Abstract
Abstract 1885
Poster Board I-908
Treatment of MGUS by hesperetin, potentially delaying/blocking the conversion of MGUS to symptomatic myeloma
Yoshitaka Kikukawa, Hiroaki Mitsuya, Hiroyuki Hata. Department of Hematology, Kumamoto Unversity Hospital
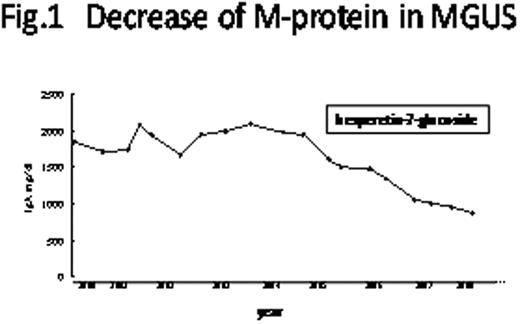
Although prognosis of multiple myeloma has recently been improved by novel therapeutic regimens, all currently available therapuetics target symptomatic myeloma only. We saw an MGUS patient, experiencing a gradual and continuous decrease of M-protein (IgA-k) from 2,080 mg/dl to 878 mg/dl over 3 years (Fig. 1). History examiantion revealed that the decrease of M-protein initiated when the patient started taking a dietary supplement containing hesperetin-7-glucoside, which is a glycosylated form of hesperetin designated to achive an approximately 104-fold increase of bioavailability comparing to aglycon structure (Hayashibara Biochemical Laboratories, Inc.). Because this compound is eventually metabolized to hesperetin, a possible anti-myeloma effect of hesperetin was examined in vitro.
Myeloma cells were obtained from myeloma patients and purified with CD138 magnetic beads. Anti-myeloma effects of test compounds on purified myeloma cells or myeloma cell lines were evaluated by WST-8 assay. Apoptosis of myeloma cells was quantified either by annexin V staining or the propidium iodide method, followed by flowcytomerty analysis or by morphological analysis of cytospin slides. Mitochondrial membrane potential was quantified using JC-1 staining Kit (Cayman Chemical Co.) and flow cytometry. Caspase activation and total ubiqutinated protein were analysed by western blotting. Proteasomal chymotrypsin-like activity was measured using 20S Proteasome Assay Kit (Enzo Life Sciences).
Hesperetin showed inhibitory effects in a dose-dependent manner on the growth of 4 myeloma cell lines and freshly isolated myeloma cells. Two myeloma cell ines, RPMI8226 and 12PE, were utilized as representative cell lines for further analysis. Hesperetin induced annexin V/PI positive cells, morphological fragmentation of the nucleus of myeloma cells, and activation of caspase-3, 8 and 9, at a concentration around 500 microM, which is clinically achievable with peroral administration of glycosylated hesperetin, suggesting that the observed anti-myeloma effects by hesperitine through apoptotic pathways. Further analysis revealed that hesperetin disrupted mitochondrial membrane potential which leads to release of cytochrome c from mitochondria to cytoplasm as assessed by western blot analysis. Caspase-8 and 9 inhibitors(Z-IETD-fmk and Z-LEHD-fmk, respectively)did not inhibit the hesperetine-induced apoptosis, although they completely inhibited anti-Fas antibody-induced apoptosis, suggesting that the hesperitine-induced apoptosis is not dependent on death receptor signaling. A pan-caspase inhibitor, Z-VAD-fmk, completely blocked the hesperetin-induced apoptosis in 12PE cells, but only partially in RPMI8226 cells, suggesting that hesperetin also mediated caspase-independent apoptosis in RPMI8226 cells. Moreover, western blot showed that hesperetin treatment induced an accumlation of poly-ubiquitine proteins. Analysis of proteasome activity revealed that hesperetin at 500 microM exerted a moderate inhibition of proteasome activity with 72.6% of the inhibition exerted by bortezomib at 40 nM.
This is the first report showing anti-myeloma effect of hesperetin. It showed anti-myeloma effects via caspase-dependent and independent apoptotic pathways and proteasome inhibition activity. Given a fact that hesperitin has been approved by Japanese Goverment as a safe supplementary compound, and the present MGUS case had been taking hesperitin over >3 years without any adverse events, this compound should be well torelated over a long period. If a delay of conversion from either MGUS or asymptomatic myeloma to symptomatic myeloma could be achieved, prognosis of MM could be improve. Based on these findings, an open-label, pilot clinical trial to test the efficacy of hespertin, enroling asymptomatic myeloma patients, has currently been underway (Approved by IRB of Kumamoto Univerisity Hospital).
Decrease of M-protein in MGUS
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.