Abstract
Abstract 3431
Poster Board III-319
Chemoimmunotherapy forms the basis of treatment for patients (pts) with CLL. FCR is an effective salvage regimen in relapsed pts who are not heavily pretreated or refractory to fludarabine (F). Alemtuzumab (A) has demonstrated efficacy in F-refractory pts and pts with 17p del. We combined A with FCR to improve remission rates for pts with relapsed and refractory CLL. We present a long-term follow-up of 80 pts who received CFAR as salvage therapy.
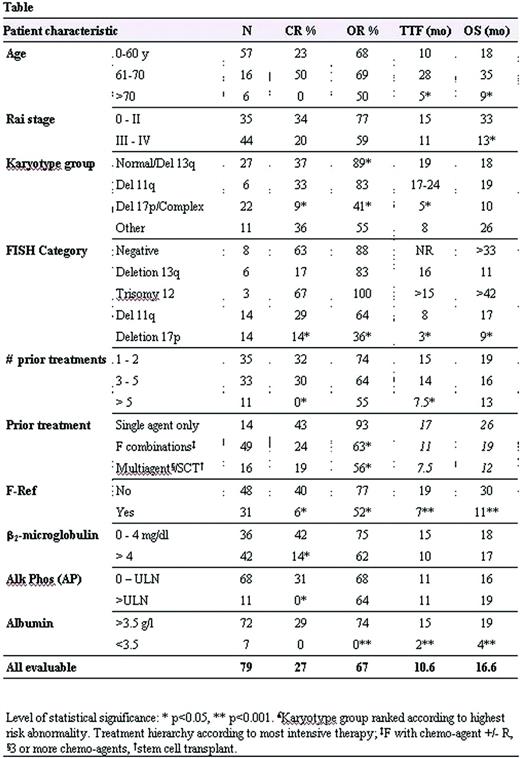
Eighty pts with previously treated CLL received CFAR consisting of C-250mg/m2 d3-5; F-25mg/m2 d-3-5; A-30mg IV d1,3,5; R 375-500 mg/m2 d2, every 28 days for an intended total of 6 courses. Premedication prior to antibody therapy was with steroids. Pts received prophylactic TMP-SMZ DS and either valacyclovir or valgancyclovir. Responses were evaluated by 1996 NCI-WG criteria. Prognostic factors (including Rai stage, B2M, LDH, CD38, metaphase karyotype, ZAP70, IgVH, FISH) were assessed prior to initiating therapy (table). Overall survival (OS) was defined as time from initiation of therapy to death. Time to treatment failure (TTF) was defined as time from treatment to resistance or progression of disease, transformation to other hematological malignancies or death from any cause.
Eighty pts received at least one courses of CFAR, 61 pts (76%) received at least 3 courses and 14 pts (18%) received all 6 courses of therapy (median 3). One patient was not evaluable for response. Median number of prior treatments was 3 (1-14) with 75% of pts having received prior F in combination with alkylator (including FCR 56%) or mitoxatrone (N); 39% pts were refractory to fludarabine.
Of 79 pts, there were 21 CR (27%), 3 NPR (4%) and 29 PR (37%) for an ORR 67%. Predictors for higher likelihood of CR included B2M<4mg/l, normal serum Alk phos (AP), sensitivity to F or alkylators, ≤5 prior treatments, absence of abnormal chromosome 17 or complex cytogenetics, or absence of 17p del on FISH. Median OS and TTF were 16.6 and 10.6 months respectively for all pts. For pts achieving CR, median OS (50+ months) and TTF (28+ months) have not been reached. Multivariate analysis correlated improved overall survival with age <70y, ANC > 1.5 ×109/l, albumin >3.5 g/dl and fludarabine sensitivity. Variables independently associated with longer time to progression in responding pts were age <70y, lower ALC, lower AP and ≤5 prior treatments. In comparison with pts who received FCR as salvage therapy, pts who had received 3 to 5 prior treatments or prior F combination chemotherapy demonstrated superior CR with CFAR.
Causes of early cessation of therapy included cytopenia (n=15), hemolytic anemia (n=2), infection (n=22), and lack of response or progression of disease (n=11). G3/4 haematological toxicity included neutropenia (62% of courses), thrombocytopenia (34%) and anemia (16%). G3/4 infectious complications included pneumonia (n=7) or sepsis (n=3). Other infections included minor infections (n=32), CMV reactivation (n=13), or other herpes virus infections (n=5). Patients on prophylactic valganciclovir had a lower rate of CMV reactivation compared to valacyclovir (24% v. 3%, p=0.01)
CFAR is an effective therapy in this group of heavily treated pts with CLL. We demonstrated favorable responses in pts younger than 70 years with F-sensitive disease who had 5 or fewer prior therapies. CFAR is a good salvage regimen for pts who have received prior FC or FN based regimen.
Garcia-Manero:Cyclacel, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.