Abstract
Abstract 3768
Poster Board III-704
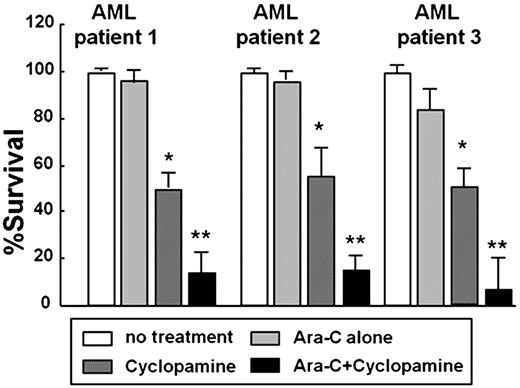
Aberrant reactivation of Hedgehog (Hh) signaling has been described in a wide variety of human cancers and its role in maintenance of self-renewal of cancer stem cells. However, it is unclear whether Hh signaling contributes to the growth, survival and drug resistance of acute myeloid leukemic (AML) cells. Regarding normal CD34+hematopoietic cells, we previously showed that Indian Hh (Ihh) and its receptor molecules, Patched and Smoothened are expressed in cord blood (CB) CD34+ cells and Ihh regulate proliferation of short-term hematopoietic repopulating cells in vivo. In the present study, we assessed the possibility that Hh pathway activation contributes to survival and drug resistance of acute myeloid leukemic (AML) cells. Hh signaling in bone marrow (BM) CD34+ cells, leukemic cell lines and primary CD34+ leukemic cells were screened by RT-PCR and a Hh signaling reporter assay. We have found that Ihh were expressed in normal BM CD34+ cells, primary CD34+leukemic cells and most of human AML cell lines, HL60, U937, KG1, Kasumi-1, Kasumi-3 and TF-1. In contrast, Hh receptor components, Patched and Smoothened, were detected in BM CD34+ cells, primary CD34+leukemic cells (n=3) and cytokine responsible CD34+ AML cell lines such as Kasumi-1, Kasumi-3 and TF-1. Moreover, the downstream transcription factor GLI1 or GLI2 were expressed in BM CD34+ cells, primary CD34+leukemic cells and these CD34+ cell lines, indicating that Hh signaling was active in these cells. We further assessed whether Hh signaling transmit to GLI1 using GLI1-responsive luciferase assay. GLI-responsive reporter plasmid (TK-6GBS-Luc) was transduced into these cells in the presence or absence of anti-Hh neutralizing antibody 5E1. TK-6GBS-Luc-transduced Kasumi-1, Kasumi-3 and TF-1 cells showed high luciferase activity. Furthermore, the luciferase activity of these cells was significantly reduced in the presence of 10 μg/ml antibody 5E1. These results clearly indicated that Hh signaling could transmit into these CD34+ leukemic cell lines in autocrine manner. We next examined the effect of Hh inhibitors on these CD34+ leukemic cells. Inhibition of Hh signaling with the naturally derived chemical Smoothened antagonist Cyclopamine, endogenous Hh inhibitor Hedgehog-interacting protein or anti-Hedgehog neutralizing antibody induces apoptosis in these cells after 48 hr exposure although these CD34+ cell lines exhibit resistance to cytarabine (Ara-C) exposure. In contrast, cyclopamine did not affect growth and survival of the U937 or HL-60 cell line that lacks expression of Hh receptor components. Furthermore, combination of 10 μM cyclopamine significantly reduced the drug resistance against Ara-C in CD34+ cell lines as well as primary CD34+AML cells (Figure). These results suggest that Hh pathway activation is a feature of CD34+ myeloid leukemic cells and inhibition of Hh signaling pathway in combination with Ara-C etc. may be a new therapeutic strategy to eradicate CD34+ AML stem cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.