Abstract
Abstract 2752
Poster Board II-728
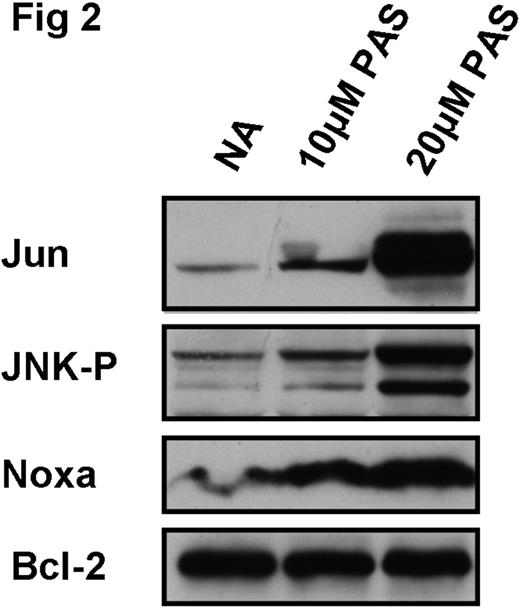
Approximately 15% of CLL patients present with a deletion of chromosome 17p, resulting in the loss of the p53 gene. The percentage of 17p-deleted patients can increase to between 30–50% following treatment with cytotoxic agents which induce CLL apoptosis via upregulation of p53. Therefore, identification of novel agents which kill CLL cells independently of p53 is of crucial importance. We have recently shown that two chemically unrelated agents, 2-phenylacetylenesulfonamide (PAS) and the sesquiterpene lactone LC1 kill CLL cells regardless of the functional status of p53. Furthermore, in contrast to the conventional drugs chlorambucil and fludarabine, PAS and LC1 induce apoptosis in the absence of p53 elevation. However, the mechanisms of action by which these agents induce p53-independent apoptosis are unclear. We have previously shown that both PAS and LC1 initiated CLL cell apoptosis within 6-10h with, maximal killing by 48h. Here we show that treatment of CLL cells with either agent results in the generation of reactive oxygen species (ROS), the activating phosphorylation of the pro-apoptotic MAP kinase family member JNK (Fig 1), resulting in turn in upregulation of its downstream target, the transcription factor c-JUN (Fig 2). The BH3-only pro-apoptotic protein Noxa was originally described as a pro-apoptotic target for upregulation by p53. However, both PAS (Figs1 and 2) and LC1 upregulated Noxa in a p53-independent manner. Addition of N-acetylcysteine (NAC), a free radical scavenger, decreased ROS generation by PAS or LC1 and also prevented phosphorylation of JNK and Noxa upregulation (Fig 1) and also the upregulation of c-JUN. NAC also strikingly abrogated apoptosis induction by either agent, as shown by quantitation of cleavage of the caspase 3 substrate poly (ADP ribose) polymerase (PARP; Fig 1). Taken together, the data suggest both PAS and LC1 induce p53-independent apoptosis via upregulation of ROS and the subsequent induction of Noxa. The data are also compatible with a role for JNK and c-JUN in the events leading to Noxa upregulation.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2009 by The American Society of Hematology
2009