presented as medians with interquartile range, unless stated otherwise. Mann-Whitney U test was applied to test differences between the groups. The Spearman rank (rs) correlation coefficient was calculated for correlation studies. P values less than .05 were considered statistically significant (SPSS; Version 16.0).
Results
Patient data
Of 45 consecutive eligible homozygous (HbSS) SCD patients who visited our outpatient clinic, 23 (14 women; median age, 26 years; range, 21-42 years) agreed to participate in the study. Sixteen age-, sex-, and ethnicity-matched healthy controls (9 women; median age, 33 years; range, 24-40 years) were also included in the study. NIRS data of 9 patients and 3 controls were excluded from analysis because of an insufficient noise-to-signal ratio (sudden and recurrent spikes during the measurements; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). SCD patients had lower body mass index, diastolic BP, O2 saturation, Hb, and Ht and higher MCA Vmean, reticulocyte percentage, LDH, and total bilirubin levels (Table 1). Age, male:female ratio, and systolic BP were comparable between groups.
Cerebrovascular CO2 responsiveness
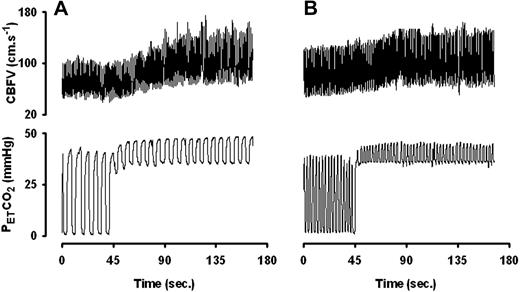
The cerebrovascular CO2 responsiveness determined with both TCD (Figure 1A) and NIRS (Figure 1B) was lower in SCD patients than in controls. Representative examples of continuous PETCO2 and CBF velocity recordings of a healthy control (Figure 2A) and a sickle cell patient (Figure 2B) are shown.
Cerebrovascular CO2 responsiveness (CCR) in SCD patients and healthy controls. (A) Cerebrovascular CO2 responsiveness expressed as relative change in mean CBF velocity (% ΔVmean) per millimeter of mercury change in PETCO2 (CCR-TCD) was lower in SCD patients (■, n = 23) than in healthy controls ( , n = 16). (B) Cerebrovascular CO2 responsiveness expressed as absolute changes (Δ μmol/L) in cerebral [O2Hb], [dHb], and [t-Hb] per millimeter of mercury PETCO2 (CCR-NIRS) were also significantly lower in SCD patients (■, n = 14) than in healthy controls (
, n = 16). (B) Cerebrovascular CO2 responsiveness expressed as absolute changes (Δ μmol/L) in cerebral [O2Hb], [dHb], and [t-Hb] per millimeter of mercury PETCO2 (CCR-NIRS) were also significantly lower in SCD patients (■, n = 14) than in healthy controls ( , n = 13). Data are mean ± SEM.
, n = 13). Data are mean ± SEM.
Cerebrovascular CO2 responsiveness (CCR) in SCD patients and healthy controls. (A) Cerebrovascular CO2 responsiveness expressed as relative change in mean CBF velocity (% ΔVmean) per millimeter of mercury change in PETCO2 (CCR-TCD) was lower in SCD patients (■, n = 23) than in healthy controls ( , n = 16). (B) Cerebrovascular CO2 responsiveness expressed as absolute changes (Δ μmol/L) in cerebral [O2Hb], [dHb], and [t-Hb] per millimeter of mercury PETCO2 (CCR-NIRS) were also significantly lower in SCD patients (■, n = 14) than in healthy controls (
, n = 16). (B) Cerebrovascular CO2 responsiveness expressed as absolute changes (Δ μmol/L) in cerebral [O2Hb], [dHb], and [t-Hb] per millimeter of mercury PETCO2 (CCR-NIRS) were also significantly lower in SCD patients (■, n = 14) than in healthy controls ( , n = 13). Data are mean ± SEM.
, n = 13). Data are mean ± SEM.
Representative continuous recordings of PETCO2 and CBF velocity (CBFV). (A) Healthy subject. (B) Sickle cell patient. In the patient, the increase in CBFV is less pronounced. Early leveling off for a comparable change in PETCO2 indicates a reduced cerebrovascular CO2 responsiveness.
Representative continuous recordings of PETCO2 and CBF velocity (CBFV). (A) Healthy subject. (B) Sickle cell patient. In the patient, the increase in CBFV is less pronounced. Early leveling off for a comparable change in PETCO2 indicates a reduced cerebrovascular CO2 responsiveness.
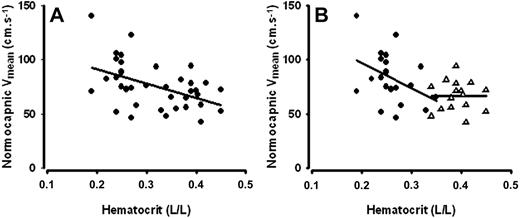
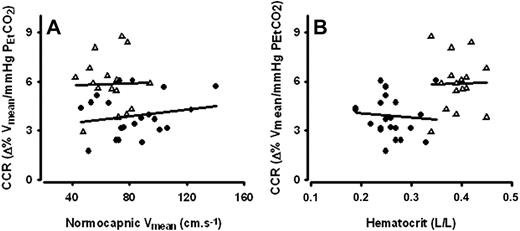
During normocapnia, Vmean correlated to Ht (rs = −0.46, P = .004; Figure 3A). When analyzed for the SCD and control groups separately, the correlations between normocapnic resting CBF velocity and Ht were rs = −0.40 (P = .068) and rs = −0.01 (P = .98), respectively (Figure 3B). Neither Ht nor Vmean at normocapnia was related to cerebrovascular CO2 responsiveness (Figure 4A and B, respectively). Other indicators of hemolytic anemia, including Hb, reticulocyte percentage, and LDH, were not related to cerebrovascular CO2 responsiveness either (data not shown). Cerebrovascular CO2 responsiveness was not related to age. Within the SCD group, patients using hydroxyurea tended to have a higher cerebrovascular CO2 responsiveness than those not on hydroxyurea, but only statistically different for [dHb] (Figure 5). Hemoglobin levels between SCD patients using and those not using hydroxyurea were comparable (9.1, 8.5-9.6 vs 9.0, 8.1-9.8). Hemoglobin F levels, available from earlier laboratory controls, were not related to cerebrovascular CO2 responsiveness (data not shown).
Normocapnic cerebral blood flow velocity (Vmean) in relation to hematocrit (Ht). (A) Normocapnic Vmean was inversely correlated to Ht across all participants (rs = −0.46, P = 0.004; n = 38). (B) The relationship between normocapnic Vmean and Ht was not statistically significant when the correlation analyses for the SCD patients (●; rs = −0.36, P = .098; n = 23) and healthy controls (Δ; rs = −0.01, P = .96; n = 16) were performed separately.
Normocapnic cerebral blood flow velocity (Vmean) in relation to hematocrit (Ht). (A) Normocapnic Vmean was inversely correlated to Ht across all participants (rs = −0.46, P = 0.004; n = 38). (B) The relationship between normocapnic Vmean and Ht was not statistically significant when the correlation analyses for the SCD patients (●; rs = −0.36, P = .098; n = 23) and healthy controls (Δ; rs = −0.01, P = .96; n = 16) were performed separately.
Cerebrovascular CO2 responsiveness (CCR) in relation to normocapnic cerebral blood flow velocity (Vmean) and hematocrit (Ht). Both in SCD patients (●; n = 23) and healthy controls (Δ; n = 16), CCR was not related to either (A) Vmean (rs = 0.07, P =.8 and rs = −0.05, P = .9 respectively) or (B) hematocrit (rs = −0.18, P = .4 and rs = 0.07, P = .8 respectively).
Cerebrovascular CO2 responsiveness (CCR) in relation to normocapnic cerebral blood flow velocity (Vmean) and hematocrit (Ht). Both in SCD patients (●; n = 23) and healthy controls (Δ; n = 16), CCR was not related to either (A) Vmean (rs = 0.07, P =.8 and rs = −0.05, P = .9 respectively) or (B) hematocrit (rs = −0.18, P = .4 and rs = 0.07, P = .8 respectively).
Cerebrovascular CO2 responsiveness (CCR) in SCD patients with and without hydroxyurea. (A) Cerebrovascular CO2 responsiveness expressed as relative change in mean CBF velocity (percentage ΔVmean) per millimeter of mercury change in PETCO2 (CCR-TCD) was higher in SCD patients using hydroxyurea (Hydroxy+,  , n = 10) than in those not using hydroxyurea (Hydroxy−, ■, n = 13), although the difference was not statistically significant. (B) Cerebrovascular CO2 responsiveness expressed as absolute changes (Δ μmol/L) in cerebral [O2Hb], [dHb], and [t-Hb] per millimeter of mercury PETCO2 were also higher in SCD patients using hydroxyurea (n = 7) than in those not using hydroxyurea (n = 7), although the difference was only statistically significant for dHb-derived cerebrovascular CO2 responsiveness. Data are mean ± SEM.
, n = 10) than in those not using hydroxyurea (Hydroxy−, ■, n = 13), although the difference was not statistically significant. (B) Cerebrovascular CO2 responsiveness expressed as absolute changes (Δ μmol/L) in cerebral [O2Hb], [dHb], and [t-Hb] per millimeter of mercury PETCO2 were also higher in SCD patients using hydroxyurea (n = 7) than in those not using hydroxyurea (n = 7), although the difference was only statistically significant for dHb-derived cerebrovascular CO2 responsiveness. Data are mean ± SEM.
Cerebrovascular CO2 responsiveness (CCR) in SCD patients with and without hydroxyurea. (A) Cerebrovascular CO2 responsiveness expressed as relative change in mean CBF velocity (percentage ΔVmean) per millimeter of mercury change in PETCO2 (CCR-TCD) was higher in SCD patients using hydroxyurea (Hydroxy+,  , n = 10) than in those not using hydroxyurea (Hydroxy−, ■, n = 13), although the difference was not statistically significant. (B) Cerebrovascular CO2 responsiveness expressed as absolute changes (Δ μmol/L) in cerebral [O2Hb], [dHb], and [t-Hb] per millimeter of mercury PETCO2 were also higher in SCD patients using hydroxyurea (n = 7) than in those not using hydroxyurea (n = 7), although the difference was only statistically significant for dHb-derived cerebrovascular CO2 responsiveness. Data are mean ± SEM.
, n = 10) than in those not using hydroxyurea (Hydroxy−, ■, n = 13), although the difference was not statistically significant. (B) Cerebrovascular CO2 responsiveness expressed as absolute changes (Δ μmol/L) in cerebral [O2Hb], [dHb], and [t-Hb] per millimeter of mercury PETCO2 were also higher in SCD patients using hydroxyurea (n = 7) than in those not using hydroxyurea (n = 7), although the difference was only statistically significant for dHb-derived cerebrovascular CO2 responsiveness. Data are mean ± SEM.
Discussion
The results of this study provide new insights regarding cerebrovascular control in SCD. The main finding of the study is that cerebrovascular CO2 responsiveness in young adult SCD patients was reduced, implying that the vasodilatory capacity of the cerebral vasculature is impaired. The defect in cerebrovascular CO2 responsiveness appeared not to be related to the Ht or normocapnic resting Vmean. Reduction of cerebrovascular CO2 responsiveness is thought to play an important role in the pathogenesis of cerebral microangiopathy, which may contribute to the increased susceptibility to cerebrovascular ischemic events,34 particularly on increased metabolic requirements or during episodes of hypoperfusion, eg, SCD-related cerebral vaso-occlusion and hypotension.35
SCD is characterized by a reduced NO bioavailability, which may contribute to the reduced cerebrovascular CO2 responsiveness in this disease.10 Elevated levels of cell-free heme as a result of chronic hemolysis induce increased NO scavenging, thereby reducing NO bioavailability in SCD.36 However, in the present study, the impaired cerebrovascular CO2 responsiveness was not related to the standard biomarkers of hemolysis. As more specific indicators of hemolysis (eg cell-free heme) were not available, a causative relation between hemolysis and cerebrovascular CO2 responsiveness cannot be ruled out. Of interest, whereas cerebrovascular CO2 responsiveness was not related to hemoglobin F levels, the use of hydroxyurea seemed to relate to a higher cerebrovascular reserve capacity in SCD patients, even though hemoglobin levels between these 2 patient groups were comparable. Although the study was not designed to assess the effect of hydroxyurea on cerebrovascular reserve capacity, this observation suggests that hydroxyurea may have a beneficial influence on the endothelial function and consequently the cerebrovascular reserve capacity.
A possible role of hyperemia-related cerebral vasodilatation as a cause of reduced cerebrovascular reserve capacity also needs to be considered. The hyperemia-related cerebral vasodilatation might be induced by an increased CBF demand as a compensation for anemia. However, in the present study, while finding a weak correlation between Ht and Vmean at normocapnia, neither Ht nor the normocapnic resting Vmean was related to cerebrovascular CO2 responsiveness, rendering a major role of hyperemia as a cause of reduced cerebrovascular reserve capacity in adult SCD patients less probable. Although we do not exclude a role of hyperemia-related cerebral vasodilatation in the reduced cerebral reserve capacity, as suggested by Prohovnik et al,37 we also call attention to other contributing factors, including chronic endothelial damage by repeated vaso-occlusion (ischemia-reperfusion),38 inflammation,8 intravascular hemolysis,10,11 and a reduced NO bioavailability.29,36 It should be taken into account that the study by Prohovnik et al,37 measuring global CBF by the 133Xe inhalation method in SCD patients, was performed in a heterogeneous group of SCD patients ranging from HbSS children with high Vmean to adults with the less severe heterozygous HbSC and patients receiving blood transfusions with consequently higher Ht and lower Vmean. In the present study, only adult homozygous HbSS SCD patients without blood transfusions in the preceding 4 months were included. Furthermore, the normocapnic resting Vmean in SCD patients of the present study (79 cm/s; 69-98 cm/s) was well below the hyperemic values normally observed in pediatric SCD patients.
The second important influence on CBF consists of cerebral autoregulation that maintains CBF more or less stable within a range of mean arterial pressure between 60 and 150 mm Hg.14 In a previous study, we demonstrated that SCD patients also have an impaired dynamic cerebral autoregulation.17 Together with the findings of the present study, these results imply that in SCD both mechanoregulation and chemoregulation as the 2 major operative mechanisms responsible for maintaining CBF are impaired, rendering SCD patients susceptible to ischemic episodes. Based on the highly sensitive control of the cerebral vasculature via changes in PETCO2 and the inverse relationship between CBF and ventilation, the alteration in CBF regulation has been proposed to contribute to breathing instability.39 This was recently verified in healthy subjects where pharmacologic reduction of brain CO2 responsiveness increased breathing instability and apneas during sleep.40 Whether this is of influence in SCD patients is unknown where the impact of the combined disarrangement of mechanoregulation and chemoregulation of brain blood flow is as yet largely unidentified and data regarding overnight pulse oximetry of the study patients were unavailable.
In the present study, the MCA Vmean was used for evaluation of changes in CBF. Because MCA is a conductance rather than a resistance vessel, changes in MCA Vmean are representative of those in CBF. NIRS is based on a different physical principle and tracks changes in cerebral frontal oxygenation with a comparable time resolution as TCD determined Vmean. In humans, NIRS has been shown as an adequate measure of cerebral oxygenation.41 Simultaneous assessment of cerebrovascular CO2 responsiveness by TCD and NIRS have shown correlating results in patients with cerebrovascular disease.28,42 This was also observed in the present study, where parallel changes of NIRS-determined cerebral blood oxygen concentrations to those in TCD determined Vmean in response to CO2 affirmed the observed differences between patients and controls. A potential influence of anemia on NIRS data in the patient group has to be considered. However, to our knowledge, the effect of reduced Hb concentration on changes in [O2Hb], [dHb], and [t-Hb] as determined by NIRS has not been investigated yet. Therefore, we can only speculate on the influence of anemia on the NIRS results, whereas the TCD results confirm the impairment of cerebrovascular CO2 responsiveness in SCD patients.
Although a history of symptomatic stroke was an exclusion criterion, the presence of silent cerebral infarcts in the study patients cannot be ruled out. Interestingly in patients with type 2 diabetes, regional white matter hyperintensities were associated with reduced cerebrovascular CO2 responsiveness.43 Despite the relatively small sample size, further limited by missing NIRS data in 9 patients and 3 controls, the strongly significant decreases in cerebrovascular CO2 responsiveness in the SCD patients suggest an important role for impaired cerebrovascular CO2 responsiveness in the increased susceptibility to stroke in SCD, regardless of the presence or absence of silent cerebral infarcts. However, whether silent cerebral infarcts are related to cerebrovascular CO2 responsiveness, needs to be elucidated in a larger study with cerebrovascular CO2 responsiveness measurements combined with cerebral MRI.
Currently, pediatric SCD patients with high stroke risk are identified by TCD screening.44,45 Even though this is an effective strategy, many of these patients are exposed to the risks of long-term transfusion programs, although not all patients will experience stroke. A better understanding of the pathophysiology of SCD related stroke is needed to identify new management strategies and to further optimize stroke risk assessment. Whether the degree of cerebrovascular CO2 responsiveness could be of additional diagnostic and prognostic value in SCD related strokes in pediatric patients is the subject of further study.
In conclusion, we demonstrated that homozygous sickle cell patients without a history of symptomatic stroke have an impaired cerebrovascular CO2 responsiveness, suggesting a reduced cerebrovascular reserve capacity that might play a role in the pathophysiology of stroke in SCD. The impaired cerebrovascular CO2 responsiveness was not related to the degree of hemolysis. Whether cerebrovascular CO2 responsiveness measurement could be of additional prognostic value next to TCD screening in identifying patients at high risk for stroke44,45 remains to be elucidated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Slotervaart Hospital Fund for Clinical Research (Stichting Klinisch Wetenschappelijk onderzoek Slotervaart Ziekenhuis to E.N.), the Dutch Diabetes Foundation (grant 2004-00-001 to Y.-S.K.), and the Dutch Heart Foundation (grant 2006B027 to J.T., S.C.A.T.D.).
Authorship
Contribution: E.N. designed and performed research, analyzed data, and wrote the manuscript; Y.-S.K. designed and performed research, participated in data analysis, and edited the manuscript; J.T. performed research and participated in data analysis; E.J.v.B. designed and performed research; S.C.A.T.D. performed research; D.P.B. participated in data analysis and edited the manuscript; and B.J.B. and J.J.v.L. designed research and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the CURAMA Study Group participants appears in the supplemental Appendix.
Correspondence: Johannes J. van Lieshout, Medium Care Unit, Department of Internal Medicine, Rm F7-205, Academic Medical Centre, University of Amsterdam, PO Box 22700, 1100 DE Amsterdam, The Netherlands; e-mail: j.j.vanlieshout@amc.uva.nl.
References
Author notes
E.N. and Y.-S.K. contributed equally to this study.

![Figure 1. Cerebrovascular CO2 responsiveness (CCR) in SCD patients and healthy controls. (A) Cerebrovascular CO2 responsiveness expressed as relative change in mean CBF velocity (% ΔVmean) per millimeter of mercury change in PETCO2 (CCR-TCD) was lower in SCD patients (■, n = 23) than in healthy controls (, n = 16). (B) Cerebrovascular CO2 responsiveness expressed as absolute changes (Δ μmol/L) in cerebral [O2Hb], [dHb], and [t-Hb] per millimeter of mercury PETCO2 (CCR-NIRS) were also significantly lower in SCD patients (■, n = 14) than in healthy controls (, n = 13). Data are mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/16/10.1182_blood-2009-05-223859/4/m_zh89990943300001.jpeg?Expires=1765951373&Signature=vabjs83Zpw89-iq022Ts09sYQDsuTu1S1e9oKzpSGOEf0IyQCNF1TOki53BuL1~NQoNvXggCk9dbJC4GOZxF7zltIdBrg-OjgQOT22UKgm~iz9beZTD8jECLpMrn8m-R45N3OichO1x3gKR2DYjKfGvCrd79aq4h9cE0YS54rHtP8bY5IhD4vhw9RQGIxQV72I-i6kIbYGRaVTAsegVhp1rngd~7kxtgnRdBLVnk5gfYXeHfLmpyuuRRW5QS6zlYb3A-xayFI7NlFOua9vW~hYTaHuAvjiFMBt1JD4~fM7YX7wtC8h1TeJq2k8UVCZpmvWUQL8XYTF1rfLMC7qRWbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Cerebrovascular CO2 responsiveness (CCR) in SCD patients with and without hydroxyurea. (A) Cerebrovascular CO2 responsiveness expressed as relative change in mean CBF velocity (percentage ΔVmean) per millimeter of mercury change in PETCO2 (CCR-TCD) was higher in SCD patients using hydroxyurea (Hydroxy+, , n = 10) than in those not using hydroxyurea (Hydroxy−, ■, n = 13), although the difference was not statistically significant. (B) Cerebrovascular CO2 responsiveness expressed as absolute changes (Δ μmol/L) in cerebral [O2Hb], [dHb], and [t-Hb] per millimeter of mercury PETCO2 were also higher in SCD patients using hydroxyurea (n = 7) than in those not using hydroxyurea (n = 7), although the difference was only statistically significant for dHb-derived cerebrovascular CO2 responsiveness. Data are mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/16/10.1182_blood-2009-05-223859/4/m_zh89990943300005.jpeg?Expires=1765951373&Signature=uGqKzgiW9N0FG0YHQFeaYFho04rOG0StjNvgwaevUoVlkJPbkighmmfxXHzYGhOPKxzKp5AVHz9iTKbm~VDXYqsIDY75UVqQMzxiAx65HRWVaPwT2touiwx0iu3lS5SuKAvsN-4k7FKXPf1SzfqT3UPclU8XNrxhiVZ3a-rRL7E~tecR0kKEVpm4GHtL6HXK3~21375fxhLtjdQdE2H1q9I0RGRZMh1c2dSVic-sy2d2Ij1qr6iF380tRijLOO5XYjQ8aZvHnBuT6j~cnOBsPNfgbuWV0vpXHm4nAk9Tngp5pdeaT5rVQeMbaX4IqeBaw8umOk9Aw~Prs-u8KBJ9HA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
