Abstract
Abstract 622
Several gene-expression signatures are predictive of prognosis in diffuse large-B-cell lymphoma (DLBCL), but the lack of practical methods for a genome-scale analysis has restricted their routine clinical applicability.
We studied genes whose expression had been reported to predict survival in DLBCL, attempting to validate genes and prognostic models with robust survival associations that are amenable to rapid diagnostic testing.
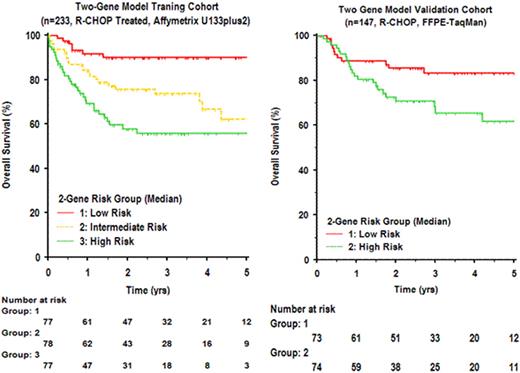
Among a previously described set of 6-genes shown to predict survival independent of measurement platform or therapy era (Lossos, et al. 2004 NEJM 350:1828), we identified LMO2 as the single gene with strongest independent prognostic value in 3 independent cohorts of patients with DLBCL. To assess the independent contribution of other genes in predicting survival, using existing microarray gene expression data (Lenz, et al. 2008 NEJM 359:2313), we evaluated all pairwise models that included LMO2 toward construction of a robust bivariate survival predictor. Among 54674 possible models, one combining expression of LMO2 with TNFRSF9 (encoding 4-1BB, also known as CD137) emerged as among the best in cross-validation when assessed in training (n=233) and test (n=181) cohorts. This bivariate predictor remained prognostic in both CHOP (p=1.7e-6) and R-CHOP (p=6.5e-8) therapy eras, was highly independent of the International Prognostic Index, Cell-of-Origin classification, 6-gene predictive model, ‘stromal' model, and added significantly to their prognostic power. While LMO2 expression was highly restricted to tumor cells and was linked to Cell-of-Origin (GCB, p=2.2e-16), TNFRSF9 expression was highest in non-tumor cells (P=0.02), particularly in an activated subset of infiltrating CD8 T-cells. To validate this bivariate model and devise a practical diagnostic assay, we used quantitative real-time polymerase-chain-reaction to measure the expression of LMO2 and TNFRSF9 as well as other components of the 6-gene model (BCL2, BCL6, FN1, CCL3, and CCND2) in diagnostic formalin fixed and paraffin-embedded samples of lymphoma from an independent set of 147 patients with de novo DLBCL treated with R-CHOP. The IPI distribution for these patients was: 0-1 factor (n=70), 2 factors (n=40), 3 factors (n=26), ≥4 factors (n=11). In univariate and multivariate analyses of this independent cohort, LMO2 and TNFRSF9 expression remained individually prognostic of both progression free and overall survival. The bivariate model combining LMO2/TNFRSF9 could be used to stratify distinct risk groups for overall survival (p=0.004), and remained independent of IPI.
Measurement of the expression of two genes integrating contributions of tumor cells and the tumor microenvironment is sufficient to predict overall survival in patients with DLBCL treated with R-CHOP.
Advani:Seattle Genetics, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.