Abstract
Abstract 2166
Poster Board II-143
Normal hematopoietic stem cells reside in the epiphyses of the bone marrow that is a low oxygenated area and are protected against ROS-induced DNA damages. Recent study showed that the hypoxic environment plays a crucial role not only in maintaining stem cells but also in tumorigenesis. Hypoxia induces dramatical changes in cell characteristics including cell cycle quiescence, self renewal potency, and shift in energy production from an aerobic to anaerobic pathway, and it induces resistance to a variety of cell death stimuli. Chronic myelogenous leukemia (CML) is a disorder of hematopoietic stem cells caused by the constitutive activation of the Bcr-Abl tyrosine kinase. Tyrosine kinase inhibitors (TKIs) have led to marked improvement in prognosis of CML patients. However, CML cells could not be eradicated completely by TKI alone because quiescent CML stem cells are less sensitive to such molecular target drugs. Therefore, we speculate that the adaptation of leukemic cells to hypoxia in the bone marrow niche alters their characteristics contributing to minimal residual disease.
We first evaluated the oxygen levels of engrafted leukemic cells by pimonidazol (hypoxia specific marker) staining.We transplanted K562 cells to the bone marrow of NOD/SCID/gcnull (NOG) mice and found that those cells engrafted and survived in the epiphysis of the bone marrow where O2 concentrations are less than 1.3%. Then, we generated two hypoxia-adapted (HA) CML subclones from K562 and KCL22 by cultivating under 1.0% O2, and were denoted as K562/HA and KCL22/HA, respectively. Both cell lines survived and proliferated continuously for years under 1.0% O2 conditions, although their growth was slower than that of their parental counterparts under 20% O2 conditions. Interestingly, HA-CML cells exhibited several unique characteristics compared to their parental cells. First, these HA cells showed higher transplantation efficacy in NOG mice. The transplanted HA cells grow more rapidly in vivo than the parental cells and mice transplanted with HA cells died earlier. Next, the percentage of G0 fractions in K562 and K562/HA cells were 0.87 ± 0.58 % and 4.9 ± 2.1 %, respectively, indicating that K562/HA cells included more quiescent fractions than the parental K562. Hoechst staining analysis confirmed that HA cell lines include more SP (side population) fractions than their parental cells, indicating that HA cells contains more dormant cells. We next examined the signaling pathway of HA cell lines. Despite the unchanged levels of AKT, STAT, and ERK phosphrylation, BCR-ABL phsophrylation was suppressed in HA cells. Both of HA cell lines showed higher expression of b-catenin which is considered essential for survival and self-renewality of CML stem cells. Furthermore, HA cells were less sensitive to TKIs (imatinib, dasatinib, and bafetinib) and chemotherapeutic agents (daunorubicin and busulfan). Taken together, our HA cell lines have characteristics of more primitive CML cell populations resistant to cytotoxic agents.
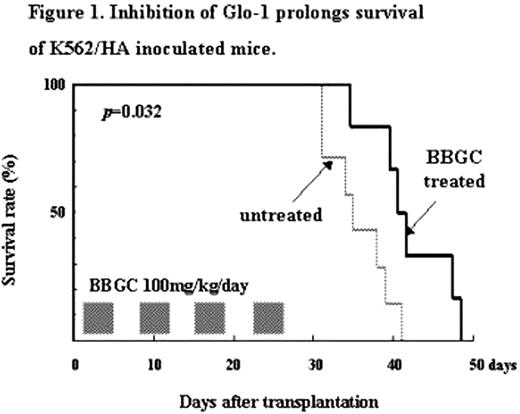
We next examined the energy metabolites such as adenosine triphosphate (ATP), glucose consumption, and lactate production in HA cells. The amounts of ATP in K562/HA and KCL22/HA cells decreased, whereas glucose consumption and lactate production increased compared with those in their parental cell lines. These findings indicate that ATP production of HA cells depends on glycolysis. Furthermore, we found higher expression and kinase activity of Glyoxalase-1 (Glo-I). Glo-I is an enzyme that detoxifies glycolysis-specific cytocidal byproducts in glycolytis system. Glo-I inhibitors such as S-p-bromobenzylglutathione cyclopentyl diester (BBGC), 2-crotonyloxymethyl-4,5,6-trihydroxycylohex-2-enone (COTC), and methyl-gerfelin were much more cytotoxic against HA-CML cells than their parental cells in vitro. Notably, when K562/HA-transplanted mice were treated with 100 mg/kg/day BBGC for 8 days, the treated mice survived longer than the untreated mice (Figure 1). These findings suggest that Glo-1 plays an important role in primitive CML cells survival under hypoxia.
In conclusion, Glo-1 is a novel attractive target against hypoxia-adapted primitive CML cells in the bone marrow milieu. Investigation of hypoxia-specific pathways and roles on CML cells could develop novel therapeutic approach targeting TKIs resistance.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.