Abstract
We show that endothelial cell (EC)–generated vascular guidance tunnels (ie, matrix spaces created during tube formation) serve as conduits for the recruitment and motility of pericytes along EC ablumenal surfaces to facilitate vessel maturation events, including vascular basement membrane matrix assembly and restriction of EC tube diameter. During quail development, pericyte recruitment along microvascular tubes directly correlates with vascular basement membrane matrix deposition. Pericyte recruitment to EC tubes leads to specific induction of fibronectin and nidogen-1 (ie, matrix-bridging proteins that link together basement membrane components) as well as perlecan and laminin isoforms. Coincident with these events, up-regulation of integrins, α5β1, α3β1, α6β1, and α1β1, which bind fibronectin, nidogens, laminin isoforms, and collagen type IV, occurs in EC-pericyte cocultures, but not EC-only cultures. Integrin-blocking antibodies to these receptors, disruption of fibronectin matrix assembly, and small interfering RNA suppression of pericyte tissue inhibitor of metalloproteinase (TIMP)-3 (a known regulator of vascular tube stabilization) all lead to decreased EC basement membrane, resulting in increased vessel lumen diameter, a key indicator of dysfunctional EC-pericyte interactions. Thus, pericyte recruitment to EC-lined tubes during vasculogenesis is a stimulatory event controlling vascular basement membrane matrix assembly, a fundamental maturation step regulating the transition from vascular morphogenesis to stabilization.
Introduction
Considerable interest has focused on determining how support cells such as pericytes affect the vasculature during development and in various disease states.1-3 An important step in vascular morphogenesis is the recruitment of pericytes, which, in conjunction with endothelial cells (ECs), establish conditions to facilitate tube stabilization.2,4-11 EC factors such as platelet-derived growth factor-BB play a critical role in these events, and failure to recruit pericytes during development leads to vascular instability and regression.4,12-14 Thus, abnormalities in EC-pericyte interactions lead to embryonic death due to failures in vascular remodeling and stabilization.2,11,12 Recently, we reported that pericyte recruitment to EC tubes induced stabilization by affecting the production and function of EC-derived tissue inhibitor of metalloproteinase (TIMP)-2 and pericyte-derived TIMP-3, which led to inhibition of both tube regression and morphogenic events through blockade of particular matrix metalloproteinases (MMPs).15 The molecular mechanisms controlling how pericytes affect vascular tube stabilization are being elucidated and include the identification of key growth factors regulating these events, such as angiopoietin-1, vascular endothelial growth factor (VEGF), and transforming growth factor (TGF)-β, signaling pathways involving Notch and Ephrins, as well as the presentation of MMP inhibitors such as TIMP-3.3,7-9,11,15-23
Recent work from our laboratory has identified a key regulatory step in vessel formation, which is a requirement for membrane type 1 (MT1)-MMP in both EC lumen and vascular guidance tunnel formation.24 Vascular guidance tunnels are generated in conjunction with EC tube morphogenesis and represent physical spaces throughout the matrix that serve as conduits for tube assembly, remodeling, and recruitment of other cell types such as pericytes.24
In this study, we show that pericyte recruitment to developing EC tubes in vitro and in vivo induces vascular basement membrane matrix assembly, which is a critical step in vessel maturation. EC-pericyte interactions regulate increased expression of basement membrane protein genes and proteins (eg, fibronectin and laminins) as well as integrins (eg, α5β1, α3β1, α6β1) that recognize the remodeled matrices to control this process. These changes occur specifically in EC-pericyte cocultures and not in EC-only cultures. Overall, our findings show that pericyte interactions with EC tubes critically regulate vascular maturation and stabilization events by (1) stimulating vascular basement membrane formation; (2) inducing integrins that recognize this newly deposited matrix; and (3) stabilizing this matrix through inhibition of proteolysis.
Methods
Reagents
Basic fibroblast growth factor was purchased from Millipore. α1-α5 integrin-blocking antibodies (MAB1973Z, 1950Z, 1952Z, 16983Z, 1956Z) were from Chemicon International; α6 blocking antibody (Go H3, ab19765) was from Abcam. Recombinant human stromal-derived factor-1α (CXCL12), stem cell factor, and interleukin-3 were from R&D Systems. Antibodies were as follows: platelet EC adhesion molecule/CD31 (DakoCytomation; M0823), TIMP-3 (Chemicon International; MAB3318), laminin (Sigma-Aldrich; L9393), laminin α4 (Alexis; C51C2), laminin β1 (Chemicon International; MAB1921P), collagen type I (Sigma-Aldrich; C2456), collagen type IV (Chemicon International; AB769), nidogen 1 and 2 (R&D Systems; AF2570, AF3385), perlecan (Zymed Laboratories; 13-4400), fibronectin (Sigma-Aldrich; poly-F3648, mono-F0916), smooth muscle actin (SMA; Sigma-Aldrich; A2547), and α-tubulin (Sigma-Aldrich; T5168). The 70-kDa fibronectin fragment (Sigma-Aldrich; F0287) and custom-designed reverse transcription–polymerase chain reaction (RT-PCR) primer sets were from Sigma-Aldrich. Antibodies for staining quail chorioallantoic membranes (CAMs) were as follows: nidogen/entactin (1G12; W. Halfter, University of Pittsburgh), EC surface (quail; QH1; F. Dieterien, Institut d'Embryologie du CNRS et du College du France), fibronectin (B3/D6; D. Fambrough, Johns Hopkins University), and laminin (3H11; W. Halfter), and were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by University of Iowa Department of Biological Science.
Cell culture
Human umbilical vein ECs (HUVECs) and dermal microvascular ECs were from Lonza and used from passages 2 to 6. Bovine retinal pericytes and human brain pericytes (Lonza) were cultured in Dulbecco modified Eagle medium/10% fetal bovine serum and used from passages 2 to 8. Bovine retinal pericytes were isolated, as previously described.25
Tube assembly (vasculogenic) assays
ECs were either suspended alone or with pericytes in 2.5 mg/mL collagen type I matrices, and assays were performed, as described,26 except that the culture media did not contain phorbol ester or VEGF, but retained reduced serum supplement and fibroblast growth factor-2 at 40 ng/mL. In addition, stem cell factor, stromal-derived factor-1α, and interleukin-3 were each added at 200 ng/mL in the collagen matrix. Pericytes were added at 20% of the total EC level. Cultures were allowed to assemble over time and fixed at predetermined time points and processed, as described.26
Transfection of ECs and pericytes with small interfering RNA
Smart pool small interfering RNAs (siRNAs) for TIMP-3, LaminA, and luciferase controls were purchased from Dharmacon and used as described previously.15,26 Stealth siRNAs to collagen IV were purchased from Invitrogen, with the sequences as follows: human No. 1, CCUCAUCU GUGAUAUAGACGGAUAU and UCCUGUAACACCUUGCUGGCCUUUC; human No. 2, AUAUCCGUCUAUAUCACAGAUGAGG and GAAAGGCCAGCAAGGUGUUACAGGA; and bovine No. 1, ACUCUUUGCUCUACGUGCAAGGCAA and CGAUAGAGCGGAGCGAGA-UGUUCAA.
Immunostaining of cultures
Cultures were fixed in 2% paraformaldehyde for at least 1 hour before the addition of blocking solution (phosphate-buffered saline containing 1% bovine serum albumin). Gels were incubated with primary antibodies overnight at 4°C and then were washed in phosphate-buffered saline and incubated for 2 hours with secondary antibodies. Final washes were performed over several hours, and the cultures were examined by immunofluorescence microscopy.
PCR analysis
Cells were suspended in three-dimensional (3D) collagen type I and allowed to assemble for 1, 3, or 5 days. Collagen gels were removed and collagenase treated, and cells were lysed using the Ambion ToTALLY RNA Kit (No. 1910) to isolate total RNA. cDNA was produced using the Stratagene AccuScript High Fidelity 1st Strand cDNA Synthesis Kit, and RT-PCR and analysis of differentially expressed genes were performed, as described.27 Primer sets were made (300-400 bp) in both a gene- and species-specific manner and are included in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Electron microscopy
Cultures were fixed in 3% glutaraldehyde (electron microscopic grade; Sigma-Aldrich) and processed for electron microscopy, as described.28 Images were acquired using a Hitachi S4700 Field Emission scanning electron microscope (FESEM; Hitachi).
Microscopy and imaging
Time-lapse videomicroscopy and fluorescence imaging were performed using a fluorescence inverted microscope (Eclipse TE2000-E; Nikon), and the analysis software MetaMorph (Molecular Devices) was used, as described.26 Fluorescence intensity of cultures was determined by tracing the borders of EC tube structures versus EC/pericyte tube structures and converting to average pixel intensity for the areas traced.
Statistical analysis
Statistical analysis of selected EC vasculogenic and lumen formation data was performed using SPSS 11.0 (SPSS) or Microsoft Excel (Microsoft). Analysis of variance was used to compare means of 2 or more groups. Statistical significance was set at minimum with P less than .05. Student t tests were used when analyzing 2 groups within a single experiment (with n ≥ 10).
Results
Vascular guidance tunnels generated during EC tube formation represent matrix templates for dynamic EC-pericyte interactive events during vascular wall assembly
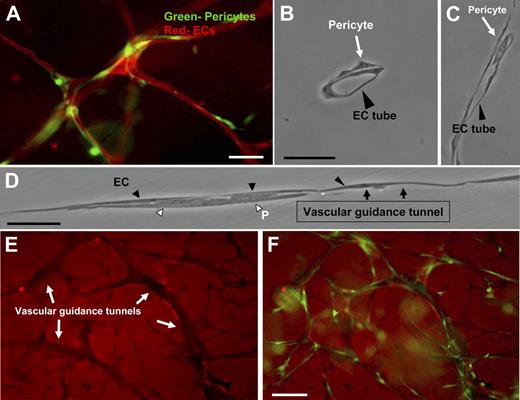
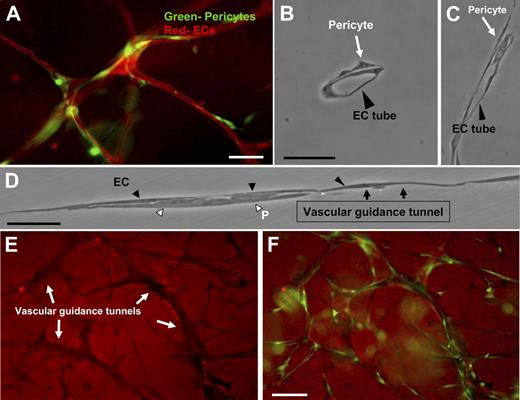
We have developed a novel EC-pericyte coculture model that strongly mimics primary vascular plexus assembly during development to study the molecular events underlying EC-pericyte interactions (Figure 1A; supplemental Figure 1). As shown in Figure 1, vascular guidance tunnel spaces, generated by ECs through MT1-MMP–dependent proteolytic events (detected using anti–collagen type I antibodies) during the EC lumen and tube formation process,24 serve as matrix conduits whereby pericytes are recruited during EC-pericyte tube coassembly. They provide a platform and physical template for subsequent events leading to vessel wall maturation (Figure 1E-F). CD31 immunostaining of cocultures containing green fluorescent protein (GFP)–labeled pericytes was used to determine the level of association of the 2 cell types in this model (Figure 1A; supplemental Figure 2B). Initially, ECs (ie, HUVECs) and pericytes (ie, bovine retinal) are randomly seeded as single cells into the 3D collagen matrix culture system, and over 5 days, coassembly of EC-lined tubes and pericytes occurs (supplemental Figures 1 and 2B). Thin plastic sections reveal these tubes to be lumen-containing structures with pericytes selectively recruited to the ablumenal tube surface (Figure 1B-D; supplemental Figure 2B). In addition, pericytes migrate within vascular guidance tunnels along both the EC basal surface and the inner surface of tunnels (supplemental Videos 2-4; Figure 1). Time-lapse movies illustrate the dynamic nature of these tube assembly events (supplemental Videos 1-4). Both ECs and pericytes are highly migratory during initial tube assembly and later maturation events. For comparison, a time-lapse movie of EC-only cultures is shown (supplemental Video 5). These results illustrate the marked ability of pericytes to influence the pattern and caliber of microvessels created in 3D collagen matrices (supplemental Videos 1-4 vs 5). Interestingly, once vascular guidance tunnels have been formed, both ECs and pericytes migrate within these conduits in a MMP-independent manner (supplemental Figure 2A).
Recruitment of pericytes to the ablumenal surface of EC-lined tubes within vascular guidance tunnels using a new model of EC-pericyte tube coassembly in 3D collagen matrices. EC-pericyte cocultures were allowed to assemble randomly within a 3D collagen matrix for 5 days, fixed, and processed for immunofluorescence or cross-sectioning (HUVECs-bovine pericytes). (A) ECs were stained with the endothelial specific marker, CD31, whereas pericytes were GFP labeled. Bar equals 25 μm. (B) Plastic sections show association of pericytes with endothelial tubes on the abluminal face. Bar equals 25 μm. (C-D) EC-pericyte cocultures were allowed to form, and a thin plastic section of the monolayer was examined along an EC tube surface. ECs form a continuous layer with pericytes recruited to the basal surface. Black arrows indicate the border of the vascular guidance tunnel and the entrance of pericytes within these borders. Bar equals 50 μm. (E) Randomly placed ECs and GFP pericytes were allowed to form within the 3D collagen matrices for 5 days. These cultures were then immunostained with an anti-collagen type I antibody. (F) Images of the GFP pericytes were also obtained and overlaid with the corresponding collagen type I stain. Images show the clear presence of pericytes within vascular guidance tunnels. Bar equals 50 μm.
Recruitment of pericytes to the ablumenal surface of EC-lined tubes within vascular guidance tunnels using a new model of EC-pericyte tube coassembly in 3D collagen matrices. EC-pericyte cocultures were allowed to assemble randomly within a 3D collagen matrix for 5 days, fixed, and processed for immunofluorescence or cross-sectioning (HUVECs-bovine pericytes). (A) ECs were stained with the endothelial specific marker, CD31, whereas pericytes were GFP labeled. Bar equals 25 μm. (B) Plastic sections show association of pericytes with endothelial tubes on the abluminal face. Bar equals 25 μm. (C-D) EC-pericyte cocultures were allowed to form, and a thin plastic section of the monolayer was examined along an EC tube surface. ECs form a continuous layer with pericytes recruited to the basal surface. Black arrows indicate the border of the vascular guidance tunnel and the entrance of pericytes within these borders. Bar equals 50 μm. (E) Randomly placed ECs and GFP pericytes were allowed to form within the 3D collagen matrices for 5 days. These cultures were then immunostained with an anti-collagen type I antibody. (F) Images of the GFP pericytes were also obtained and overlaid with the corresponding collagen type I stain. Images show the clear presence of pericytes within vascular guidance tunnels. Bar equals 50 μm.
Pericytes catalyze extracellular deposition of basement membrane matrix components during vessel wall assembly in 3D collagen matrices
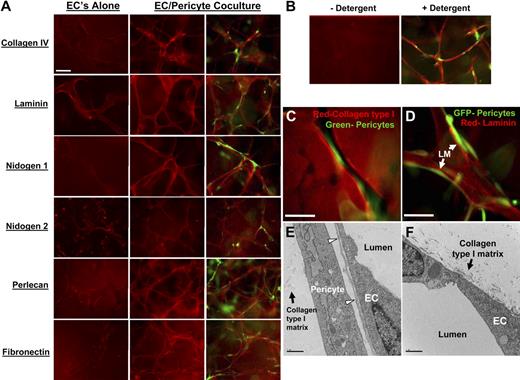
A critical step during vasculogenesis and other blood vessel assembly events is the development of an endothelial basement membrane matrix.27,29-31 The fact that pericytes are recruited into EC-generated vascular guidance tunnels provides insight into how basement membrane matrix deposition between ECs and pericytes may be controlled. Having both ECs and pericytes located in the same matrix space, with no predefined matrix in between, allows for both cell types to move and scan the surface of the opposing cell type. To address whether pericytes affect EC basement membrane protein deposition, several experimental approaches were used. We used a detergent-free immunofluorescence staining approach so that we would only be examining basement membrane components that are deposited extracellularly (rather than a combination of intracellular and extracellular staining). As shown in Figure 2A, extracellular deposition of these components is markedly increased in EC-pericyte cocultures compared with EC-only cultures. In addition, pericyte-only cultures showed no staining (supplemental Figure 3D). Collagen type IV, laminin, nidogens, perlecan, and fibronectin are all deposited to a much greater extent when ECs and pericytes are present together during tube assembly. The intracellular protein α-tubulin was used as a control (Figure 2B); it was only detected after detergent permeabilization and not detected without detergent. Quantitation of staining intensity was performed to verify the visual findings (supplemental Figure 4A-B). These data show that a strong synergistic relationship exists between these 2 cell types during critical extracellular remodeling events necessary for vasculogenic plexus assembly. To determine the position of basement membrane matrix components in relation to ECs, pericytes, and vascular guidance tunnels (Figures 1 and 2), we stained for laminin, which shows its presence between the EC and pericyte surfaces within tunnel spaces (Figure 2D). ECs and pericytes from different sources such as human dermal microvascular ECs and human brain-derived pericytes will also coassemble into tube networks and deposit basement membrane matrices (supplemental Figure 3A), in the same manner as HUVECs and bovine retinal pericytes (Figure 2). In addition, HUVECs combined with human brain pericytes deposit basement membrane matrices (supplemental Figure 3B). Thus, under the culture conditions that we have established, different combinations of ECs and pericytes will coassemble into tube structures with an associated basement membrane matrix.
Extracellular deposition of basement membrane components is observed only when EC are cocultured with pericytes during EC-pericyte tube coassembly. ECs were cultured alone or with pericytes and allowed to randomly assemble in a 3D collagen for 5 days, fixed, and processed for immunofluorescence (HUVECs-bovine pericytes). Staining was done without detergent to assure extracellular staining only. (A) Basement membrane matrices were stained for the following: collagen IV, laminin, nidogens 1 and 2, perlecan, and fibronectin (red staining), and GFP pericytes (green staining). EC-only cultures show very little extracellular deposition of the indicated molecules. EC-pericyte cocultures show a dramatic increase in extracellular deposition of the basement membrane components along with fibronectin. (B) α-Tubulin staining was conducted as a methods control. (C) Pericytes are localized within vascular guidance tunnels; EC-GFP pericyte cocultures in 3D collagen gels were allowed to coassemble, and then stained with an anti-collagen type I antibody. Bar equals 25 μm. (D) Separate gels from the same cultures were then immunostained with an anti-laminin antibody that shows localized laminin staining between the 2 cell types. Bar equals 25 μm. (E-F) Electron microscopy was performed, and representative images are shown revealing the deposition of basement membrane matrix material (arrowheads) between ECs and pericytes, corresponding to the position of anti-laminin staining shown in (D); this deposition and organization is not seen when ECs are cultured alone (F). Bar equals 2 μm.
Extracellular deposition of basement membrane components is observed only when EC are cocultured with pericytes during EC-pericyte tube coassembly. ECs were cultured alone or with pericytes and allowed to randomly assemble in a 3D collagen for 5 days, fixed, and processed for immunofluorescence (HUVECs-bovine pericytes). Staining was done without detergent to assure extracellular staining only. (A) Basement membrane matrices were stained for the following: collagen IV, laminin, nidogens 1 and 2, perlecan, and fibronectin (red staining), and GFP pericytes (green staining). EC-only cultures show very little extracellular deposition of the indicated molecules. EC-pericyte cocultures show a dramatic increase in extracellular deposition of the basement membrane components along with fibronectin. (B) α-Tubulin staining was conducted as a methods control. (C) Pericytes are localized within vascular guidance tunnels; EC-GFP pericyte cocultures in 3D collagen gels were allowed to coassemble, and then stained with an anti-collagen type I antibody. Bar equals 25 μm. (D) Separate gels from the same cultures were then immunostained with an anti-laminin antibody that shows localized laminin staining between the 2 cell types. Bar equals 25 μm. (E-F) Electron microscopy was performed, and representative images are shown revealing the deposition of basement membrane matrix material (arrowheads) between ECs and pericytes, corresponding to the position of anti-laminin staining shown in (D); this deposition and organization is not seen when ECs are cultured alone (F). Bar equals 2 μm.
To further confirm the conclusion that pericytes stimulate EC basement membrane assembly, electron microscopy was performed using HUVEC and bovine pericyte cocultures. As shown in Figure 2E, continuous deposition of basement membrane matrix material was detected between ECs and pericytes (Figure 2E arrowheads; supplemental Figure 2C), which is consistent with the immunofluorescence staining (Figure 2A,D). Basement membrane matrix deposition was not observed in EC-only cultures in 3D collagen matrices by electron microscopy in our current (Figure 2F) or past studies.28 Overall, our data strongly support the concept that pericyte recruitment to EC-lined tubes catalyzes extracellular deposition of basement membrane matrix components using both immunostaining and electron microscopic methodologies.
Pericyte recruitment to microvascular tubes in the quail CAM reveals coincident vascular basement membrane matrix assembly
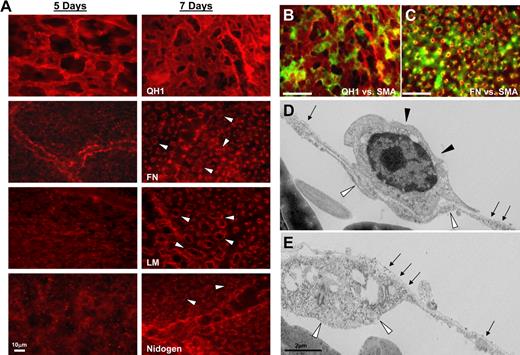
To address the role of pericyte recruitment in regulating vascular basement membrane matrix assembly in vivo, we performed experiments using quail embryos at different developmental stages (Figure 3; supplemental Figure 5A). Double staining of pericyte markers (ie, anti–α-SMA in green) versus EC-specific markers (ie, anti-QH1 in red) reveals that there is strong association between the 2 cell types by day 7 of development (Figure 3B,D) in quail CAMs, whereas there is much less association at day 5 (supplemental Figure 5). Electron microscopic images at day 5 reveal ECs still undergoing tube morphogenesis, with evidence of tube assembly and EC vacuolation (an indicator of ongoing lumenogenesis) during these events. Staining of quail CAMs using the detergent-free immunofluorescence protocol allowed us to assess extracellular deposition of basement membrane matrix components (Figure 3) as in our in vitro experimental models (Figure 2). At 7 days of development, both immunostaining analysis and electron microscopy revealed extracellular deposition of basement membrane proteins around capillary-like microvessels (Figure 3A,D-E), which is not observed at embryonic day 5 (Figure 3A). Only by the 7-day time point is there a recognizable and continuous basement membrane by electron microscopy around these vessels (Figure 3E), a finding that concurs with our immunofluorescence results (Figure 3A). The 2 images shown are adjacent fields (Figure 3D-E) and are consistent with our findings that pericytes actively migrate along the vessel to catalyze continuous basement membrane assembly (supplemental Videos 3-4). Furthermore, double staining at this time point for fibronectin and pericytes reveals considerable overlap in the staining for basement membrane fibronectin in relation to the position of pericytes surrounding the tube structures (Figure 3D). Therefore, analysis of quail CAMs in vivo shows that pericyte recruitment to microvessels during vasculogenic tube assembly correlates with basement membrane matrix deposition as observed in our in vitro models of EC-pericyte tube coassembly (Figure 2; supplemental Figure 3).
Pericyte recruitment to microvascular tubes during vasculogenesis in quail CAMs correlates with basement membrane matrix assembly. (A) Quail CAMs were isolated at 5 and 7 days of development and fixed for detergent-free immunostaining. Images reveal basement membrane deposition (arrowheads) around developing microvessels at the 7-day time point. Bars equal 10 μm. (B-C) Double staining of quail CAMs at 7 days for QH1, an EC-specific marker in red, versus SMA, recognizing pericytes at the microvascular level in green. (B) Reveals the coassociation of the 2 cells at this time point. (C) Staining of SMA (in green) versus the basement membrane component protein, fibronectin (in red), reveals the location of the pericytes in relation to the basement membrane. (D-E) Electron microscopy of quail CAMs reveals a continuous basement membrane (arrows) along the ablumenal surface of developing microvessels and between the perivascular cells (closed arrowheads) and the endothelium (open arrowheads). Bar equals 2 μm.
Pericyte recruitment to microvascular tubes during vasculogenesis in quail CAMs correlates with basement membrane matrix assembly. (A) Quail CAMs were isolated at 5 and 7 days of development and fixed for detergent-free immunostaining. Images reveal basement membrane deposition (arrowheads) around developing microvessels at the 7-day time point. Bars equal 10 μm. (B-C) Double staining of quail CAMs at 7 days for QH1, an EC-specific marker in red, versus SMA, recognizing pericytes at the microvascular level in green. (B) Reveals the coassociation of the 2 cells at this time point. (C) Staining of SMA (in green) versus the basement membrane component protein, fibronectin (in red), reveals the location of the pericytes in relation to the basement membrane. (D-E) Electron microscopy of quail CAMs reveals a continuous basement membrane (arrows) along the ablumenal surface of developing microvessels and between the perivascular cells (closed arrowheads) and the endothelium (open arrowheads). Bar equals 2 μm.
Molecular events underlying the ability of pericytes to catalyze EC basement membrane matrix assembly
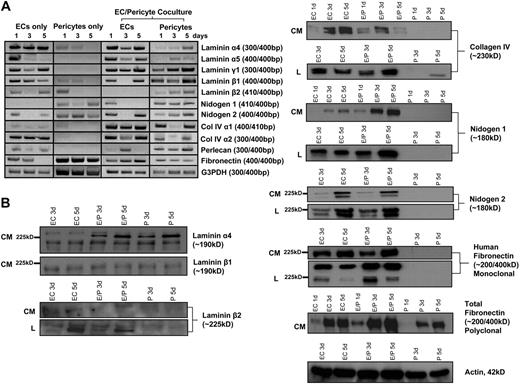
To address how pericyte recruitment catalyzes basement membrane matrix assembly around EC-lined tubes, we performed a detailed time course analysis of basement membrane matrix molecule mRNA and protein production by both ECs and pericytes during these events. For the RT-PCR analysis, the different species of the 2 cell types (human ECs vs bovine pericytes) was exploited to generate species-specific primer sets that allow us to independently assess the contribution of the 2 cell types when the cells are cultured individually or in combination. Interestingly, each of the basement membrane components is produced at the mRNA level by either ECs or pericytes (as shown by RT-PCR [Figure 4A]) and confirmed at the protein level by Western blot and immunofluorescence (Figures 2, 4B). As shown in Figure 4A, there are many mRNA expression changes that selectively occur in EC-pericyte cocultures compared with either EC- or pericyte-only cultures. In particular, mRNA changes are observed in EC fibronectin; pericyte nidogen-1; pericyte laminin α4, α5, β2, and γ1 subunits; pericyte perlecan; and EC laminin α5 subunit when ECs and pericytes are cocultured. Western blots reveal increased EC fibronectin (detected with an anti-human specific monoclonal antibody), total fibronectin, nidogen-1, and laminin α4 in EC-pericyte cocultures compared with EC-only cultures (Figure 4B). These data strongly suggest that both increased synthesis and deposition of basement membrane matrix proteins occur when pericytes and ECs coassemble to form microvascular tubes.
Up-regulation of fibronectin, nidogen-1, laminin isoforms, and perlecan occurs selectively in EC-pericyte cocultures to regulate EC basement membrane assembly. ECs alone, pericytes alone, or both ECs and pericytes were seeded randomly in 3D collagen matrices and allowed to assemble into tube networks. These networks were then either isolated for RNA or lysed for Western blot analysis. (A) mRNA expression of basement membrane components (using species-specific primer sets to HUVECs versus bovine pericytes) was examined at 1, 3, and 5 days of culture. ECs and pericytes alone in culture were examined as well as EC-pericyte cocultures. Relative expression patterns are shown, with glyceraldehyde-3-phosphate dehydrogenase (G3PDH) as a control. (B) Western blot analysis of collagen IV, laminin isoforms, nidogen 1/2, and fibronectin are shown demonstrating protein levels in EC-only (HUVECs), EC-pericyte coculture (HUVECs, bovine pericytes), and pericyte-only cultures (bovine pericytes). CM refers to conditioned medium; L refers to lysates. Actin blots are included as a loading control.
Up-regulation of fibronectin, nidogen-1, laminin isoforms, and perlecan occurs selectively in EC-pericyte cocultures to regulate EC basement membrane assembly. ECs alone, pericytes alone, or both ECs and pericytes were seeded randomly in 3D collagen matrices and allowed to assemble into tube networks. These networks were then either isolated for RNA or lysed for Western blot analysis. (A) mRNA expression of basement membrane components (using species-specific primer sets to HUVECs versus bovine pericytes) was examined at 1, 3, and 5 days of culture. ECs and pericytes alone in culture were examined as well as EC-pericyte cocultures. Relative expression patterns are shown, with glyceraldehyde-3-phosphate dehydrogenase (G3PDH) as a control. (B) Western blot analysis of collagen IV, laminin isoforms, nidogen 1/2, and fibronectin are shown demonstrating protein levels in EC-only (HUVECs), EC-pericyte coculture (HUVECs, bovine pericytes), and pericyte-only cultures (bovine pericytes). CM refers to conditioned medium; L refers to lysates. Actin blots are included as a loading control.
Changes in integrin requirements regulating EC-pericyte tube coassembly and maturation directly parallel basement membrane matrix deposition events
Previous work has shown that ECs when cultured alone in 3D collagen matrices are dependent on the collagen-binding integrin, α2β1, for lumen formation and tube morphogenesis.28,32 In this study, we assessed the integrin requirements in EC-pericyte cocultures (where dramatic matrix remodeling is occurring) compared with EC-only cultures. First, mRNA analysis of integrin expression was performed to examine known integrin α-chains with affinity for basement membrane components from either ECs alone, pericytes alone, or EC-pericyte cocultures (Figure 5A). RT-PCR analysis displays increased expression of select α integrin chains within the 2 cell types. There is strong induction of α3, α5, and α6 integrin mRNAs in ECs, whereas there is a dramatic increase in pericyte α1, α3, and α6 integrin mRNAs only when the 2 cells are cultured together and coincident with the deposition of basement membrane matrices (Figure 5A). These data support the concept that matrix-remodeling events lead to up-regulation of integrins that recognize this newly remodeled basement membrane matrix. In contrast, as ECs lose contact with the collagen type I matrix in the cocultures (due to ECM remodeling), there is concomitant down-regulation of the integrin α2 subunit compared with EC-only cultures (Figure 5A). The induced integrin subunits recognize multiple components of this basement membrane matrix, including fibronectin (α5β1), laminins (α1β1, α3β1, α6β1), nidogens (α3β1), and collagen type IV (α1β1).
Selective induction and functional requirement of integrins with affinity for basement membrane matrices during EC-pericyte tube coassembly. (A) PCR analysis of selected α integrin chains was performed to examine expression in both the bovine pericytes and ECs (HUVECs) when cultured alone or in coculture. G3PDH is shown as a loading control. (B) Five-day images from time lapse movies are shown, highlighting the differences in vessel width between control EC-only cultures and EC-pericyte cocultures. Arrows show the differences in width. (C) Average vessel widths were measured from images obtained from time lapse movies for quantitation. (D) Integrin-blocking antibodies were added to the culture media of EC alone versus EC-pericyte cocultures either from the beginning of the experiment or starting at day 3 or 5 at 20 μg/mL. Analysis of total vessel area is shown, demonstrating a requirement for α2β1 using EC-only cultures. Analysis of average vessel width is reported showing increases in EC tube width with blockade of α5β1, α3β1, and α6β1 only when pericytes are present, and more significantly increased tube widths when antibodies to α1β1, α3β1, and α6β1 are mixed (bottom panels). Statistical significance, P < .01. Species specificity of the antibodies can be found in supplemental Table 2.
Selective induction and functional requirement of integrins with affinity for basement membrane matrices during EC-pericyte tube coassembly. (A) PCR analysis of selected α integrin chains was performed to examine expression in both the bovine pericytes and ECs (HUVECs) when cultured alone or in coculture. G3PDH is shown as a loading control. (B) Five-day images from time lapse movies are shown, highlighting the differences in vessel width between control EC-only cultures and EC-pericyte cocultures. Arrows show the differences in width. (C) Average vessel widths were measured from images obtained from time lapse movies for quantitation. (D) Integrin-blocking antibodies were added to the culture media of EC alone versus EC-pericyte cocultures either from the beginning of the experiment or starting at day 3 or 5 at 20 μg/mL. Analysis of total vessel area is shown, demonstrating a requirement for α2β1 using EC-only cultures. Analysis of average vessel width is reported showing increases in EC tube width with blockade of α5β1, α3β1, and α6β1 only when pericytes are present, and more significantly increased tube widths when antibodies to α1β1, α3β1, and α6β1 are mixed (bottom panels). Statistical significance, P < .01. Species specificity of the antibodies can be found in supplemental Table 2.
To address a functional role for integrins in EC-pericyte tube coassembly, we performed integrin-blocking experiments at different time points using EC-only versus EC-pericyte cocultures (Figure 5D). Initially, we performed quantitative experiments to assess how pericytes affected EC tube formation and diameter. Quantification of the normal curves for average EC tube width over time in culture versus average EC-pericyte tube width is shown (Figure 5C) along with representative images of the 2 systems (Figure 5B) as a basis for comparison. These data show that pericytes play an important role in negatively regulating EC tube width by restricting the tendency of ECs to expand the lumenal compartment during tube morphogenesis (Figure 5B-C).
For this analysis, we examined both tube widths as well as total EC lumen area in the coculture as well as EC-only cultures in the presence or absence of integrin-blocking antibodies. As basement membrane matrix proteins are deposited around EC-pericyte tubes with time, there is a decrease in the α2β1 requirement and an increase in the requirements for other key integrins, including α1β1, α3β1, α5β1, and α6β1 (Figure 5D). Blockade of α3β1, α5β1, α6β1, and α2β1 (in the EC-pericyte coculture, but not in the EC-only cultures; Figure 5D) reveals a shift in the EC-pericyte tube width closer to that of ECs alone, with no affect on EC-alone cultures, whereas α1β1, α5β1, and α6β1 blockade result in increased EC tube area in the EC-pericyte cocultures. Thus, multiple basement membrane-binding integrins appear to be necessary for both ECs and pericytes to recognize this new matrix and to then affect and control the behavior of adjacent EC tube structures. In contrast, α2β1 alone controls both tube width and EC lumen area in the EC-only cultures.
To address the possibility that different laminin-binding integrins might be working together during these events, mixtures of blocking antibodies directed to α1β1, α3β1, or α6β1 were tested. Combinations of anti-α1 with either anti-α3 or anti-α6 led to significant increases in tube width as well as the combination of anti-α3 and anti-α6 antibodies (which showed the greatest effect; Figure 5D, bottom panel). These data suggest that laminin receptors from both ECs and pericytes are important in the ability of EC-lined tubes to mature in response to deposited basement membrane matrices. Thus, remodeling of basement membrane matrix surrounding EC tubes leads to alterations in EC integrin requirements for recognition of this matrix only when ECs and pericytes are present together as a consequence of pericyte-catalyzed EC basement membrane matrix assembly.
Critical functional role of fibronectin in vascular basement membrane assembly
Basement membrane assembly is contingent on several processes, including synthesis and deposition of the proper molecules as well as their maintenance. We have shown that fibronectin synthesis and deposition are markedly affected by EC-pericyte interactions (Figures 2 and 4), and furthermore, that the fibronectin receptor, α5β1, is both induced and functionally relevant in controlling tube diameter (Figure 5D). To further address the role of fibronectin in this process, we have used an N-terminal fragment of fibronectin that is known to block fibronectin matrix assembly.33 Figure 6A demonstrates that blockade of fibronectin matrix assembly also leads to impaired collagen type IV deposition, whereas laminin was affected to a lesser extent (Figure 6A,C; supplemental Figure 4D). Under these conditions, marked increases in vessel tube width occur, suggesting that tube maturation is impaired. Interestingly, there is no effect of this treatment on EC tube widths in EC-only cultures (Figure 6B). Similar effects are observed by the addition of anti-α5 integrin-blocking antibodies, which also significantly increase vessel width by disrupting the ability of ECs to recognize this fibronectin matrix (see Figure 5D). Importantly, fibronectin splice variant knockouts as well as α5β1 integrin knockouts in mice have been previously shown to result in marked increases in vascular tube widths compared with control mice.34
Fibronectin matrix assembly is required for vascular basement membrane formation during EC-pericyte tube coassembly. EC-pericyte cocultures were allowed to assemble for 5 days in a 3D collagen matrix either in the presence or absence of 50 μg/mL 70-kDa fragment of fibronectin (using HUVECs-bovine pericytes). (A) Immunofluorescent staining of fibronectin, collagen IV, and laminin demonstrates that disruption of fibronectin assembly leads to disrupted collagen IV assembly (first column in red, with the second column showing overlays denoting the position of GFP-pericytes). (B) Quantification of average vessel width reveals that blockade of fibronectin assembly leads to increased vessel width of EC tubes in the cocultures, but not EC-only cultures (P < .01). (C) Intensity mapping of representative fibronectin and collagen IV stains is shown to demonstrate the reduced levels of assembly/deposition of these proteins.
Fibronectin matrix assembly is required for vascular basement membrane formation during EC-pericyte tube coassembly. EC-pericyte cocultures were allowed to assemble for 5 days in a 3D collagen matrix either in the presence or absence of 50 μg/mL 70-kDa fragment of fibronectin (using HUVECs-bovine pericytes). (A) Immunofluorescent staining of fibronectin, collagen IV, and laminin demonstrates that disruption of fibronectin assembly leads to disrupted collagen IV assembly (first column in red, with the second column showing overlays denoting the position of GFP-pericytes). (B) Quantification of average vessel width reveals that blockade of fibronectin assembly leads to increased vessel width of EC tubes in the cocultures, but not EC-only cultures (P < .01). (C) Intensity mapping of representative fibronectin and collagen IV stains is shown to demonstrate the reduced levels of assembly/deposition of these proteins.
Pericyte TIMP-3 controls collagen type IV deposition and/or stability during EC-pericyte tube coassembly and basement membrane formation
TIMP-3, a pericyte-derived MMP inhibitor that we have recently shown plays a role in EC tube stabilization,15 is also a basement membrane matrix-binding TIMP.29 To address its functional relevance during these events, TIMP-3 expression was suppressed using siRNA techniques. When pericyte TIMP-3 expression is suppressed, immunostaining of EC-pericyte cocultures reveals that collagen IV deposition is markedly decreased, whereas fibronectin and laminin deposition are less affected (Figure 7A; supplemental Figure 4C) compared with controls. In addition, pericyte TIMP-3 suppression resulted in dramatic increases in EC tube width, suggesting that TIMP-3 is required to control deposition or maintenance of collagen type IV within the vascular basement membrane (Figure 7B). Western blot analysis was performed to confirm TIMP-3 siRNA suppression (Figure 7C), whereas PCR analysis was used to show that pericyte TIMP-3 mRNA is modestly induced in the cocultures (Figure 7D). Overall, we have identified 2 constituents, fibronectin and TIMP-3, that are critical to the functional ability of vascular basement membranes to control EC tube maturation. Interestingly, a key target of both molecules is collagen type IV, a major basement membrane component.29,30 Additional experiments were designed to determine which cell type was the predominant contributor of deposited collagen type IV during EC-pericyte tube coassembly (supplemental Figure 6). siRNAs were generated to either the bovine or human collagen IV α1 chain, and their inhibitory effects on collagen type IV production were confirmed by Western blot (supplemental Figure 6D). Immunostaining analyses reveal that ECs are the major contributor of collagen-type IV during EC-pericyte tube coassembly (supplemental Figure 6B-C). However, knockdown of collagen type IV from either cell type leads to increased vessel width (supplemental Figure 6A), suggesting that both cells contribute to collagen type IV assembly and, furthermore, that collagen type IV deposition itself appears to play an important functional role in determining vessel tube width.
Pericyte TIMP-3 regulates basement membrane formation during EC-pericyte coassembly events within vascular guidance tunnels during tube remodeling and maturation events. (A) Immunostaining of basement membrane components reveals that in cocultures in which pericytes were treated with a siRNA to TIMP-3, there is marked disruption of collagen IV (first column in red, with the second column showing overlays denoting the position of GFP-pericytes). (B) Furthermore, suppression of pericyte TIMP-3 leads to increased vessel width (P < .01). (C) RT-PCR analysis of pericyte TIMP-3 suggests regulation over time in EC-pericyte cocultures. G3PDH is shown as a loading control. (D) Western blot analysis of TIMP-3 is shown to demonstrate siRNA suppression versus controls. (E) This schematic diagram illustrates the function of vascular guidance tunnels24 that affect EC tube remodeling, recruitment of pericytes, and dynamic EC-pericyte interactions that are necessary for deposition of basement membrane matrix as well as EC and pericyte integrin expression changes that control vascular tube maturation events. Vascular guidance tunnels form as a consequence of EC lumen and tube formation that occurs through a signaling cascade involving Cdc42, MT1-MMP, and α2β1 integrin and the indicated downstream kinase effectors.35,36 Asterisks indicate basement membrane components and integrins that are up-regulated specifically by EC-pericyte interactions during tube coassembly in 3D collagen matrices. Cross indicates the down-regulation of α2β1, which is observed in EC-pericyte cocultures and not EC-only cultures.
Pericyte TIMP-3 regulates basement membrane formation during EC-pericyte coassembly events within vascular guidance tunnels during tube remodeling and maturation events. (A) Immunostaining of basement membrane components reveals that in cocultures in which pericytes were treated with a siRNA to TIMP-3, there is marked disruption of collagen IV (first column in red, with the second column showing overlays denoting the position of GFP-pericytes). (B) Furthermore, suppression of pericyte TIMP-3 leads to increased vessel width (P < .01). (C) RT-PCR analysis of pericyte TIMP-3 suggests regulation over time in EC-pericyte cocultures. G3PDH is shown as a loading control. (D) Western blot analysis of TIMP-3 is shown to demonstrate siRNA suppression versus controls. (E) This schematic diagram illustrates the function of vascular guidance tunnels24 that affect EC tube remodeling, recruitment of pericytes, and dynamic EC-pericyte interactions that are necessary for deposition of basement membrane matrix as well as EC and pericyte integrin expression changes that control vascular tube maturation events. Vascular guidance tunnels form as a consequence of EC lumen and tube formation that occurs through a signaling cascade involving Cdc42, MT1-MMP, and α2β1 integrin and the indicated downstream kinase effectors.35,36 Asterisks indicate basement membrane components and integrins that are up-regulated specifically by EC-pericyte interactions during tube coassembly in 3D collagen matrices. Cross indicates the down-regulation of α2β1, which is observed in EC-pericyte cocultures and not EC-only cultures.
Discussion
Pericyte recruitment to developing EC-lined tubes stimulates EC basement membrane matrix assembly, a fundamental vascular stabilization event during vasculogenesis
The studies presented in this work are consistent with a model of vascular morphogenesis whereby molecular signals downstream of integrin signaling such as Cdc42 and Rac1 activation29,35-37 in coordination with MT1-MMP lead to EC tube and vascular guidance tunnel formation in 3D collagen matrices (Figure 7E). Vascular guidance tunnels serve as a critical matrix conduit for the recruitment and migration of pericytes along the abluminal surface of EC-lined tubes. In turn, these dynamic events result in continuous deposition of EC basement membrane matrices, a necessary step in the progression of tube assembly toward maturation and stabilization. Importantly, this mechanism needs to be dynamic to ensure that each region of the tube is appropriately covered with basement membrane matrix (Figure 7E). Because pericyte coverage of EC tubes is variable in different vascular beds,38 this mechanism would allow for fewer pericytes necessary to scan along EC tubes to stimulate continuous basement membrane matrix deposition. This is important during vascular tube formation and assembly because addition of pericytes at high levels relative to ECs (ie, > 30% of ECs) provides inhibitory signals that interfere with the tube assembly process (data not shown).
EC-pericyte heterotypic cell-cell interactions control basement membrane matrix assembly
Within the context of vascular guidance tunnels, both ECs and pericytes are free to move in a MMP-independent manner, and thus, are able to remodel cell-matrix and cell-cell contacts in a dynamic fashion to facilitate tube assembly and maturation. The cell-cell and cell-matrix contacts that are necessary for this process remain to be determined. Previous work has implicated N-cadherin,39 α4β1–vascular cell adhesion molecule interactions,40 as well as alternatively spliced fibronectin isoforms34 in EC-pericyte interactions. The nature of the signaling processes regulating the motility of either cell type within vascular guidance tunnels during these events will be a major direction in future studies.
Our finding that heterotypic cell-cell interactions facilitate basement membrane assembly has precedent in other systems.30,41 Most notably, epidermal basement membrane formation on a 3D collagen matrix surface depends on the presence of fibroblasts embedded in the underlying collagen matrix.41,42 Their contribution of nidogen-1 and an appropriate cytokine milieu are thought to be reasons for their influence.41,42 Thus, it appears that vascular basement membrane matrix assembly also depends on heterotypic interactions, and pericytes can serve in this role to facilitate this process in conjunction with ECs.
Previous work suggests that microvascular beds with few pericytes have minimal basement membrane protein, whereas those with abundant pericytes such as retina show substantial basement membrane deposition.38 The fact that pericytes represent only one-fifth to one-fourth of the total of ECs in our model suggests that to deposit a continuous basement membrane matrix catalyzed by EC-pericyte contact, active motility of pericytes along EC-lined tubes must occur. We hypothesize that these responses are important in the establishment of the initial vasculogenic plexus during vascular development, which is a necessary cell/matrix template that responds to flow stimuli to remodel this initial plexus into arterio-capillary-venous networks.43 Furthermore, it is possible that many mouse knockout phenotypes, which result in a lethal vascular phenotype at this stage of development,44 are in many cases deficiencies in the assembly of this primary vascular plexus and its associated vascular basement membrane. Our model system (mimicking this plexus) allows for specific molecular experiments to be performed to address mechanistic questions regarding the role of a variety of molecules (eg, TGF-β and angiopoietins)1,9,10,16,22,44 that are known to control these events. One of the intriguing aspects of the role of growth factor functions in this context is that increasing information suggests that specific growth factors bind to components of the basement membrane matrix.45,46 This allows for the possibility that cosignaling events from growth factor receptors that are directly paired with integrins with affinity for particular basement membrane components will lead to unique signal transduction events that affect vascular maturation. Examples include fibronectin, which binds VEGF;46 collagen type IV, which binds bone morphogenic protein-4;45 and angiopoietin-1, which binds α5β1.47 Furthermore, α5β1 is known to form complexes with the angiopoietin-receptor, Tie-2.48 Thus, it is likely that EC-pericyte interactions, which catalyze vascular basement membrane matrix assembly, establish a matrix environment that anchors and spatially organizes particular growth factors. Such a modified matrix could then lead to unique EC and pericyte signaling events necessary for vascular tube maturation and stabilization.
Another finding in this work is that the stability and integrity of the vessel wall downstream of EC-pericyte interactions depend on the MMP inhibitor, TIMP-3.15 In this study, we demonstrate a role for TIMP-3 in basement membrane matrix deposition/stability in that siRNA suppression of the molecule in pericytes leads to a marked decrease in collagen type IV deposited around EC tubes, and consequently, marked increases in EC tube width. Thus, inhibition of matrix proteolysis is one mechanism by which pericytes can promote basement membrane formation and maintenance.
Fibronectin matrix assembly plays a critical role in vascular basement membrane formation and EC tube maturation in events catalyzed by EC-pericyte interactions
Functional blockade of fibronectin assembly (using the 70-kDa N-terminal fragment) and blockade of the α5β1 integrin caused increased EC tube widths in EC-pericyte cocultures, but not in EC-only cultures (Figures 5 and 6). Furthermore, disrupted fibronectin assembly also leads to disrupted collagen IV deposition (Figure 6A), suggesting a role for fibronectin in the vascular basement membrane assembly process. Data in vivo corroborate these findings using fibronectin splice variant or α5β1 integrin knockout mice.34,49,50 These data reveal that there are marked increases in vessel width associated with the vasculature, similar to what we observe. Importantly, our new data reveal the novel observation that disruption of fibronectin assembly also leads to defects in collagen type IV deposition that together could account for the vascular tube abnormalities. Furthermore, we show that EC-pericyte interactions lead to increased mRNA and protein for EC-expressed fibronectin and increased mRNA for the integrin α5 subunit in both ECs and pericytes only when they are cocultured together. We also showed that anti-integrin α5 antibodies cause marked increases in EC tube diameter selectively in EC-pericyte cocultures.
Furthermore, we show that EC-pericyte interactions induce basement membrane matrix synthesis of laminin isoforms, nidogen-1, and perlecan, and induce integrin subunits with affinity for these matrix proteins (Figures 2 and 4). Importantly, EC-pericyte cocultures induce expression of laminin α5 chains that are necessary in laminin-10/11 isoforms to enable self-assembly into basement membrane structures,30 a function not shared with laminin α4 chain containing laminin-8/9 isoforms.30 Our data support the concept that increased laminin-10/11 production, which occurs selectively in EC-pericyte cocultures, could play a key role by serving as a nidus to catalyze basement membrane assembly like we observe in vitro and in vivo. In support of these concepts, functional blockade of integrins α3, α6, and α1, which bind laminins, nidogens, and collagen type IV, affects tube maturation events only in the cocultures (either when added alone or to a greater extent when added in combination; Figure 5). These results are similar to what we observe with blockade of integrin α5 or fibronectin assembly (Figures 5 and 6). In conclusion, our data suggest that EC-pericyte interactions are necessary for vascular tube assembly, maturation, and stabilization in 3D matrices through basement membrane matrix-remodeling events, and through key changes in EC and pericyte integrin expression that facilitates recognition of these matrices (Figure 7E). These changes most likely act in conjunction with growth factor-signaling pathways, mediated by molecules such as TGF-β and angiopoietins, to provide unique signals that are necessary for the transition from vascular tube morphogenesis to stabilization.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the University of Missouri's Electron Microscopy Core Facility for assistance in this research.
This work was supported by National Institutes of Health grants HL79460, HL59373, and HL87308 (G.E.D.).
National Institutes of Health
Authorship
Contribution: A.N.S. designed and performed experiments, analyzed data, and wrote the paper; K.M.M. performed experiments; R.D.M. performed experiments; M.J.D. analyzed experiments and wrote the paper; and G.E.D. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George E. Davis, Department of Medical Pharmacology and Physiology, School of Medicine, One Hospital Dr, MA415 Medical Sciences Bldg, University of Missouri-Columbia, Columbia, MO 65212; e-mail: davisgeo@health.missouri.edu.