Chronic inflammatory conditions may augment tumor growth, but in this issue of Blood, Kryczek and colleagues1 find that endogenous IL-17 may not be one of the contributing factors.
The association between chronic inflammatory conditions and the development of cancer was postulated by Rudolph Virchow in 1863. Indeed, many cancers arise at the sites of chronic irritation by chemical compounds or physical factors, infections, or autoimmune diseases through a plethora of mechanisms. Subsequent tumor progression is associated with invasion of surrounding tissues that causes further inflammatory changes, triggering recruitment of myeloid and lymphoid immune cells into the tumor microenvironment. Host cells can then release factors that contribute to either tumor progression or tumor inhibition.2 The precise regulation of the balance between cancer-promoting and antitumor factors involved in this process has yet to be understood, but overall it appears that the immune system does a rather poor job in eliminating the established tumors on its own.
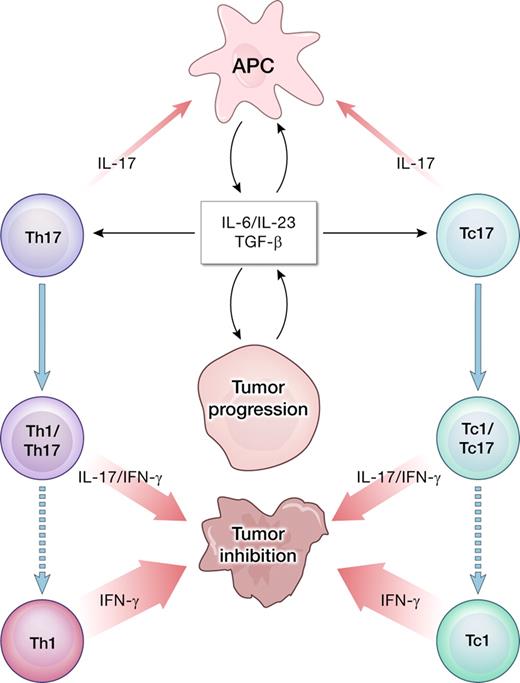
Cancer progression is associated with the release of inflammatory cytokines, including IL-6 and IL-23, which might promote further growth of the tumor cells in a microenvironment dominated by STAT3 signaling. However, the same cytokines in combination with TGF-β might be involved in polarizing tumor-infiltrating lymphocytes into type 17 cells. The IL-17 produced by infiltrating T cells may have multiple proinflammatory effects. Importantly, Th17 CD4+ T cells can be unstable and evolve into IFN-γ–producing Th1-like cells capable of tumor destruction.7 A similar pathway is postulated for CD8+ IL-17–producing cells (Tc17) evolving into cytotoxic effectors (Tc1).12
Cancer progression is associated with the release of inflammatory cytokines, including IL-6 and IL-23, which might promote further growth of the tumor cells in a microenvironment dominated by STAT3 signaling. However, the same cytokines in combination with TGF-β might be involved in polarizing tumor-infiltrating lymphocytes into type 17 cells. The IL-17 produced by infiltrating T cells may have multiple proinflammatory effects. Importantly, Th17 CD4+ T cells can be unstable and evolve into IFN-γ–producing Th1-like cells capable of tumor destruction.7 A similar pathway is postulated for CD8+ IL-17–producing cells (Tc17) evolving into cytotoxic effectors (Tc1).12
It is postulated that proinflammatory cytokines, including IL-6 and IL-23, contribute to the carcinogenesis and tumor growth directly or indirectly via STAT3-related mechanism.3,4 These cytokines induce the differentiation of CD4+ T cells into the Th17 subset, whose name derives from the capacity to produce IL-17. Th17 cells are closely related to induced FOXP3+ regulatory T cells (iTregs), as both are generated in the presence of TGF-β that is readily available in the tumor bed and is considered a potent immunosuppressant. Other sources of IL-17 include CD8+ T cells, γδ T cells, and natural killer (NK) cells. IL-17–secreting cells have been identified in many types of human cancers and murine models. Their exact roles in cancer remain unclear. Th17 cells have been linked to tissue damage associated with many autoimmune diseases, graft-versus-host reaction, and allo-graft rejection. IL-17 elicits diverse proinflammatory effects including induction of GM-CSF, TNF-α, IL-1β, IL-6, and IL-23, as well as production of matrix metalloproteinases and various chemokines. IL-17 attracts neutrophils into the site of inflammation, contributing to end organ destruction in autoimmunity.
Transfection of IL-17 into human tumor cell lines augmented the progression of the disease in nude mice via the effects on vascular endothelium and increased neovascularization. In sharp contrast, the same kind of experiments using syngeneic tumors in immunocompetent mice yielded the opposite result, attributed to the antitumor function of T cells.5 In another case, IL-17 derived from CD8+ T cells was shown to act directly on a breast cancer cell line, where it had prosurvival and antiapoptotic effect.6 Overall, these confusing and contradictory results are similar to findings reported for IL-6 or IL-23 where both beneficial and detrimental direct and indirect effects occurred in various experimental settings.
In this issue of Blood, Kryczek et al clearly demonstrate that the growth of MC38 colon adenocarcinoma in mice deficient for IL-17A is not suppressed. To the contrary, the growth of subcutaneous tumors and pulmonary metastases was significantly slower in wild-type animals capable of producing endogenous IL-17. In both hosts, the number of tumor-infiltrating cells, including FOXP3+ Tregs and IL-10 producers, was comparable. The number of NK cells and MC38-specific T cells capable of releasing IFN-γ was markedly reduced in IL-17–deficient animals. The study did not focus on the cellular sources of IL-17, but the authors conclude that given the close relationship between Th17 cells and IL-17, it is likely that type 17 polarized tumor-infiltrating T cells may contribute to the protective anticancer immunity.
In the adoptive transfer model, our group reported that Th17-polarized, tumor-specific T cells effectively rejected large, established B16 murine melanoma approaching 1cm in diameter.7 Treatment was critically dependent on the IFN-γ secreted by the transferred Th cells, thus implying plasticity of the in vitro– generated Th17 population. Recent detailed reports clearly indicate that Th17 and the related iTreg phenotypes are relatively unstable,8,9 and might easily acquire Th1-like properties upon exposure to IL-12 or IL-23.10 The observation made by Kryczek et al hints that even if naturally occurring tumor-infiltrating lymphocytes develop in the proinflammatory conditions that favor induction of type 17 responses, the overall outcome might yield a relatively significant IFN-γ–mediated type-1 reaction (see figure).
These results suggest that the TGF-β/IL-6/IL-23/IL-17 axis of inflammation does not always and in every case trigger tumor progression. Instead, inflammatory cytokines should perhaps be viewed as pluripotent factors, and their net effects might vary depending on the context. Anti-inflammatory therapies are of obvious interest in the cancer prevention setting. On the other hand, exploring and manipulating the Th17-mediated autoimmune-like antitumor response might lead to more effective immunotherapies for patients with advanced cancer.11
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■