Abstract
Abstract 523
The advent of reduced intensity conditioning (RIC) regimens permits the delivery of allogeneic stem cell transplant (alloSCT) with a potentially curative graft-versus-leukaemia effect in patients with acute myeloid leukaemia (AML) whose outcome with conventional chemotherapy or conventional myeloablative SCT would be poor. Although widely utilised in the management of older patients with AML in 1st complete remission (CR) there are no randomised trials or “donor v. no donor” analyses supporting a role for RIC alloSCT in this setting.
We have examined the impact of RIC allograft in 1st CR on the outcome of patients over the age of 45 treated within the UK MRC AML15 trial. Patients with favourable cytogenetics (t8;21, inv16, t15;17) were not eligible for transplant and were thus excluded from the analysis, which was restricted to patients with intermediate and adverse cytogenetics (defined as: -5, -7, 5q-, abn3q, or 5 or more abnormalities). Patients who were not considered suitable for transplant at the tissue typing stage were excluded. The data was initially analysed by treatment actually received, using the method of Mantel-Byar to allow for time to transplant. The analysis was stratified by presence of a sibling donor, and by cytogenetic risk group. End points were death in 1st CR (DCR), relapse risk (RR), relapse free survival (RFS) and overall survival (OS). A hazard ratio (HR) less than 1 indicates benefit for alloSCT/donor.
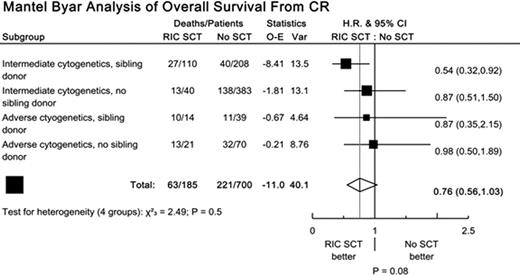
A total of 700 patients were eligible, of whom 185 received RIC alloSCT in 1st CR (120 sibling, 65 MUD), 109 patients had adverse cytogenetics, and 247 patients had a matched sibling donor. Median follow up from 1st CR was 2.5 years. Mantel-Byar analysis showed increased DCR for RIC alloSCT (HR 6.73; CI 3.49-12.97; p<0.001), but better RR (HR 0.40; CI 0.29-0.55; p<0.001) and RFS (HR 0.68; CI 0.51-0.90; p=0.007). There was some evidence of a benefit on OS (HR 0.76; CI 0.56, 1.03; p=0.08) but the difference was not significant. Whilst the observed treatment effect was greatest in patients with intermediate cytogenetics and a matched sibling donor, there was no significant heterogeneity of effect between groups (figure). To partially allow for selection biases, Cox proportional hazards modelling was performed. Results were in line with the Mantel-Byar analyses, but with a significant benefit for RIC alloSCT on OS (p=0.03). Finally, a sibling donor vs no sibling donor analysis was carried out. 50% of patients in the sibling donor arm received RIC SCT, compared with 13% in the no sibling donor arm. This analysis showed no significant benefit or detriment for any outcome measure (OS: HR 0.94; CI 0.74, 1.19; p=0.6).
In the absence of randomised trials, evaluation of the benefit of SCT is difficult. Mantel-Byar analyses may well be subject to selection biases; sibling donor versus no sibling donor analyses are difficult to interpret in this context, since non-receipt of SCT in the donor group and use of MUD SCT (in the no donor group) both tend to reduce any effect. Hence, these results need to be interpreted cautiously. While our data are consistent with the possibility that RIC alloSCT in 1st CR may be effective in some patients aged 45+ (those with both a sibling donor and intermediate cytogenetics being the most likely candidates based on the current evidence), further data are clearly required, ideally from large scale prospective randomised controlled trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.