Abstract
The differentiation of human peripheral blood monocytes into resident macrophages is driven by colony-stimulating factor-1 (CSF-1), which upon interaction with CSF-1 receptor (CSF-1R) induces within minutes the phosphorylation of its cytoplasmic tyrosine residues and the activation of multiple signaling complexes. Caspase-8 and -3 are activated at day 2 to 3 and contribute to macrophage differentiation, for example, through cleavage of nucleophosmin. Here, we show that the phosphatidylinositol-3 kinase and the downstream serine/threonine kinase AKT connect CSF-1R activation to caspase-8 cleavage. Most importantly, we demonstrate that successive waves of AKT activation with increasing amplitude and duration are required to provoke the formation of the caspase-8–activating molecular platform. CSF-1 and its receptor are both required for oscillations in AKT activation to occur, and expression of a constitutively active AKT mutant prevents the macrophage differentiation process. The extracellular receptor kinase 1/2 pathway is activated with a coordinated oscillatory kinetics in a CSF-1R–dependent manner but plays an accessory role in caspase activation and nucleophosmin cleavage. Altogether, CSF-1 stimulation activates a molecular clock that involves phosphatidylinositol-3 kinase and AKT to promote caspase activation. This oscillatory signaling pathway, which is coordinated with extracellular receptor kinase 1/2 oscillatory activation, involves CSF-1 and CSF-1R and controls the terminal differentiation of macrophages.
Introduction
Monocytes have the unique property among peripheral blood cells to migrate into tissues and to further differentiate into morphologically and functionally heterogeneous cells that include macrophages, myeloid dendritic cells, and osteoclasts. In contrast with the differentiation of bone-marrow progenitors and precursors, this development generally proceeds in the absence of proliferation. The differentiation of peripheral blood monocytes into resident tissue macrophages can be recapitulated ex vivo by incubation in the presence of macrophage colony-stimulating factor, also known as colony-stimulating factor-1 (CSF-1). This homodimeric glycoprotein stimulates the proliferation, differentiation, and survival of multipotential, bipotential, and unipotential bone marrow progenitors as they progress throughout the monocyte differentiation pathway.1 CSF-1 may be sufficient in physiologic conditions to generate macrophages with a M2 phenotype, which are involved in debris scavenging, angiogenesis, tissue remodeling, and wound healing, whereas M1 macrophages with an inflammatory phenotype could be produced independently of CSF-1 under stimulation with granulocyte-macrophage colony-stimulating factor, interferon γ, or microbial products.2 Mice lacking active CSF-1 production as the result of a null mutation in coding region of the CSF-1 gene, known as op/op osteopetrotic mice, demonstrate severe deficiency of macrophages3 that is restored by the injection of CSF-1 in several tissues.4
The biologic effects of CSF-1 are mediated by a unique receptor, CSF-1R, which is encoded by the c-fms proto-oncogene. This trans-membrane glycoprotein of 130 to 170 kDa exhibits an extracellular ligand binding domain, a hydrophobic trans-membrane domain, and an intracellular tyrosine kinase domain. Upon CSF-1 binding, the receptor dimerizes, leading to the activation of its intrinsic tyrosine kinase activity that results in trans-phosphorylation of 7 specific tyrosine residues within its intracytoplasmic portion.5 The phosphorylated tyrosine residues serve as docking sites for a variety of intracellular signaling molecules that contain Src homology 2 domains. Much is known about the signaling pathways that can be activated downstream, but their respective importance is still under stringent analysis because the outcome depends on the cell type and the experimental conditions. CSF-1R–deficient mice display a pleiotropic phenotype that includes a decreased macrophage number,6 and impaired CSF-1 signaling activity has been involved in several medical disorders (for reviews, see Hamilton1 and Chitu and Stanley7 ).
Downstream signaling pathways that are activated by CSF-1R upon ligand binding include the mitogen-activated protein kinase (MAPK) pathway that leads to the activation of extracellular receptor kinases (ERK) 1 and 2 through Ras and Raf, and the phosphatidylinositol 3-kinase (PI3K) pathway. Grb2 is the adaptor molecule that may couple the activated CSF-1R to MAPK activation, whereas PI3K can be activated by direct binding of its p85 regulatory subunit to phosphorylated tyrosine 723 of CSF-1R or through interaction of p85 with a Src family kinase (SFK) member that binds phosphorylated tyrosine 559 of the CSF-1R. Active PI3K, which is formed by recruiting one of the p110 isoforms of the class I A catalytic subunit to p85, stimulates downstream targets that include the serine/threonine kinase AKT.8 Negative regulators of PI3K pathways include inositol phosphatases such as phosphatase and tensin homolog9,10 and the Src homology 2 domain-containing inositol 5′-phosphatases SH2 inositol 5′-phosphatase 1 and SH2 inositol 5′-phosphatase 2.11,12 These negative regulators of CSF-1 signaling reduce AKT activity and inhibit nuclear factor (NF)-κB–regulated gene transcription in CSF-1–stimulated cells. ERK1/2-negative regulators in monocytes may include protein phosphatase 2A,13 T-cell protein tyrosine phosphatase,14 and some of the MAPK phosphatase family members (MKP1, 2, and 4).15 Both the MAPK and the PI3K/AKT pathways are required for the commitment of monocytes to macrophages, but the interplay between them in this specific context remains unclear.16
Transient activation of NF-κB is part of the transcriptional program that drives CSF-1–induced macrophage differentiation.17 We have shown that CSF-1–mediated differentiation requires the activation of caspase-8 in a multimolecular platform whose formation culminated 2 to 3 days after CSF-1 stimulation.17-20 As a consequence, the serine/threonine kinase RIP1 is cleaved, allowing the release of a cleavage fragment involved in the down-regulation of NF-κB activity that characterizes terminal macrophage differentiation.17 Caspase-8 activates downstream caspase-3 that cleaves a limited number of intracellular proteins.19 Here, we demonstrate that the synchronized oscillatory activation of PI3K/AKT and ERK1/2 amplifies these signaling pathways and that PI3K/AKT waves end with caspase-8 activation, suggesting that a molecular clock is involved in the control of caspase activity along CSF-1–driven macrophage differentiation.
Methods
Reagents and antibodies
CSF-1 was purchased from PromoCell; kinase inhibitors MG132 and epoxomicin from Calbiochem; and Q-VD-OPH from MBL. Mouse monoclonal antibodies (Abs) used include anti–human heat shock cognate (HSC) 70, CD71, SRC, HCK, LYN, and FYN (from Santa Cruz Biotechnology); caspase 8 (from MBL); and Rieske iron-sulfur protein (RIP1) and Fas-associated death domain (FADD; from PharMingen). Anti-ERK1/2, phospho-ERK1/2, AKT, phospho-AKT, p38 MAPK, phospho-p38 MAPK, CSF-1R, phospho-CSF-1R, phospho-p70 S6 kinase, I-κBα, phospho-I-κBα, nucleophosmin (NPM), caspase-3, and peroxidase-conjugated anti–rabbit Abs were from Cell Signaling Technology. Secondary Abs included horseradish peroxidase–conjugated goat anti–mouse IgG1 and IgG2b (Southern Biotechnology). In flow cytometric experiments, we used APC-conjugated anti-CD71, -CD16, and isotype IgG1-matched control; PE-conjugated anti-CD163, -CD14, and -CD115 (CSF-1R); and IgG1 isotype control (PharMingen). A goat anti–caspase-8 (Santa Cruz Biotechnology) was used for immunoprecipitation experiments.
Cell culture and differentiation
Human peripheral blood monocytes were obtained from healthy donors with informed consent according to recommendations of an independent scientific review board, in accordance with the Declaration of Helsinki. The ethical board of Dijon Hospital approved this protocol. Cells were enriched by the use of a monocyte isolation kit with autoMACS Separator according to the manufacturer's instructions (Miltenyi Biotec), grown in RPMI 1640 medium with glutamax-I (Lonza) supplemented with 10% or 2% (vol/vol) fetal bovine serum (Lonza) in standard conditions, and exposed to 100 ng/mL CSF-1. Macrophage differentiation (adhesion to culture flasks and fibroblast-like shape) was visualized by the use of an inverted microscope (Axiovert 25; Zeiss) equipped with an Axiocam HRm camera (Zeiss). Phase images of culture were recorded with a 20×/0.30 PH1 objective with AxioVision 4.7 software (Zeiss). Trypan blue was used to check cell viability.
Flow cytometry
To detect caspase activity, we used FAM-IETD-fmk and FAM-DEVD-fmk detection kits (PromoCell). The expression of CD115 was studied on cells washed with ice-cold phosphate-buffered saline (PBS), incubated at 4°C for 1 hour in PBS/bovine serum albumin (bovine serum albumin 0.1%) with a phycoerythrin-conjugated CD115 or an isotype control, washed, and fixed in 2% paraformaldehyde. Macrophage differentiation was studied by use of the same protocol with anti-CD71, -CD163, -CD14, and -CD16 Abs. Exposure of phosphatidylserine on the outer plasma membrane leaflet was identified by annexin V–FITC staining in the presence of propidium iodide according to the manufacturer's instruction (annexin V–FITC apoptosis detection kit I; Pharmingen). Fluorescence was measured by the use of an LSRII flow cytometer (Becton Dickinson).
Immunoblot assays
Cells were lysed for 30 minutes at 4°C in lysis buffer (50 mmol/L HEPES, pH 7.4; 150 mmol/L NaCl; 20 mmol/L ethylene diamine tetraacetic acid; 100 mmol/L NaF; 10 mmol/L Na3VO4; complete protease inhibitor mixture; and 1% Triton X-100). Lysates were centrifuged at 20 000g (15 minutes, 4°C), and supernatants were supplemented with concentrated sodium dodecyl sulfate. Then, 50 μg of proteins were separated and transferred following standard protocols before analysis with chemiluminescence detection kit (Santa Cruz Biotechnology).
Transient transfection and siRNA knockdown
Small interfering (si) RNAs were introduced into monocytes by nucleoporation (Amaxa) of 5 × 106 monocytes in 100 μL of nucleofector solution with 2 μg of siRNA. Cells were incubated overnight with 4 mL of prewarmed complete medium, and CSF-1 was subsequently added. We used siRNAs (Eurogentec) targeting AKT (AKT1: 5′-CAGCCCUGAAGUACUCUUUtt-3′ and AKT2: 5′-CGGGCACAUUAAGAUCACttA-3′), p85 PI3K subunit (PI3K1: 5′-GCAGCCAGCUCUGAUAAUAtt-3′, PI3K2: 5′-GCAGCCGUUUACAGUGAAAtt-3′), and luciferase as a negative control (Sense: 5′-CUUACGCUGAGUACUUCGAtt-3′). Other siRNA sequences are accessible upon request (Invitrogen). Transient transfections were performed by nucleoporation of 5 × 106 monocytes in 100 μL of nucleofector solution with 150 ng of pcDNA4 or pcDNA4-Myr-AKT plasmid (a kind gift of Dr Jean-François Tanti, Inserm U895).
Reverse-transcription and real-time polymerase chain reaction
Total RNA was isolated with TRIzol (Invitrogen) and reverse transcribed by Moloney murine leukemia virus reverse transcriptase (Promega) with random hexamers (Promega). Specific forward and reverse primers are accessible upon request. Real-time polymerase chain reaction (PCR) was performed with AmpliTaq Gold polymerase in an Applied Biosystems 7500 Fast thermocycler by use of the standard SyBr Green detection protocol (Applied Biosystems). In brief, 12 ng of total complementary DNA, 50 nmol/L (each) primers, and 1× SyBr Green mixture were used in a total volume of 20 μL.
Immunoprecipitation
Monocytes (50 × 106) were stimulated with 100 ng/mL CSF-1 in the presence or absence of kinase inhibitor, washed with cold PBS, and lysed for 30 minutes on ice in 1 mL of lysis buffer containing 1% NP-40, 20 mmol/L Tris-HCl, pH 7.5; 150 mmol/L NaCl; 10% glycerol; and complete protease inhibitor mixture. After centrifugation at 20 000 g (4°C, 30 minutes), 6 mg of supernatant proteins were precleared in the presence of Sepharose 6B (Sigma-Aldrich, Sigma), centrifuged, and incubated with an anti-caspase 8 antibody (1 μg) at 4°C for 20 hours in the presence of 60 μL of protein G (Amersham). Beads were washed 4 times with the lysis buffer, and proteins were analyzed by immunoblotting.
Densitometry and statistical analysis
The densitometry of blots was realized by the use of ImageJ software (National Institutes of Health). The statistical analysis was performed by the use of the Student t test, and significance was considered when P values were lower than .05. Results are expressed as mean plus or minus SEM.
Results
CSF-1–induced caspase activation is sensitive to specific kinase inhibitors
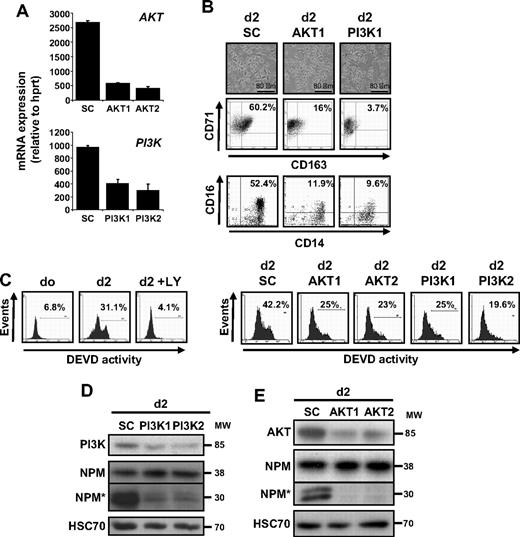
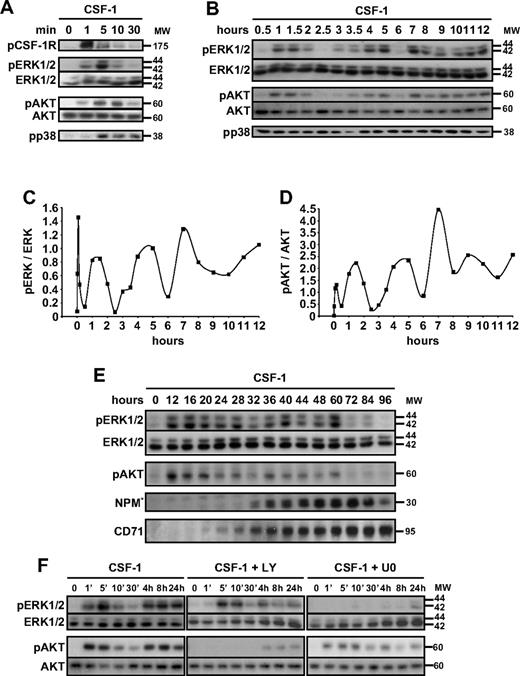
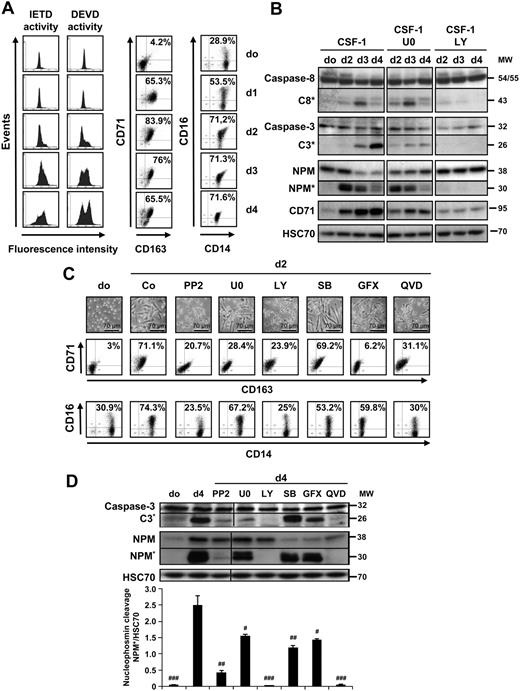
We and others have shown that CSF-1–induced differentiation of peripheral blood monocytes into macrophages required the activation of caspase-821 and downstream caspase-3.17,19,20 Figure 1A shows the time-dependent accumulation of FAM-IETD-fmk and FAM-DEVD-fmk cleavage activities, which may indicate caspase-8 and caspase-3 activation, along the differentiation process. These activities were detected at day 2 to 3 after the beginning of cell exposure to CSF-1 and increased with time (Figure 1A). The differentiation of monocytes into macrophages is assessed by the increased expression of CD71 and CD163 at the cell surface, as well as the increase fraction of CD14-positive cells that express CD16 (Figure 1A). Immunoblot analysis of monocytes exposed to CSF-1 for 4 days demonstrated the differentiation-associated procaspase-8 and -3 cleavages into intermediate fragments that precede the formation of active enzymes and the downstream proteolysis of the 38-kDa NPM, whereas transferrin receptor (CD71) accumulated in the cells (Figure 1B).
Specific kinase inhibitors prevent CSF-1–induced caspase activation. Enriched human monocytes were exposed for indicated times (days) to 100 ng/mL CSF-1. (A left) Flow cytometry analysis of FAM-IETD and FAM-DEVD cleavage activities. (A right) Indicated cell-surface marker expression (percentages indicate double-positive cells). One representative of 5 independent experiments is shown. (B) Monocytes were exposed for 0 to 4 days to 100 ng/mL CSF-1 alone or in association with U0126 (U0, 10 μmol/L), or LY294002 (LY, 10 μmol/L), added 30 minutes before CSF-1 treatment, and the expression of indicated proteins was analyzed by immunoblot. HSC70 indicates loading control. *Cleavage fragments. (C) Monocytes were exposed to 100 ng/mL CSF-1 alone or in association with PP2 (10 μmol/L), U0126 (U0, 10 μmol/L), LY294002 (LY, 10 μmol/L), SB202190 (SB, 5 μmol/L), GF109203X (GFX, 5 μmol/L), or Q-VD-OPH (QVD, 50 μmol/L) added to the culture medium 30 minutes before CSF-1, and differentiation was studied by morphologic examination and FACS analysis at day 2. (D) Monocytes were exposed for 4 days (d4) as in panel C, and the expression of indicated proteins was analyzed by immunoblot. HSC70 indicates loading control. Molecular weight (MW) is given in kilodaltons. *Cleavage fragments. A vertical line has been inserted to indicate a repositioned gel lane. One representative blot is shown, and the ratio of NPM* to the HSC70 protein level was measured by the analysis of 3 independent experiments with ImageJ software. #P < .01, ##P < .001, ###P < .001 (vs d4). Results obtained with wortmaninn were similar to those obtained with LY294002 (not shown). The vertical line indicates repositioned gel lanes caused by the removal of an irrelevant control.
Specific kinase inhibitors prevent CSF-1–induced caspase activation. Enriched human monocytes were exposed for indicated times (days) to 100 ng/mL CSF-1. (A left) Flow cytometry analysis of FAM-IETD and FAM-DEVD cleavage activities. (A right) Indicated cell-surface marker expression (percentages indicate double-positive cells). One representative of 5 independent experiments is shown. (B) Monocytes were exposed for 0 to 4 days to 100 ng/mL CSF-1 alone or in association with U0126 (U0, 10 μmol/L), or LY294002 (LY, 10 μmol/L), added 30 minutes before CSF-1 treatment, and the expression of indicated proteins was analyzed by immunoblot. HSC70 indicates loading control. *Cleavage fragments. (C) Monocytes were exposed to 100 ng/mL CSF-1 alone or in association with PP2 (10 μmol/L), U0126 (U0, 10 μmol/L), LY294002 (LY, 10 μmol/L), SB202190 (SB, 5 μmol/L), GF109203X (GFX, 5 μmol/L), or Q-VD-OPH (QVD, 50 μmol/L) added to the culture medium 30 minutes before CSF-1, and differentiation was studied by morphologic examination and FACS analysis at day 2. (D) Monocytes were exposed for 4 days (d4) as in panel C, and the expression of indicated proteins was analyzed by immunoblot. HSC70 indicates loading control. Molecular weight (MW) is given in kilodaltons. *Cleavage fragments. A vertical line has been inserted to indicate a repositioned gel lane. One representative blot is shown, and the ratio of NPM* to the HSC70 protein level was measured by the analysis of 3 independent experiments with ImageJ software. #P < .01, ##P < .001, ###P < .001 (vs d4). Results obtained with wortmaninn were similar to those obtained with LY294002 (not shown). The vertical line indicates repositioned gel lanes caused by the removal of an irrelevant control.
To identify the signaling pathways connecting CSF-1R interaction with its ligand to downstream caspase activation, we tested a series of kinase inhibitors, including SFK inhibitors PP1 and PP2, mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor U0126, PI3K inhibitors LY294002 and wortmaninn, p38 MAPK inhibitor SB202190, and protein kinase C inhibitor GF109203X. The concentration chosen depended on preliminary experiments demonstrating the kinase inhibitory activity of these compounds at doses that did not induce caspase-dependent apoptosis of peripheral blood monocytes (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The pan-caspase inhibitor Q-VD-OPH was used as a positive control. Kinase and caspase inhibitors were added to the medium at the beginning of the culture, together with CSF-1. SFK inhibitor PP2 and PI3K inhibitors such as LY294002 (Figure 1C-D) and wortmaninn (not shown) all prevented procaspase-8 and -3 and NPM cleavage, similarly to Q-VD-OPH. The ERK kinase inhibitor U0126 demonstrated a limited effect, and the others did not show any inhibitory action on these events (Figure 1C-D). These results suggested a role for SFKs and the PI3K/AKT pathway in caspase activation along the differentiation of monocytes into macrophages.
CSF-1 activates oscillatory signaling pathways in monocytes
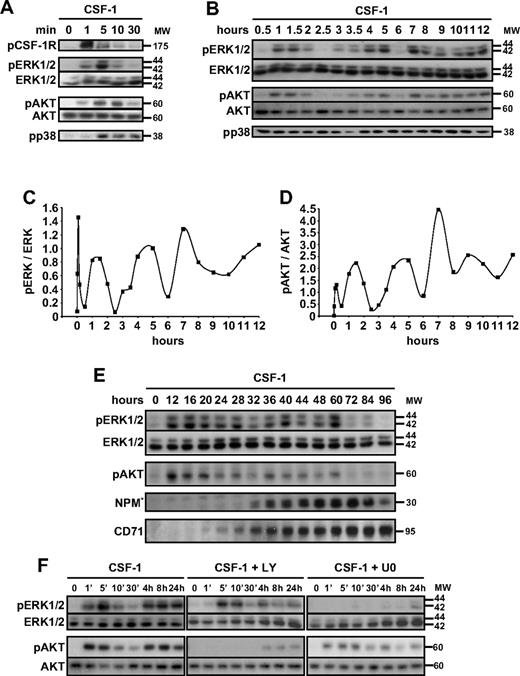
The interaction of CSF-1 with CSF-1R rapidly and transiently activates, within the first 30 minutes, a series of signaling events starting with an auto-/trans-phosphorylation of CSF-1R (seen in Figure 2, 1 minute after the addition of ligand) that precedes the transient phosphorylation of ERK1/2 and AKT (maximal phosphorylation detected here 5 minutes after the cytokine addition). These phosphorylating events decreased or virtually disappeared after 30 minutes. Meanwhile, phosphorylated p38 MAPK progressively accumulated (Figure 2A). Because caspase activation could be detected only 2 to 3 days after the engagement of CSF-1 receptor, we wondered whether these kinase pathways could be reactivated at later time points. Therefore, we performed immunoblot (Figure 2B,E) and flow cytometry (data not shown) analyses at various time points after the addition of CSF-1 to cultured monocytes during 2.5 days. Although the phosphorylation of p38 MAPK remained stable, successive waves of AKT and ERK1/2 phosphorylation with increasing amplitude could be detected. After the initial transient burst, the phosphorylated forms of AKT and ERK1/2 reappeared between 1 and 2 hours after the beginning of cell treatment, disappeared at 2 hours to reappear at 3 hours, with a peak at 5 hours and a subsequent dephosphorylation at 6 hours. These 3 first synchronizes waves (in the first 30 minutes, then between 0.5 and 2.5 hours, and then between 2.5 and 6 hours) were identified with a remarkably similar kinetics in 5 independent experiments. A new wave of phosphorylation of AKT and ERK1/2 appeared at 7 hours, then decreased progressively during a 48-hour period with some variations that could indicate that additional periods of phosphorylation/dephosphorylation could have been missed by these experiments (Figure 2B,E). These later waves of AKT and ERK1/2 activation preceded the appearance of CD71, a marker of monocyte differentiation into macrophages, and the proteolytic cleavage of NPM by caspases (Figure 2E). The interplay between PI3K/AKT and ERK1/2 activation in CSF-1–stimulated human primary monocytes is only partly understood. Here, we show that U0126 added at the beginning of cell culture completely blocks the phosphorylation of ERK1/2 but marginally affects the oscillatory AKT phosphorylation. Conversely, LY294002 added at the beginning of cell culture inhibits AKT phosphorylation and marginally affects oscillations in ERK1/2 phosphorylation (Figure 2F). These results suggested that oscillations in both pathways were synchronized but independent.
CSF-1 generates oscillatory activation of AKT and ERK1/2. (A-E) Human monocytes were exposed for indicated times to 100 ng/mL CSF-1 before immunoblot analysis of indicated proteins, with p indicating phosphorylated proteins. One representative of 5 independent experiments is shown. (C-D) Ratio of phosphorylated ERK1/2 and phosphorylated AKT, respectively, to the corresponding total protein level measured by analysis of the blots with ImageJ software. (F) Monocytes were exposed to CSF-1 in the absence (left) or presence of U0 (U0126, 10 μmol/L, right), or LY (LY294002, 10 μmol/L, middle) added to the culture medium 30 minutes before CSF-1, and the expression of indicated proteins was analyzed by immunoblot at indicated time points. Molecular weight (MW) is given in kilodaltons. *Cleavage fragments.
CSF-1 generates oscillatory activation of AKT and ERK1/2. (A-E) Human monocytes were exposed for indicated times to 100 ng/mL CSF-1 before immunoblot analysis of indicated proteins, with p indicating phosphorylated proteins. One representative of 5 independent experiments is shown. (C-D) Ratio of phosphorylated ERK1/2 and phosphorylated AKT, respectively, to the corresponding total protein level measured by analysis of the blots with ImageJ software. (F) Monocytes were exposed to CSF-1 in the absence (left) or presence of U0 (U0126, 10 μmol/L, right), or LY (LY294002, 10 μmol/L, middle) added to the culture medium 30 minutes before CSF-1, and the expression of indicated proteins was analyzed by immunoblot at indicated time points. Molecular weight (MW) is given in kilodaltons. *Cleavage fragments.
CSF-1R is required for the oscillatory activation of ERK1/2 and AKT
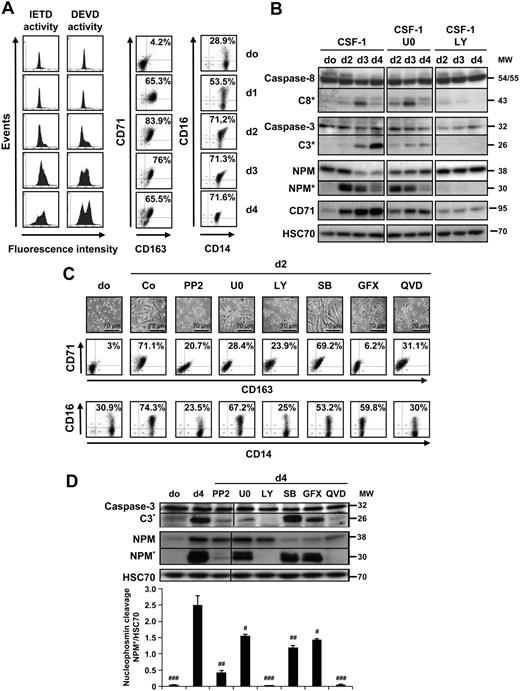
In response to CSF-1, phosphorylated CSF-1R dimers are ubiquitinylated by the E3 ligase c-Cbl and rapidly degraded in the lysosomes.22 An alternative intramembrane proteolysis of CSF-1R also was described in CSF-1–stimulated cells.23 Here, we found a rapid 30% decrease in CSF-1R expression at the surface of monocytes exposed to CSF-1 (Figure 3A-B). This decrease remained unaffected by 2 proteasome inhibitors, MG132 and epoxomicin (supplemental Figure 2). Thirty minutes later, the receptor was re-expressed at its initial level, although the total level of cellular protein explored by immunoblot had decreased. CSF-1R expression at the cell surface then diminished in a time-dependent manner (Figure 3A). The phosphorylation of tyrosine 723, used as a marker for CSF-1R activation, was detected 5 minutes after exposure to CSF-1 and decreased, to reappear with a lower intensity at 4 and 8 hours after cell exposure to the cytokine, suggesting oscillatory activation (Figure 3C). GW2580, an ATP-competitive inhibitor of CSF-1R,24 completely inhibited the phosphorylation of the receptor on tyrosine 723 as well as that of ERK1/2 and AKT when added to the culture with CSF-1 (Figure 3C). GW2580 also prevented the following waves of phosphorylation of ERK1/2 and AKT when added 30 minutes, 2 hours, or 6 hours after the beginning of cytokine treatment (Figure 3C). These results suggested that, despite partial down-regulation, CSF-1R was required for the oscillatory activation of ERK1/2 and AKT pathways. Accordingly, when CSF-1 was removed from the culture medium 2 hours after the beginning of cell stimulation, the amplitude of the following waves of AKT phosphorylation was decreased, and no subsequent wave of AKT activation could be detected (Figure 3D).
Oscillations in AKT and ERK1/2 phosphorylation require CSF-1R. (A) Monocytes were treated for indicated times with 100 ng/mL CSF-1. (Top) Flow cytometric analysis of CSF-1R expression at the surface of monocytes exposed for indicated times to 100 ng/mL CSF-1 (mean fluorescence indexes [MFIs] normalized to untreated cells). (Bottom) Immunoblot analysis of CSF-1R expression in total cell lysates. HSC70 indicates loading control. One representative of 3 independent experiments is shown. (B) Two examples of flow cytometric analyses are shown, before (0) and after 8 hours of CSF-1 treatment (8h). Isotype-matched control: empty histogram. (C) Monocytes were cultured for indicated times in the presence of 100 ng/mL CSF-1 alone or with 1 μmol/L GW2580 (GW) added either 30 minutes before CSF-1 treatment (P) or 30 minutes, 2 hours, or 6 hours after CSF-1. Immunoblot analysis of indicated proteins is shown. Two exposures of pCSF-1R are shown. Molecular weight (MW) is given in kilodaltons. HSC70 indicates loading control. (D) Monocytes were treated as shown previously and CSF-1 was kept (left) or removed from the culture medium (washing) at 2 hours before immunoblot analysis of indicated proteins.
Oscillations in AKT and ERK1/2 phosphorylation require CSF-1R. (A) Monocytes were treated for indicated times with 100 ng/mL CSF-1. (Top) Flow cytometric analysis of CSF-1R expression at the surface of monocytes exposed for indicated times to 100 ng/mL CSF-1 (mean fluorescence indexes [MFIs] normalized to untreated cells). (Bottom) Immunoblot analysis of CSF-1R expression in total cell lysates. HSC70 indicates loading control. One representative of 3 independent experiments is shown. (B) Two examples of flow cytometric analyses are shown, before (0) and after 8 hours of CSF-1 treatment (8h). Isotype-matched control: empty histogram. (C) Monocytes were cultured for indicated times in the presence of 100 ng/mL CSF-1 alone or with 1 μmol/L GW2580 (GW) added either 30 minutes before CSF-1 treatment (P) or 30 minutes, 2 hours, or 6 hours after CSF-1. Immunoblot analysis of indicated proteins is shown. Two exposures of pCSF-1R are shown. Molecular weight (MW) is given in kilodaltons. HSC70 indicates loading control. (D) Monocytes were treated as shown previously and CSF-1 was kept (left) or removed from the culture medium (washing) at 2 hours before immunoblot analysis of indicated proteins.
The PI3K/AKT pathway plays a key role in macrophage differentiation-associated caspase activation
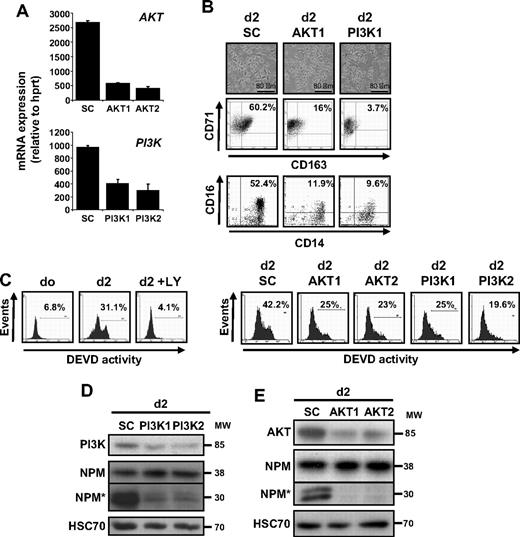
To further confirm the role of the PI3K/AKT pathway in caspase activation that occurs along CSF-1–driven macrophage differentiation of monocytes, we used siRNA targeting AKT and the p85 regulatory subunit of PI3K. We selected 2 siRNAs that efficiently decreased the targeted mRNA level in monocytes (Figure 4A). As observed with LY294002, down-regulation of AKT or p85 prevented the differentiation of monocytes into macrophages, as indicated by the decreased number of cells with a fibroblast-like shape and the appearance of CD163 at the cell surface (Figure 4B). The down-regulation of AKT and PI3K also limited the differentiation associated appearance of a DEVD-fmk cleavage activity, as observed with LY294002 (Figure 4C). The down-regulation of targeted kinases was confirmed by immunoblot together with NPM cleavage inhibition (Figure 4D-E).
PI3K and AKT down-regulation prevents CSF-1–mediated caspase activation. (A) Monocytes were transfected with siRNA targeting Luciferase (SC) or AKT (AKT1 or AKT2) or the p85 subunit of PI3K (PI3K1 or PI3K2) 2 days before real-time qPCR analysis of the corresponding mRNAs (mean ± SD of 3 independent experiments). (B) Monocytes were exposed for 2 days to CSF-1 after indicated siRNA transfection. Macrophage differentiation was examined morphologically (fibroblastic-like shape) and by 2-color flow cytometric analysis. Percentages indicate cells that express both CD71 and CD163 or both CD14 and CD16. Similar results were obtained with the 2 siRNAs targeting indicated genes. (C) FAM-DEVD cleavage activity was studied by flow cytometry in monocytes treated for 2 days with CSF-1 in the absence or presence of 10 μmol/L LY added 30 minutes before CSF-1 or in cells transfected at day 0 with siRNA targeting AKT (AKT1 and AKT2) or p85 PI3K subunit (PI3K1 and PI3K2). Numbers: percentages of cells with DEVD activity. One representative of at least 3 independent experiments is shown. (D-E) Expression of p85 PI3K subunit, NPM, and its cleavage fragment (NPM*) in cells transfected with indicated siRNA and treated for 2 days with CSF-1 as above. HSC70 indicates loading control. Molecular weight (MW) is in kilodaltons. In panel D, vertical line indicates repositioned gel lanes caused by the removal of an inefficient control.
PI3K and AKT down-regulation prevents CSF-1–mediated caspase activation. (A) Monocytes were transfected with siRNA targeting Luciferase (SC) or AKT (AKT1 or AKT2) or the p85 subunit of PI3K (PI3K1 or PI3K2) 2 days before real-time qPCR analysis of the corresponding mRNAs (mean ± SD of 3 independent experiments). (B) Monocytes were exposed for 2 days to CSF-1 after indicated siRNA transfection. Macrophage differentiation was examined morphologically (fibroblastic-like shape) and by 2-color flow cytometric analysis. Percentages indicate cells that express both CD71 and CD163 or both CD14 and CD16. Similar results were obtained with the 2 siRNAs targeting indicated genes. (C) FAM-DEVD cleavage activity was studied by flow cytometry in monocytes treated for 2 days with CSF-1 in the absence or presence of 10 μmol/L LY added 30 minutes before CSF-1 or in cells transfected at day 0 with siRNA targeting AKT (AKT1 and AKT2) or p85 PI3K subunit (PI3K1 and PI3K2). Numbers: percentages of cells with DEVD activity. One representative of at least 3 independent experiments is shown. (D-E) Expression of p85 PI3K subunit, NPM, and its cleavage fragment (NPM*) in cells transfected with indicated siRNA and treated for 2 days with CSF-1 as above. HSC70 indicates loading control. Molecular weight (MW) is in kilodaltons. In panel D, vertical line indicates repositioned gel lanes caused by the removal of an inefficient control.
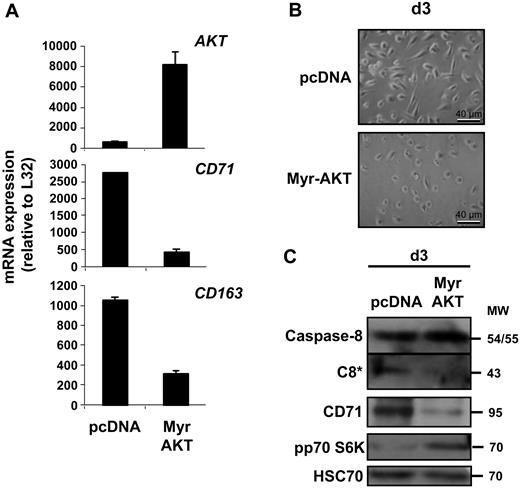
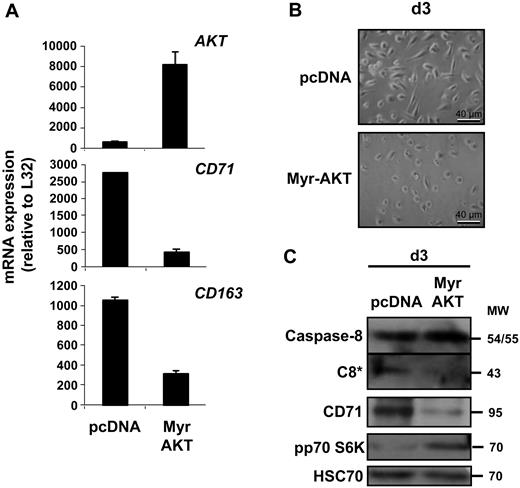
Because the role of the other signaling pathways initially was explored with pharmacologic compounds of limited specificity, we also tested a series of siRNA targeting specific SFK, including SRC, HCK, LYN, and FYN, as well as ERK1 and ERK2 or the combination of both. Schematically, down-regulation of HCK and, to a lesser extent, down-regulation of LYN negatively interfered with NPM proteolysis into a 30-kDa fragment by caspase-3. Down-regulation of FYN, SRC, ERK1, and ERK2 did not demonstrate any effect, although they could interfere with other aspects of macrophage differentiation such as the expression of some cell-surface markers, and the combination of ERK1 and ERK2 siRNA had limited efficacy (supplemental Figure 3). We then transfected primary monocytes with a constitutively active mutant of AKT (Myr-AKT). The efficacy of the transfection was checked by quantitative PCR (qPCR), and the kinase activity was demonstrated by identification of the phosphorylated form of p70 S6K. This transfection inhibited CSF-1–induced macrophage differentiation of monocytes, as assessed by qPCR analysis of CD71 and CD163 genes expression, morphologic analysis of the cells, and immunoblot analysis of CD71, which was associated with an inhibition of caspase-8 cleavage (Figure 5).
A constitutively activated AKT inhibits macrophage differentiation. Monocytes were transfected with an empty vector (pcDNA) or the vector encoding a constitutively active mutant of AKT (Myr-AKT). Cells were then treated for 3 days with CSF-1 before qPCR analysis of indicated gene expression (mean ± SD of triplicates; A), morphologic examination of cells (B), and immunoblot analysis of indicated proteins (C). Molecular weight (MW) stated as kilodaltons. *Cleavage fragment. pp70 S6K is used as a marker of AKT activity.
A constitutively activated AKT inhibits macrophage differentiation. Monocytes were transfected with an empty vector (pcDNA) or the vector encoding a constitutively active mutant of AKT (Myr-AKT). Cells were then treated for 3 days with CSF-1 before qPCR analysis of indicated gene expression (mean ± SD of triplicates; A), morphologic examination of cells (B), and immunoblot analysis of indicated proteins (C). Molecular weight (MW) stated as kilodaltons. *Cleavage fragment. pp70 S6K is used as a marker of AKT activity.
Successive waves of AKT activation are required for interaction of procaspase-8 with FADD
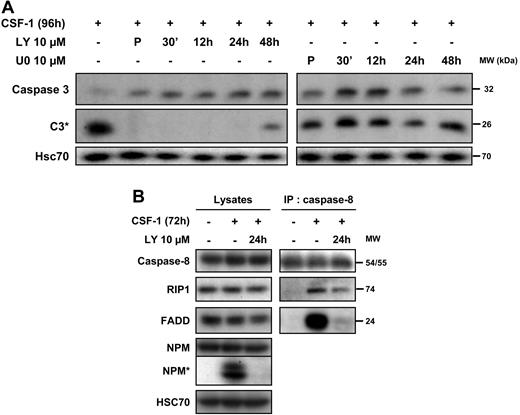
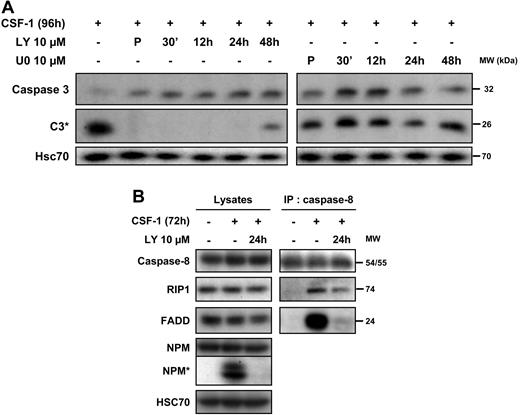
We then explored the importance of the oscillations in AKT signaling in the macrophage differentiation-associated caspase activation. The PI3K/AKT inhibitor LY294002 was added at different time points after the beginning of cell treatment with the cytokine and was still able to completely prevent the cleavage of caspase-3 when added 24 hours after the initiation of CSF-1 treatment (Figure 6A). Conversely, the addition of the ERK inhibitor U0126 at different time points after stimulation with CSF-1 did not affect differentiation-associated caspase-3 cleavage (Figure 6A). We have shown previously that caspase-8 was activated upstream of caspase-3 through interaction with the adaptor FADD and the serine/threonine kinase RIP1. In turn, RIP1 is cleaved and its cleavage fragment is involved in the down-regulation of NF-κB. Using caspase-8 immunoprecipitation, we show that, added 24 hours after the beginning of cell treatment with CSF-1, LY294002 inhibited the procaspase-8/FADD interaction and partially prevented the recruitment of RIP1 (Figure 6B). These results indicated that oscillations of AKT phosphorylation might be needed to trigger the formation of the caspase-8 activating platform.
CSF-1–mediated caspase-8 interaction with FADD and RIP1 depends on PI3K. (A) Monocytes were exposed for 4 days in the absence or presence of Y294002 (LY, 10 μmol/L) or U0126 (U0, 10 μmol/L) added either 30 minutes before CSF-1 (P) and at indicated times after the beginning of CSF-1 treatment and the expression of procaspase-3 and caspase-3 cleavage (C3*) was analyzed by immunoblot. HSC70 indicates loading control. (B) Monocytes were analyzed before any treatment or after exposure to CSF-1 for 72 hours in the presence or absence of LY (10 μmol/L) added 24 hours after the beginning of CSF-1 treatment. Cell lysates were used for immunoblotting before (lysates) or after (IP: caspase 8) immunoprecipitation with an anti–caspase-8 antibody. Molecular weight (MW) is given in kilodaltons. One representative of 3 independent experiments is shown.
CSF-1–mediated caspase-8 interaction with FADD and RIP1 depends on PI3K. (A) Monocytes were exposed for 4 days in the absence or presence of Y294002 (LY, 10 μmol/L) or U0126 (U0, 10 μmol/L) added either 30 minutes before CSF-1 (P) and at indicated times after the beginning of CSF-1 treatment and the expression of procaspase-3 and caspase-3 cleavage (C3*) was analyzed by immunoblot. HSC70 indicates loading control. (B) Monocytes were analyzed before any treatment or after exposure to CSF-1 for 72 hours in the presence or absence of LY (10 μmol/L) added 24 hours after the beginning of CSF-1 treatment. Cell lysates were used for immunoblotting before (lysates) or after (IP: caspase 8) immunoprecipitation with an anti–caspase-8 antibody. Molecular weight (MW) is given in kilodaltons. One representative of 3 independent experiments is shown.
Discussion
CSF-1 regulates the development of macrophages found in tissues undergoing active morphogenesis and/or tissue remodeling. The activation of caspase-8 and -3 is part of the specific differentiation program activated in monocytes by CSF-1.20,21 The present study demonstrates that CSF-1 interaction with its receptor is connected to procaspase-8 interaction with FADD by the PI3K/AKT pathway and, to a much lower extent, the ERK1/ERK2 pathway. Importantly, amplification of the PI3K/AKT signaling pathway through waves of phosphorylation/dephosphorylation is required for caspase-8 recruitment in the multimolecular platform in which the protease is activated. The oscillatory activation of AKT signaling pathway may act as a molecular clock that accompanies the differentiation process.
Molecular clocks regulate several biologic processes. One is the segmentation that establishes in vertebrate embryo during somitogenesis, which is associated with the periodic activation of 3 major signaling pathways, Notch, fibroblast growth factor, and Wnt.25 Another is the circadian clock that involves transcriptional/posttranscriptional feedback loops together with cytosolic rhythms in small signaling molecules.26 Oscillations in Ras and ERK activities do exist in some cell systems through negative feedback phosphorylation of Sos by ERK27 or the Raf kinase inhibitor protein.28 Analysis of a murine myeloid cell line ectopically expressing CSF-1R demonstrated that CSF-1 could induce 2 temporally distinct phases of MAPK activation.16 Such oscillations have not been described for the PI3K/AKT pathway. Here, we demonstrate that, in primary human monocytes exposed to CSF-1, several waves of phosphorylation/activation of AKT with increasing amplitude and duration are required to activate caspases. In turn, these enzymes cleave specific proteins19 and modulate the transcription program that drives the differentiation process.17 Expression of a constitutively activated kinase abolishes CSF-1–driven macrophage differentiation, which suggests that oscillations in the kinase activity are an absolute requirement for the differentiation process to progress.
The Src family of kinases plays an essential role in CSF-1 signaling,29 but the kinases involved are still a matter of debate. CSF-1 stimulation results in the phosphorylation of SRC, FYN, and YES, whereas LYN and HCK could negatively regulate CSF-1 signaling in hematopoietic cells because their loss skews HSC differentiation toward monocytes.30,31 SFK inhibitors PP1 and PP2 were observed to prevent caspase activation in primary monocytes exposed to CSF-1. Because SFK connects the activated CSF-1R to the PI3K/AKT pathway, PP1 and PP2 effects could be related to the inhibition of downstream waves of AKT inactivation. Accordingly, down-regulation of HCK could play a role in the initiation of this oscillatory signaling pathway, whereas neither SRC nor LYN nor FYN appear to be involved, which indicates distinct roles of SFK in HSC differentiation toward monocytes and monocyte differentiation into resident macrophages. Class I PI3Ks play an essential role at various stages of hematopoietic cell differentiation.32,33 In normal human peripheral blood monocytes, PI3K regulates differentiation through activation of AKT and hexokinase II–mediated glycolysis,34,35 and AKT activation was proposed to up-regulate Mcl-1 to prevent caspase-dependent cell death.36 Here, we show that suppression of AKT1 or PI3K in human primary monocytes actually prevents the activation of caspases required for CSF-1–induced differentiation into macrophages.
The interplay between PI3K/AKT and ERK1/2 pathways in CSF-1–stimulated human primary monocytes remains poorly understood.16 The ERK1/2 activation was suggested to depend on both PI3K products and the production of radical oxygen species,37 but a Raf-dependent, PI3K-independent pathway of ERK1/2 activation also could exist.38,39 The parallel but independent oscillations in PI3K/AKT and ERK1/2 phosphorylation identified in the present study led us to explore whether CSF-1R was a common starting point for these oscillations. CSF-1 was reported to stabilize noncovalent dimers of CSF-1R, which precedes the receptor dephosphorylation and the internalization of the receptor-ligand complex.40 The degradation of CSF-1R could involve the E3 ubiquitin ligase c-Cbl that interacts with CSF-1R through the Src-like adaptor protein 222 and whose mutation recently was identified in a subsets of patients with chronic myelomonocytic leukemia.41 CSF-1 receptor also was described to undergo intramembrane proteolysis upon Toll-like receptor activation through ERK activation.42 Here, we show that the expression level of CSF-1R at the cell surface decreases with time upon stimulation of human monocytes with CSF-1 through a proteasome-independent mechanism. However, the receptor remains expressed at the cell surface and is dephosphorylated/rephosphorylated on tyrosine 723. GW2580, an ATP-competitive inhibitor of CSF-1R,24 prevents the oscillatory amplification of PI3K/AKT and ERK1/2 pathways when added at various time points after CSF-1 stimulation. In addition, the removal of the cytokine from the culture medium after 2 hours of stimulation decreases the intensity of the following AKT activation waves and prevents any subsequent activation of the pathway. These observations suggest that, despite its partial down-regulation at the cell surface, CSF-1R interaction with its ligand is required for the waves of signaling amplification that occurs 2 to 3 days after cell stimulation and contribute to the differentiation process, eg, through caspase activation.
In some situations, procaspase-8 interacts with the p85 alpha regulatory subunit of PI3K and with c-Src, which induces its phosphorylation on tyrosine 380. This phosphorylation prevents the conversion of procaspase-8 to proteolytically active enzyme and confers to the protein the ability to control calpain activation, lamellipodial assembly, cell interaction with the extracellular matrix, and cell migration.43-47 A distinct mechanism may operate in monocytes undergoing differentiation into macrophages in which a series of intracellular proteins are cleaved by proteolytically active caspases,19 including the serine/threonine kinase RIP1, whose caspase-mediated cleavage contributes to the down-regulation of NF-κB activity.17 Similarly, the proteolytic activity of caspase-3 appears to be required for optimal erythroid cell differentiation.48 Thus, procaspase-8 could contribute to hematopoietic progenitor-cell maturation through nonproteolytic functions, whereas monocytes maturation into macrophages as well as terminal erythroid differentiation may require the proteolytic activity of one or several caspases.
Altogether, our results demonstrate that CSF-1 interaction with CSF-1R activates oscillatory and coordinated AKT and ERK1/2 signaling pathways, probably through a HCK-dependent mechanism. The waves of phosphorylation appear to be reactivated at the CSF-1R level by interaction with the cytokine. After 2 to 3 days, oscillations in AKT phosphorylation end with caspase-8 and caspase-3 activation, whereas the functions of ERK1/2 waves remain elusive. Tight regulation of these molecular clocks that could involve one or several phosphatases9-15,49,50 may be required for appropriate renewal of tissue macrophages, and the deregulation of these finely regulated oscillations could account for the pathogenesis of various human diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Our group is supported by grants from the Ligue Nationale Contre le Cancer (équipe labellisée, to E.S.), the association Cent Pour Sang La Vie, the Association Nationale de la Recherche (ANR), and the National Institute of Cancer (INCA). O.M. is supported by research grants from the ANR and the European community. A.J. and L.G. were supported by fellowships from the Ligue Nationale Contre le Cancer. J.P. and N.L. were supported by fellowships from the Association pour la Recherche sur le Cancer.
Authorship
Contribution: A.J. performed the experimental work and data analysis; N.B., J.P., N.L., L.G., E.K.D., M.C., and C.R. contributed to some experiments; O.M., L.D., and N.D. participated with helpful discussion; and E.S. directed the work and wrote the paper. All authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eric Solary, Inserm UMR866, Faculty of Medicine, 7, boulevard Jeanne d'Arc, 21000 Dijon, France; e-mail: esolary@u-bourgogne.fr.

![Figure 3. Oscillations in AKT and ERK1/2 phosphorylation require CSF-1R. (A) Monocytes were treated for indicated times with 100 ng/mL CSF-1. (Top) Flow cytometric analysis of CSF-1R expression at the surface of monocytes exposed for indicated times to 100 ng/mL CSF-1 (mean fluorescence indexes [MFIs] normalized to untreated cells). (Bottom) Immunoblot analysis of CSF-1R expression in total cell lysates. HSC70 indicates loading control. One representative of 3 independent experiments is shown. (B) Two examples of flow cytometric analyses are shown, before (0) and after 8 hours of CSF-1 treatment (8h). Isotype-matched control: empty histogram. (C) Monocytes were cultured for indicated times in the presence of 100 ng/mL CSF-1 alone or with 1 μmol/L GW2580 (GW) added either 30 minutes before CSF-1 treatment (P) or 30 minutes, 2 hours, or 6 hours after CSF-1. Immunoblot analysis of indicated proteins is shown. Two exposures of pCSF-1R are shown. Molecular weight (MW) is given in kilodaltons. HSC70 indicates loading control. (D) Monocytes were treated as shown previously and CSF-1 was kept (left) or removed from the culture medium (washing) at 2 hours before immunoblot analysis of indicated proteins.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/17/10.1182_blood-2009-03-208843/4/m_zh89990943900003.jpeg?Expires=1767935292&Signature=usMd5qmbYJJ-TcKeucNBEVm5JTDkq-LDfDHRrJUh-Is~L83gLLI788JEGnlJFSMrkDXf8owkQ0bHLiYoHpDwTsmIwFwR-DNH-TqnnK0VBGzzhL2k~EFQNMfUmZMZ1emN--h4dSXCzu0mAz3L8JsPB~OXTfNNvE6wx5vyA1npVB5DtJA2EXbSVWUzbIYfod3nBElvQckuQaKzFILeys7aXL9TbXrCqu8kUO20Px7T36PgkBhBaP2OZKWSPciBuqrfluS2u6xe81ZKxp0c1BjnC4phQ2qKiQRwcrXKyFIzFkLORekOUa4QHJGQHd0OawZNpjpLU~EfxS9mrCNCOa0dqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Oscillations in AKT and ERK1/2 phosphorylation require CSF-1R. (A) Monocytes were treated for indicated times with 100 ng/mL CSF-1. (Top) Flow cytometric analysis of CSF-1R expression at the surface of monocytes exposed for indicated times to 100 ng/mL CSF-1 (mean fluorescence indexes [MFIs] normalized to untreated cells). (Bottom) Immunoblot analysis of CSF-1R expression in total cell lysates. HSC70 indicates loading control. One representative of 3 independent experiments is shown. (B) Two examples of flow cytometric analyses are shown, before (0) and after 8 hours of CSF-1 treatment (8h). Isotype-matched control: empty histogram. (C) Monocytes were cultured for indicated times in the presence of 100 ng/mL CSF-1 alone or with 1 μmol/L GW2580 (GW) added either 30 minutes before CSF-1 treatment (P) or 30 minutes, 2 hours, or 6 hours after CSF-1. Immunoblot analysis of indicated proteins is shown. Two exposures of pCSF-1R are shown. Molecular weight (MW) is given in kilodaltons. HSC70 indicates loading control. (D) Monocytes were treated as shown previously and CSF-1 was kept (left) or removed from the culture medium (washing) at 2 hours before immunoblot analysis of indicated proteins.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/17/10.1182_blood-2009-03-208843/4/m_zh89990943900003.jpeg?Expires=1768615125&Signature=sB-Lp4ZavSmYsjC6Atsqfc-~WVAdw4ES1T2gMRlYNtNtaz86-L3IhH5C-Vpv9hn3z93NhVCiKUttCtgwpWVYbtn~I1xdcd43ojltNLJiM~mCETq1QlRZn-clMZ0oTvRcKWAsnD4bTieg33Rm42Rb5GYmA4RuOBd1rUk3Nwdgr3hPNjnxJ~Wml6zyY47uLJ3nH7HL4FFIaU~4IGTHzBM0SWmdcQTj9nnX-FlKmepSSSo3ngZstS34hfi1xiBr-8p2yvmjhZz9jRGerBuzVP~vOCvSZ76koZZlXzb3aAS5HsOoi3EDKpnYRyYRCWS8RHxBtS06lmynHW2N~SUd7htoeQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)