Abstract
Abstract 4670
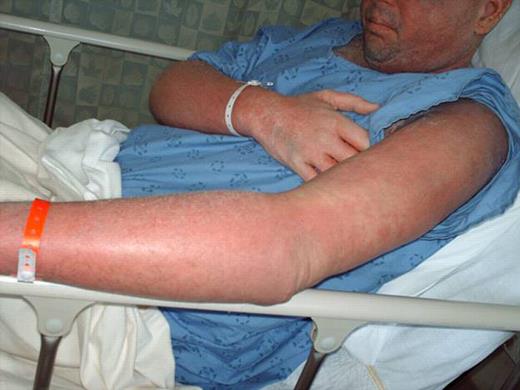
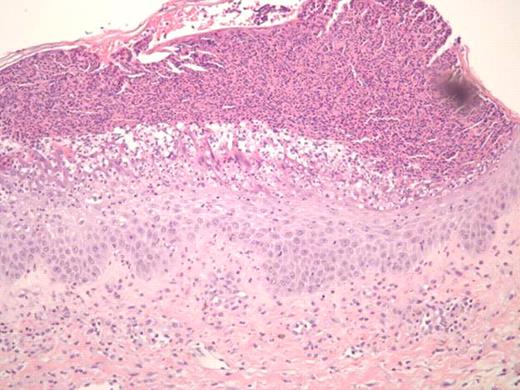
Treatment of sclerodermatous chronic graft versus host disease (cGVHD) remains a daunting challenge. Despite developments in prophylaxis for cGVHD, supportive care measures remain as the primary mode of therapy, with little evidence of treatments that reverse the process. Steroids and immunosuppressants are often ineffective, and extracorporeal photopheresis has been used with varying success. Recently, case reports have reported on the successful use of imatinib in treating sclerodermatous GVHD (Moreno-Romero et al, 2008; Leonardo Magro et al., 2009). We report a case of a 40-year-old male patient with extensive sclerodermatous cGVHD. In 2005 he underwent an unrelated stem cell transplant (SCT) for chronic myeloid leukemia. His transplant course was complicated by thrombotic thrombocytopenic purpura (TTP) associated with all standard immunosuppressive drugs (cyclosporine, tacrolimus and sirolimus). Two years later, the patient developed sclerodermatous GVHD and was given plaquenil, steroids, and photopheresis to manage this complication. Unfortunately, after months of treatment, he showed progressive disease, including increased contractures, and difficulties with inspiration. Based on case reports, and the patient's tolerance of imatinib prior to SCT, he was started on imatinib for his sclerodermatous GVHD. Within 5 days he developed generalized erythema, pustular rash, and skin sloughing (Figure 1). He was hospitalized for this severe reaction and was treated with high dose steroids after which, his symptoms began to improve. Resolution of erythema did not occur until months following initial exposure. Histological differential diagnosis included pustular psoariasis and Sneedon-Wilkinson syndrome. The skin biopsy revealed subcorneal pustular dermatosis (Figure 2). In view of this history, it is suggested that the exanthematous pustulosis was induced by imatinib in this patient. We recommend that close attention is paid to patients with cGHVD who are treated with imatinib and to intervene early in those who develop severe cutaneous reactions by stopping imatinib and starting appropriate therapy.
SEQ – Imatinib induced generalized erythema, pustular rash, and skin sloughing developed five days into treatment.
SEQ – Imatinib induced generalized erythema, pustular rash, and skin sloughing developed five days into treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.