Abstract
Abstract 197
Supported by an unrestricted grant from Genzyme Corporation.
Fludarabine (Flu) melphalan-alemtuzumab is a well tolerated, reduced intensity conditioning regimen for HCT. Clofarabine (Clo), a second generation nucleoside analog with excellent activity in acute leukemia, might enhance disease control over Flu. We report outcomes of a completed phase I and ongoing phase II study of CMA conditioning for allogeneic peripheral blood HCT. Tacrolimus was administered as GVHD prophylaxis. For the phase I cohort, one pt was enrolled per dose level, until the first DLT or until 2 had grade 3 toxicity. Dose level 1 consisted of: clo 10 mg/m2/day on d −7 to −3 and melphalan 100 mg/m2 on day −2. Clo was increased by 10 mg/m2/day per cohort until 40 mg/m2/day. Then, melphalan was increased by 20 mg/m2 until 140 mg/m2. Alemtuzumab was given at a fixed dose of 20 mg/day on d −7 to −3. Twelve pts were accrued in the phase I portion of whom three remain in remission at 26, 22 and 21 months.
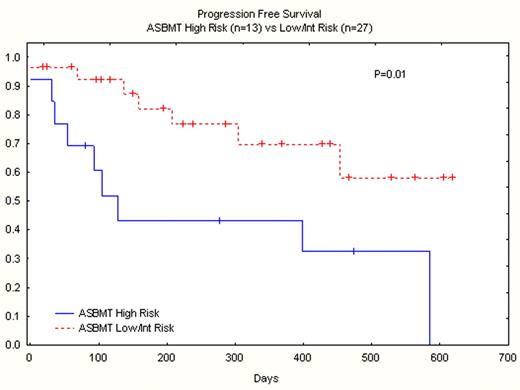
Forty pts, median age 53 (24–69), have been accrued in the phase II study of whom 16 had related and 24 unrelated donors. 20 had AML/MDS (6 refractory, 4 CR2, 9 CR1, 1 untreated MDS), 16 NHL (6 refractory, 9 chemosensitive relapse, 1 CR1) 2 CLL, 2 MPD. ASBMT risk score was high in 14, intermediate in 14, and low in 12. Performance score was 0 in 19, 1 in 17, 2 in 2, and not documented in 2 patients. The phase II dose was initiated at Clo 40 mg/m2/day x 5 days and melphalan 140 mg/m2. Twenty-four pts received this dose. Grade 3 renal toxicity occurred between day −7 and day +7 in 4 of 24 (17%) pts receiving this dose. The phase II dose was then reduced to Clo 30 mg/m2/day x 5 days and melphalan 140 mg/m2, and used to treat 16 pts. One pt with preexisting cardiomyopathy and refractory AML died during conditioning from cardiovascular failure. No grade 3 renal toxicity has been observed in this cohort and 3 pts had reversible grade 2 renal failure. Other toxicities included: gr 2–3 reversible ALT elevation between day −2 and day +5 in 8 pts; gr 2 reversible bilirubin elevation in 1 pt. No grade 3–4 hand foot syndrome or VOD occurred in this cohort.
Off Label Use: clofarabine for transplant conditioning. Kline:Genzyme corporation: Membership on an entity's Board of Directors or advisory committees. Odenike:Genzyme corporation: Membership on an entity's Board of Directors or advisory committees. Stock:Genzyme: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.