Abstract
Abstract 2878
Poster Board II-854
Significant advances have been made in the treatment of myeloma with the introduction of thalidomide, its analogue, lenalidomide, and the proteasome inhibitor bortezomib. While these new drugs have improved the outcome of patients with myeloma, the disease still remains incurable. Effectiveness of upcoming new therapies will need to be assessed in the context of anticipated outcome with the currently available therapies. However, given the rapid pace of development over the past decade, outcome of patients who have relapsed on the new therapies remain unclear.
In order to further study this group of patients, we undertook a study of patients who have relapsed on or are refractory to one of the IMiDs (thalidomide or lenalidomide) as well as bortezomib. A total of 300 cases from 8 sites in the US, 5 sites in Europe and one site in Asia were identified for this study. Enrolled patients had relapsed and refractory multiple myeloma, per the EBMT or IMWG response criteria, after at least 2 prior treatments. Patients were refractory to bortezomib, defined as having no response on a prior bortezomib-containing regimen or experiencing disease progression within 60 days of a bortezomib-containing regimen. Patients also should have relapsed, refractory, intolerant, and/or ineligible in the opinion of the investigator, to other therapies, including an IMiD (thalidomide or lenalidomide). Clinical and laboratory data pertaining to the time of diagnosis and from the time of individual relapses were obtained from the clinical records. The date patients satisfied the above entry criteria was defined as time zero.
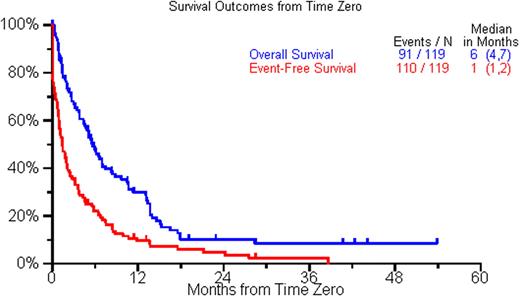
The current analysis includes the first 119 patients with complete data available. The median age at initial diagnosis was 59 years (range, 31-86 yr); 71% were males. The ISS stage distribution at diagnosis was 33%, 39% and 28% for stages 1, 2, and 3 respectively. 19 patients (37%) had high risk disease by FISH [t(4;14), t(14;16), del17p] or by cytogenetics [del 13, hypodiploidy]. The median time from diagnosis to time zero was 3.8 yrs (range 0.2-18.7 yr) and the median reported lines of therapy before time zero was 2. 74% patients had at least 1 autologous stem cell transplant prior to becoming eligible for the study. Following time zero, 77 (65%) pts had a treatment recorded, including an autologous SCT in 21 (18%), with the treatment regimen starting at a median of 1 month after time zero. A variety of treatment regimens were utilized beyond time zero (median=1; range 0-7). Thirty-two (27%) patients had a response any time following time zero, and only 8 had a subsequent response. Response to the first treatment, or supportive care for patients not treated, recorded following time zero included minor response or stable disease (n=14; 12%), PR (n=17, 14%), and VGPR or better (n=6, 5%). Patients treated with SCT had a higher rate of PR or better compared to patients receiving other treatments (85%; 17/20 vs. 26%; 15/57). In a multivariate analysis, the only significant predictor of a PR or better after time zero was younger age (<60 yrs). The median overall survival (OS) and event free survival (EFS) were 6 mos (95% CI, 4-7 mos) and 1 month (95% CI,1-2 mos) respectively. In a multivariate analysis normal creatinine predicted for a better overall survival and having an IgG M protein type predicted for better EFS. A complete analysis from all 300 patients will be presented at the meeting.
This study confirms the poor outcome of patients once they become relapsed and refractory to agents that have become the mainstay of myeloma therapy. The findings highlight the incurable nature of the disease and urgent need to develop newer effective therapeutic agents for this group of patients, who currently do not have effective treatment options.
Overall and Event Free survival form the time point they were considered refractory to bortezomib and relapsed, refractory, or intolerant to thalidomide or lenalidomide (Time zero).
Overall and Event Free survival form the time point they were considered refractory to bortezomib and relapsed, refractory, or intolerant to thalidomide or lenalidomide (Time zero).
Kumar:CELGENE: Research Funding; MILLENNIUM: Research Funding; BAYER: Research Funding; GENZYME: Research Funding; NOVARTIS: Research Funding. Jagannath:Merck: Membership on an entity's Board of Directors or advisory committees; Millennium: Membership on an entity's Board of Directors or advisory committees. Laubach:Novartis: . Palumbo:Ortho Biotech, Janssen-Cilag: Honoraria; Celgene: Honoraria, Speakers Bureau. Richardson:Millennium Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Johnson and Johnson: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Keryx: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. San Miguel:Millennium: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Siegel:Celgene: Speakers Bureau; Millennium: Speakers Bureau. Sonneveld:Janssen-Cilag: Honoraria. Durie:Celgene: Membership on an entity's Board of Directors or advisory committees; Millennium: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.