Abstract
Abstract 3035
Poster Board II-1011
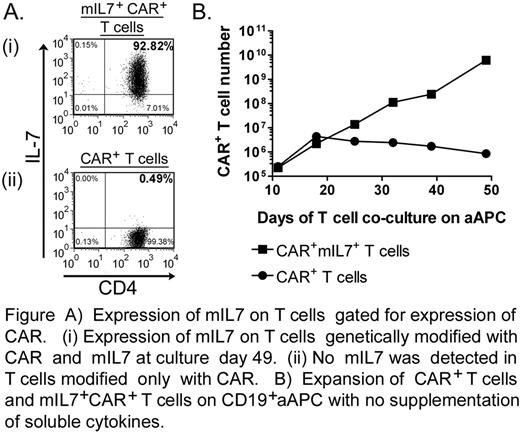
Redirecting specificity to a selected cell surface tumor-associated antigen can be accomplished by the genetic modification of T cells to express a chimeric antigen receptor (CAR). Despite systematic modifications to the CAR endodomains to provide T cells with competent signaling, CAR-dependent T-cell activation may remain incomplete resulting in inferior in vivo persistence leading to a suboptimal therapeutic response. To improve T-cell survival and therefore an anti-tumor response, investigators have co-infused soluble recombinant cytokines such as IL-2 and IL-7. IL-7 is a homeostatic cytokine for T cells and supports survival of memory T cells. To provide IL-7 mediated signaling to T cells in the tumor microenvironment and thus enhance the proliferation and survival of CAR+ T cells, we constructed a version of IL-7 as a novel membrane-bound molecule (mIL7) designed to stimulate T cells in cis and trans. The mIL7 construct was electro-transferred with a CD19-specific CAR (on day 0) into primary T cells via multiple transposition of Sleeping Beauty DNA plasmids. These genetically modified T cells could be numerically expanded ex vivo without additional soluble cytokine supplementation on CD19+ artificial antigen presenting cells (aAPC) derived from K562. This resulted in the preferential outgrowth of T cells expressing both mIL7 and CAR (Figure A and B) while CAR+ T cells receiving no soluble cytokine supplementation did not sustain proliferation (Figure B). These mIL7+CAR+ T cells exhibited redirected specific lysis of CD19+ tumor targets. Significantly, the kinetics of propagation of mIL7+CAR+ T cells in the absence of exogenous cytokine was comparable to CAR+ T cells that were numerically expanded in the presence of soluble IL-2. Signaling by IL-7 receptor signal induction in mIL7+CAR+ T cells appeared comparable to signaling by soluble IL-7 in CAR+ T cells, as assessed by phosphorylation of signal transducer and activator of transcription 5 (STAT5). These data demonstrate that mIL7 can be expressed by CAR+ tumor-redirected T cells to enhance their proliferation without the need for additional cytokine support. These results have implications for the design of clinical trials to evaluate whether mIL7+CAR+ T cells can exhibit enhanced persistence and thus improved therapeutic potential.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.