Abstract
Abstract 3070
Poster Board III-7
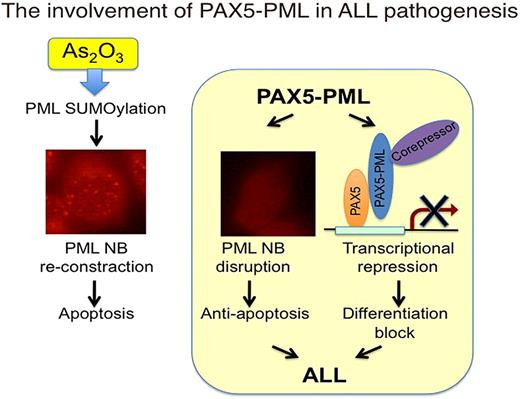
PAX5 is a transcription factor expressed in B lymphoid lineage from pro-B cell to mature B cell, and is required for B-cell development and maintenance. De-regulated and reduced PAX5 activity has been implicated in B cell malignancies both in human disease and mouse models. Recently, approximately 30% of childhood and adult B cell acute lymphoblastic leukemia have aberrancies in PAX5 gene such as deletions, point mutations, or chromosomal translocations. Chromosomal translocation t(9;15)(p13;q24) was found in 2 cases of childhood acute lymphoblastic leukemia and resulted in an in-frame fusion of PAX5 to PML gene. PAX5 moiety of PAX5-PML retains its DNA binding domain but loses its transactivation domain suggesting PAX5-PML will be a dominant negative form of PAX5. PML is originally found as a fusion partner of RARαa in PML-RARαa, an oncoprotein found in acute promyeloid leukemia (APL), and is now thought to be a tumor suppressor and a pro-apoptotic factor. PML-RARαa dominant-negatively affects PML function by disrupting PML nuclear bodies (NBs) where PML exerts its function and gives APL cells survival advantage. These findings give rise a speculation that PAX5-PML not only causes differentiation block by transcriptional repression of PAX5 target genes but also confers a survival advantage by inhibition of PML function. However, no functional analysis has been done for PAX5-PML.
Here, we demonstrate that PAX5-PML had a dominant negative effect on both PAX5 and PML. PAX5-PML inhibited transcriptional activity of PAX5 in luciferase reporter assay. PAX5-PML expression also suppressed expression of CD19, one of the transcriptional targets of PAX5, in B-lymphoid cell line. Surprisingly, PAX5-PML hardly showed DNA binding activity in electro mobility shift assay although it retains DNA binding domain of PAX5, suggesting that inhibition of PAX5 DNA binding by occupation of PAX5 binding sites would not be the mechanism for PAX5-PML to inhibit PAX5 transcriptional activity. On the other hand, co-expression of PAX5-PML inhibited PML sumoylation, an essential post-translational modification for PML to form NBs, and altered PML localization from NB pattern to diffuse nuclear pattern. Furthermore, treatment with arsenic trioxide, a therapeutic reagent for APL which induces enhancement of PML sumoylation, reconstitution of PML NBs, and apoptosis in APL cells, induced recovery of PML sumoylation and reconstitution of PML NBs also in cells expressing PAX5-PML. More importantly, arsenic trioxide treatment of PAX5-PML expressing HeLa cells, which showed resistance to PML dependent apoptosis, overcame anti-apoptotic effect of PAX5-PML. These data suggest the involvement of this fusion protein in the leukemogenesis of B-ALL in dual-dominant negative manner and the possibility that some cases of ALL can be treated with arsenic trioxide.
Naoe:Kyowa Hakko Kirin, Wyeth and Chugai: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.