Abstract
The MHC class I–related chain A (MICA) molecules exist as membrane-bound and soluble isoforms and are encoded by a polymorphic gene. Their genetic and phenotype characteristics have been studied in various pathologic settings but not in the context of hematopoietic stem cell transplantation (HSCT). Here, we evaluated whether MICA-related features namely MICA-129 gene polymorphism, serum levels of soluble MICA (sMICA) and anti-MICA antibodies (MICA Abs) before and after HSCT could influence the incidence of chronic graft-versus-host disease (cGVHD) and relapse of their disease in 211 HLA-identical sibling pairs and in a subset of 116 recipients, respectively. Although the MICA-129 val/val genotype and elevated sMICA serum levels after HSCT are independently associated with the incidence of cGVHD (P = .002 and .001) regardless of history of acute GVHD, the presence of MICA Abs before transplantation confers protection against cGVHD (P = .04). There is an inverse relationship between MICA Abs and sMICA, suggesting an antibody-based neutralization of deleterious effects of sMICA. Similarly, these genetic and phenotype characteristics of MICA influence the incidence of relapse. Altogether, these data suggest that the studied MICA genotype and phenotype specificities could be used as relevant biomarkers for cGVHD monitoring.
Introduction
Chronic graft-versus-host disease (cGVHD) is a long-term and often life-threatening complication affecting 25% to 80% of patients surviving 3 months after hematopoietic stem cell transplantation (HSCT)1,2 and can occur after the acute form or de novo.3,4 On the basis of its clinical and biologic features, cGVHD is viewed as a disorder of alloimmune and autoimmune systems.5-7 Because predictive biomarkers for an impending cGVHD must allow timely implementation of effective prophylactic measures, a variety of genetic and phenotype markers were explored.8-11 In this regard, the MHC class I–related chain A (MICA) molecules are of particular interest given their participation in non–T cell receptor–mediated immune function.12,13 MICA rather engages NKG2D, a C-type lectin expressed on effector cells, including natural killer (NK), αβ- and γδ-T cells.14 Such engagement triggers NK cells and costimulates T lymphocytes to mount adequate immune response. Further, a soluble isoform of MICA (sMICA), resulting from the proteolytic shedding of the membrane-bound molecules, was implicated in the pathogenesis of a variety of cancers, autoimmune, and other organ transplant-related disorders through NKG2D receptor down-regulation.15-18 The ensuing immune escape highlights the functional duality of the membrane-bound and soluble MICA isoforms. Although MICA expression, induced by cellular stress, was initially considered to be restricted to gastrointestinal epithelium and epithelial tumors, a more ubiquitous expression was latter shown, at least at the transcriptional level.19 Functionally relevant polymorphism of the MICA gene (with more than 60 alleles reported to date)20 may also contribute to interpatient variability in alloimmune responses as observed in various clinical settings, including organ transplantation.21 Interestingly, a methionine (met) to valine (val) change at position 129 of the α2-heavy chain domain categorized the MICA alleles into strong (MICA-129 met) and weak (MICA-129 val) binders of NKG2D receptor.22 Recently, we have shown that the MICA-129 dimorphism could influence the incidence of early onset ankylosing spondylitis (AS)23 and nasopharyngeal cancer (NPC).24
Given the demonstrated importance of MICA in immune pathways, in this study, we explored the MICA-related parameters namely MICA-129 gene polymorphism, serum levels of sMICA and anti-MICA antibodies (MICA Abs) before and after HSCT in a HLA-matched sibling HSCT setting as potential predictive biomarkers of cGVHD.
Methods
Study population
Two hundred eleven consecutive patients who underwent non–T cell–depleted allogeneic HSCT, between January 1994 and January 2002 in the Bone Marrow Transplant Department of Saint Louis Hospital, Paris, France, were included in this study. Among them, 146 received bone marrow (BM) and 65 mobilized peripheral blood stem cells (PBSCs) from an HLA-identical sibling donor. All donor/recipient (D/R) pair DNAs were submitted to MICA-129 genotyping procedure, whereas phenotype studies, that is, sMICA and MICA Abs analyses, were limited to a subgroup of 116 patients for whom sera before and after transplantation were available. All sera before transplantation were collected before the initiation of any conditioning regimens (day 0) to exclude potential interferences in phenotype marker analysis. In all cases specimen collection after transplantation was made at day 100. This subset did not differ significantly from the whole cohort in term of patient-, disease-, and transplant-related features (data not shown). The characteristics of D/R pairs are given in Table 1. The supportive therapy as well as the criteria used to define the outcomes, namely GVHD (acute and chronic) and relapse, were as previously detailed.25 The study was approved by the Saint Louis Hospital Ethical Review Board. Informed consent was obtained according to the Declaration of Helsinki.
MICA-129 genotyping
Genomic DNA was extracted from EDTA-treated peripheral blood samples with the use of the standard proteinase K–phenol extraction method. Genotyping was performed by a TaqMan 5′-nuclease assay (Applied Biosystems) with allele-specific fluorogenic oligonucleotide probes for MICA-129 alleles, allowing to discriminate the genotypes of each studied pair of alleles. Control samples, checked by nucleotide sequencing, consisted of heterozygotes and homozygotes for the variant allele. The primer and probe sequences designed by assay on demand (Applied Biosystems) were as follows: MICA-129–MICAF, GGAGCTCTTCCTCTCCCAAAAC; MICA-129–MICAR, AGTCTGCATGCATAGCGTGATAG; and Vic-5′-ATGGACAATGCCCC; Fam-5′-TGGACAGTGCCCC. Although herein we explored the DNA alleles, for reasons of clarity, the explored MICA variants are designated as MICA-129 val and MICA-129 met.
Soluble MICA enzyme-linked immunoabsorbent assay
Serum levels of sMICA were determined using commercially available MICA enzyme-linked immunosorbent assay kits (Immatics) according to the manufacturer's instructions. Briefly, interactions between capture anti-MICA mAb and sMICA in serial dilutions of serum samples before and after transplantation were detected using a second anti-MICA mAb and then revealed with the use of HRP-conjugated anti–mouse Ab and tetramethylbenzidine as chromogenic substrate. Absorbance was measured at 450 nm and the cutoff was established at 80 pg/mL (see “Posttransplantation sMICA increased the risk of GVHD”). A standard curve of the logarithmic relationship between concentration and absorbance was used to calculate the concentration of sMICA in samples. All samples were tested in triplicates.
Analysis of MICA antibodies
MICA antibody assay was performed using LABScreen assay, following manufacturer's recommendations (One Lambda). Briefly, 2 microbeads coated, respectively, with the following groups of purified MICA antigens: MICA *001, *004, *012, *018, *027 (27/08) and MICA *002, *007, *009, *017, *019 were used to screen sera before and after transplantation as well as those of healthy donors. The fluorescent signal for each MICA allele–coated bead was measured using LABScan 100 Flow Cytometry (Luminex Corporation) and analyzed by HLA-Visual 1.1 software (One Lambda). A cutoff fluorescent signal value of 3, calculated with the use of sera of healthy male individual donors (mean value and 3 SDs), consistent with the manufacturer's recommended value, was chosen as the threshold of positivity. Sera with borderline values, ranging from 2.0 to 3.0, were retested. Therefore, both values, that is, 2 and 3, were used for statistical analysis.
Statistical analysis
Differences in categorical variables between 2 groups were evaluated by χ2 analysis. Univariate and multivariate proportional hazard regression models were used to identify independent risk factors of death, respectively, by log-rank tests and Cox proportional hazard models. Nonparametric tests (Kruskall-Wallis) were also used to compare quantitative data. The univariate Kaplan-Meier analysis was used for describing death risk factors. Cumulative incidence using the competing risk method, as described by Fine and Gray,26 was used for the assessment of prognostic factors of cGVHD and relapse with death or death without relapse for relapse analysis as a competing event. Cox regression analysis was also used in multivariate analysis of risk factors for death.27 All tests were 2-sided, with type I error rate fixed at 0.05. Statistical analyses were performed with SPSS 15 software (SPSS Inc), Stata 10 (Stata Corporation), and R (R Project) packages “cmprsk” (competing risks) or “coxph” (Cox regression to maximize a penalized partial likelihood). D/R pairs as well as transplant-related characteristics such as source of stem cells, severity of the disease, conditioning regimen, D/R age and sex, and CMV serology that could potentially influence the outcomes after HSCT were evaluated in all pairs. Those that were found significantly associated with cGVHD or relapse by univariate analysis (P < .05) were further included in the multivariate analysis involving MICA-related variables.
Results
Chronic graft-versus-host disease
Recipient MICA-129 val/val genotype is a risk factor for cGVHD.
A total of 211 patient and donor pairs were typed for MICA-129 alleles. As expected, the sibling pairs fully matched for MICA-129 alleles and genotypes. The observed overall frequencies were as follows: MICA-129 val, 308 (73%) of 422; MICA-129 met, 113 (27%) of 422; MICA-129 met/met, 15 (7%) of 211; MICA-129 met/val, 99 (47%) of 211; MICA-129 val/val, 97 (46%) of 211.
In our study group, 100 patients experienced cGVHD (47%) with a cumulative incidence of 53% at 3 years. Sixty-two of them developed an extensive form. The onset of cGVHD was within the first year in 86% of cases (< 6 months, 48%; 6-11 months, 38%) and latter in 14% (12-17 months, 5%; 18-23 months, 4%; and > 23 months, 5%). These patients were categorized as follows: early onset (3-8 months after HSCT; 80%) and late onset (> 9 months; 20%).
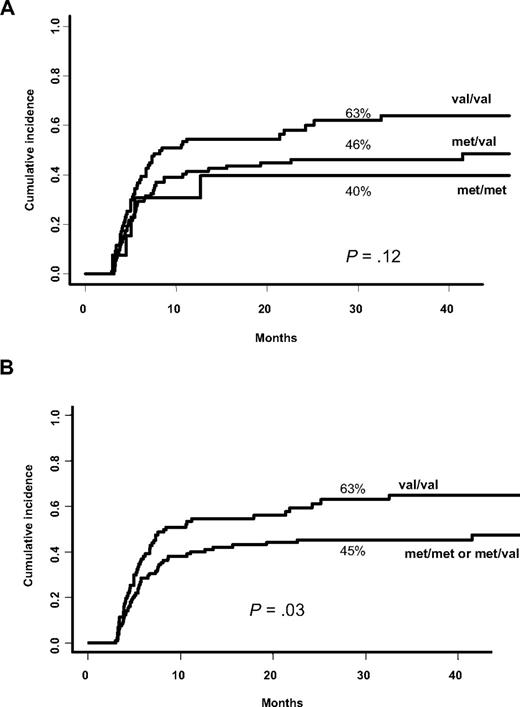
Univariate analysis using competing risk showed that MICA-129 val/val genotype is more frequent in patients who experienced cGVHD without reaching significance. Because the susceptibility status seemed to be dose dependent (Figure 1A), we analyzed the predictive effect of the MICA-129 val/val genotype versus others and found that patients bearing the MICA-129 val/val genotype were at a higher risk of developing cGVHD (63% vs 45% at 3 years; P = .03; Figure 1B). These data were further confirmed in multivariate analysis adjusted for confounding variables. Three factors reached significance: recipient MICA-129 val/val genotype (HR, 1.52; 95% confidence interval [CI] 95%, 1.02-2.24; P = .04), older recipient age (≥ 15 years; HR, 3.36; 95% CI, 1.65-6.84; P = .001), and source of stem cells (PBSCs vs BM; HR, 1.67; 95% CI, 1.10-2.53; P = .017). Because acute GVHD (aGVHD) is a major risk factor for subsequent cGVHD, we then introduced aGVHD as a time-dependent covariate in the multivariate analysis model. This analysis confirmed that the risk conferred by the MICA-129 val/val genotype is independent from aGVHD [the factors found to increase independently the risk of cGVHD were: aGVHD (HR, 1.96; 95% CI, 1.34-2.87; P = .001); MICA-129 val/val genotype (HR, 1.61; 95% CI, 1.08-2.40; P = .019), older age (≥ 15 years; HR, 2.97; 95% CI, 1.45-6.07; P = .003), and the source of stem cells (PBSCs vs BM; HR, 1.66; 95% CI, 1.09-2.52; P = .018)].
Cumulative incidence for cGVHD based on MICA-129 genotype. (A) MICA-129 val allele increased the risk of chronic graft-versus-host disease (cGVHD) in a dose-dependent manner (univariate analysis). (B) MICA-129 val/val genotype is more represented in patients who developed cGVHD (univariate analysis).
Cumulative incidence for cGVHD based on MICA-129 genotype. (A) MICA-129 val allele increased the risk of chronic graft-versus-host disease (cGVHD) in a dose-dependent manner (univariate analysis). (B) MICA-129 val/val genotype is more represented in patients who developed cGVHD (univariate analysis).
Posttransplantation sMICA increased the risk of cGVHD.
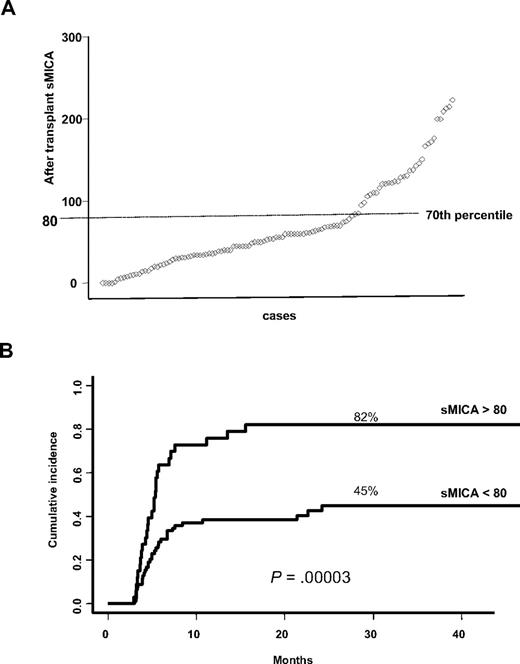
We then investigated sMICA levels in sera of a subset of 116 patients for whom serum aliquots before and after transplantation (at day 100) were available as well in 35 healthy donors. The analysis of sMICA serum level in healthy donors showed an overall absence of sMICA, whereas in patients before and after transplantation the mean values were, respectively, 52.95 pg/mL (95% CI, 42.77-63.13 pg/mL) and 71.82 pg/mL (95% CI, 60.58-83.05 pg/mL). From the distribution profile, a cutoff value for sMICA at the 70th percentile was noted (Figure 2A). Subsequent univariate analysis showed a highly significant association between posttransplantation sMICA higher than 80 pg/mL and the incidence of cGVHD (82% vs 46%, at 3 years; P < .00111 ; Figure 2B). Multivariate analysis using similar methods as described earlier found an increased risk of cGVHD associated with the following factors: aGVHD (HR, 1.74; 95% CI, 1.07-2.84; P = .03), MICA-129 val/val genotype (HR, 2.60; 95% CI, 1.48-4.56; P = .001), sMICA (HR, 2.75; 95% CI, 1.59-4.76; P < .001), and older age (≥ 15 years; HR, 2.89; 95% CI, 0.99-8.37; P = .05). Moreover, comparison between sMICA levels before and after HSCT did not show any statistically significant correlation (data not shown).
sMICA distribution profile among patients and cumulative incidence for cGVHD based on sMICA serum levels after transplantation. (A) The analysis of the distribution profile of post-HSCT sMICA allowed us to pinpoint a cutoff at the 70th percentile. (B) Patients having sMICA serum level greater than 80 pg/mL after transplantation are those at high risk of developing cGVHD (univariate analysis).
sMICA distribution profile among patients and cumulative incidence for cGVHD based on sMICA serum levels after transplantation. (A) The analysis of the distribution profile of post-HSCT sMICA allowed us to pinpoint a cutoff at the 70th percentile. (B) Patients having sMICA serum level greater than 80 pg/mL after transplantation are those at high risk of developing cGVHD (univariate analysis).
MICA-129 and sMICA exert an independent but additive effect on cGVHD incidence.
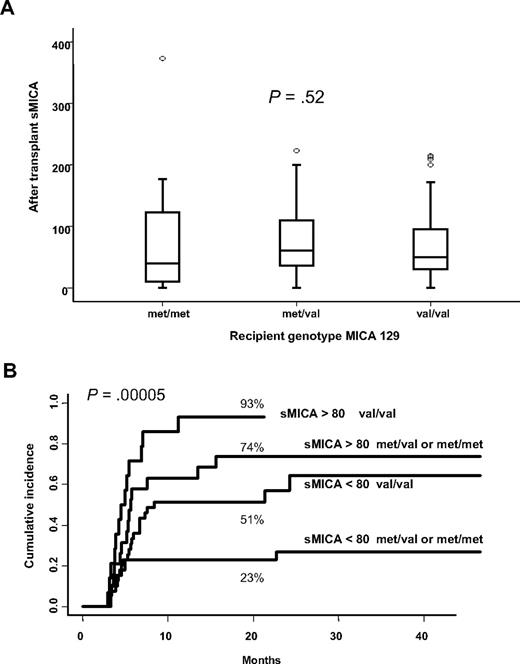
Given that both MICA-129 genotype and sMICA serum levels are associated with a high incidence of cGVHD, we questioned if MICA-129 genotype could influence sMICA levels, but nonparametric testing failed to show any relationship (Figure 3A). However, the cumulative incidence of cGVHD accounting for the different combinations of MICA-129 genotypes (val/val, met/val, or met/met) and serum levels of sMICA (< 80 pg/mL and > 80 pg/mL) showed that each of these risk factors raised the risk of cGVHD in an additive manner. Patients bearing the val/val genotype together with serum sMICA level greater than 80 pg/mL was at highest risk of developing cGVHD (93% at 20 months), whereas the lower risk was observed for those with MICA-129 met/met or met/val in combination with sMICA level below 80 pg/mL (23% at 20 months; P < .001; Figure 3B).
Relationship between MICA-129 genotypes and sMICA serum levels and cumulative incidence for cGVHD based on their combinations. (A) Kruskall-Wallis nonparametric testing did not show any association between MICA-129 genotypes and sMICA serum levels. (B) Patients bearing the val/val genotype together with sMICA serum level greater than 80 pg/mL are at high risk of developing cGVHD, whereas the lowest risk was observed for those with MICA-129 met/met or met/val in combination with sMICA level below 80 pg/mL (univariate analysis).
Relationship between MICA-129 genotypes and sMICA serum levels and cumulative incidence for cGVHD based on their combinations. (A) Kruskall-Wallis nonparametric testing did not show any association between MICA-129 genotypes and sMICA serum levels. (B) Patients bearing the val/val genotype together with sMICA serum level greater than 80 pg/mL are at high risk of developing cGVHD, whereas the lowest risk was observed for those with MICA-129 met/met or met/val in combination with sMICA level below 80 pg/mL (univariate analysis).
Pretransplantation anti-MICA antibodies protect against cGVHD.
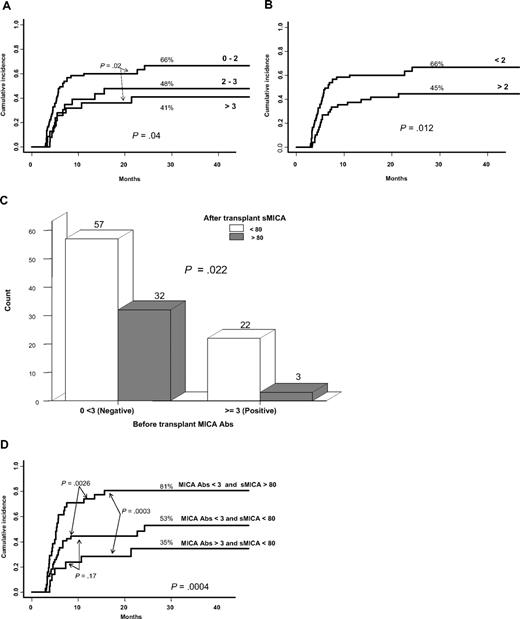
We stratified, as recommended by the manufacturer, the threshold of MICA antibodies as either 0 to 2 (negative), 2 to 3 (borderline), and greater than 3 (positive) or less than 2 (negative) and greater than 2 (positive). In univariate analysis, patients positive for pretransplantation MICA Abs had a lower incidence of cGVHD whatever the stratification schedule used in the analysis (P = .04 and .01; Figure 4A-B). This was further confirmed in multivariate analysis without affecting the status of previously identified risk factors [aGVHD (HR, 1.88; 95% CI, 1.12-3.13; P = .01), MICA-129 val/val genotype (HR, 2.45; 95% CI, 1.39-4.33; P = .002), sMICA (HR, 2.55; 95% CI, 1.47-4.41; P = .001), MICA Abs (HR, 1.80; 95% CI, 1.01-3.20; P = .04)]. These data prompted us to analyze if there was any relationship between MICA Abs and sMICA level on the one hand and the incidence of cGVHD on the other hand. We found an inverse relationship between pretransplantation MICA Abs and sMICA level at day 100 (values > 3 correlate with serum levels < 80 pg/mL; Figure 4C), potentially reflecting a neutralizing effect of such isoforms by MICA antibodies with a subsequent decrease on cGVHD incidence. Indeed, the lower risk of cGVHD was noted in patients with MICA Abs greater than 3 and sMICA less than 80 pg/mL, whereas the highest in those having MICA Abs less than 3 with sMICA greater than 80 pg/mL (35% vs 81%; Figure 4D). Note that a sex-based comparison of patients immunized against MICA did not show significant differences between males and females albeit a slight increase among females older than 15 years (data not shown).
Cumulative incidence for cGVHD based on pretransplantation MICA Abs using different cutoffs. Relationship between pretransplantation MICA Abs, sMICA serum level, and cGVHD. (A-B) Patients having the lower titer of pretransplantation MICA Abs are those at high risk of cGVHD whatever the cutoff values, ie, 2 or 3 (univariate analysis). (C) Nonparametric testing showed an inverse relationship between MICA Abs and sMICA levels (values > 3 correlates with serum levels < 80 pg/mL). (D) The lower risk for cGVHD was noted in patients with MICA Abs greater than 3 and sMICA less than 80 pg/mL, whereas the highest was in those having MICA Abs less than 3 with sMICA greater than 80 pg/mL (univariate analysis). Of note, the patient group with MICA Abs greater than 3 and sMICA greater than 80 pg/mL is not represented in the figure because of the low number of patients (n = 3).
Cumulative incidence for cGVHD based on pretransplantation MICA Abs using different cutoffs. Relationship between pretransplantation MICA Abs, sMICA serum level, and cGVHD. (A-B) Patients having the lower titer of pretransplantation MICA Abs are those at high risk of cGVHD whatever the cutoff values, ie, 2 or 3 (univariate analysis). (C) Nonparametric testing showed an inverse relationship between MICA Abs and sMICA levels (values > 3 correlates with serum levels < 80 pg/mL). (D) The lower risk for cGVHD was noted in patients with MICA Abs greater than 3 and sMICA less than 80 pg/mL, whereas the highest was in those having MICA Abs less than 3 with sMICA greater than 80 pg/mL (univariate analysis). Of note, the patient group with MICA Abs greater than 3 and sMICA greater than 80 pg/mL is not represented in the figure because of the low number of patients (n = 3).
Relapse
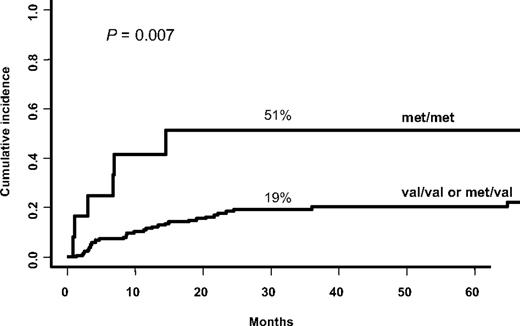
Because it is well admitted that cGVHD is closely related to decreased relapse rate28 and because MICA has been associated with malignancies, we examined the relapse rate in this patient population along with incidence of cGVHD despite the absence of a relationship between the 2 complications in our study group (data not shown). The cumulative incidence of relapse at 3 years was 21%. Forty-one patients (22% of 186 who received a transplant for malignant diseases) had relapsed or progressed with their disease. MICA-129 met/met genotype, sMICA, and posttransplantation MICA Abs less than 3 were all associated with increased risk of relapse, albeit for the 2 latter the statistical significance was borderline (ie, MICA-129 met/met vs MICA-129 met/val or MICA-129 val/val, 51% vs 19%, P = .007; Figure 5; sMICA > 80, 37% vs 17%, P = .05; and MICA Abs < 3, 26% vs 6%, P = .05). In multivariate analysis, only MICA-129 met/met genotype (HR, 2.69; 95% CI, 1.12-6.43; P = .02) remained significant together with relapse risk of the underlying malignant disease (high or intermediate vs low; HR, 1.91; 95% CI, 1.02-3.58; P = .04).
Cumulative incidence for relapse, based on MICA-129 genotype. MICA-129 met/met genotype is associated with high risk of relapse (univariate analysis).
Cumulative incidence for relapse, based on MICA-129 genotype. MICA-129 met/met genotype is associated with high risk of relapse (univariate analysis).
Discussion
The underlying immune mechanisms involved in cGVHD pathogenesis remain elusive. Nevertheless, there is consensus that immune dysfunction involves alloreactive T- and B-cell compartments as well as autoimmune responses.29-31 In such context, MICA molecules, proven to play prominent roles in immune processes, attracted our attention by their potential implication in the development of cGVHD and prompted us to analyze whether their genetic and phenotypic diversity could serve as potent biomarkers.
In particular, we focused our attention on functionally relevant characteristics of MICA, namely MICA-129 genetic polymorphism and propensity to elaborate sMICA formation and MICA Abs. We found that the MICA-129 val in an allele dose-dependent manner increased the risk of cGVHD, suggesting that the effect is recessive. Because such MICA-associated risk was independent of aGVHD occurrence, the pathogenesis of cGVHD could be considered as a de novo event in our study group. These findings and interpretations are in line with other pathologic contexts, namely AS and NPC. Indeed, we found that the MICA-129 met/met genotype was a risk factor for the early onset of AS independent of HLA-B27 antigen, whereas the homozygous state for MICA-129 val allele increased the risk of developing NPC.23,24 The functional relevance of this variant in NK and T-cell activation, led us to hypothesize that NKG2D and MICA interaction either with high-affinity (MICA-129 met) or low-affinity (MICA-129 val) alleles favor, respectively, chronic inflammation or tumor escape in persons otherwise genetically predisposed to these immune disorders. Similarly, in the context of cGVHD, the weak engagement of NKG2D receptors by the weak binder MICA-129 val allele may impair NK/cytotoxic T lymphocyte cell activation/costimulation, possibly skewing the TH1 pathway toward TH2 with consequent B-cell activation and Ab production (2 hallmarks of cGVHD pathogenesis), albeit such possibility was challenged in a recent report implying both pathways.32 The inability of MICA-129 val allele to activate NK cells corroborate with the finding that the NK-cell dose inversely correlates with the incidence of cGVHD.33 Indeed, we have reported that the risk of cGVHD was high in patients receiving low-donor NK-cell load.34 These data corroborate our findings in D/R sibling pairs fully matched for the MICA-129 polymorphism, wherein donor cell compartment possesses relatively low MICA-mediated capacity of NK-cell activation through the MICA-129 val allele. The ultimate consequence of such weak interaction could lead to overexpression of NKG2D receptors as previously shown in autoimmune settings such as rheumatoid arthritis,17 celiac disease,35 and type 1 diabetes,36 events believed to mediate autoimmunity,37 and much more recently in early the post-HSCT setting.38 This situation could favor the binding of other ligands such as UL16 binding proteins.13 Thus, in a highly IL-15–enriched microenvironment, repeated T and NK stimulation favors an autoimmune-like situation breaking down the self-tolerance with consequent auto-Ab production. Interestingly, a recent study has shown that CD4+ autoreactive donor T cells emerge during cGVHD. It was also shown that, although the host antigen-presenting cells are important for the initiation of aGVHD, donor antigen-presenting cells are involved in the development of cGVHD.39
We also found that the homozygous state for the strong NKG2D binder MICA-129 met allele was associated with a high incidence of relapse (it is important to highlight that among 15 patients bearing the MICA-129 met/met genotype, 12 (80%) relapsed for their initial disease). Because this variant protects against cGVHD, it could favor in turn a diminished graft-versus-leukemia effect. Indeed, in murine allo–BM transplantation model, alloreactive NK cells had been successfully used to protect against GVHD and to enhance graft-versus-leukemia effect.40 Similarly, in human transplantation, alloreactive NK cells protected patients against rejection, GVHD, and relapse in KIR ligand-mismatched transplantations.41 From our data obtained in MICA-matched context, the activated NK cells (by the met/met genotype), albeit protective against cGVHD, seem inefficient to mediate antitumor activity against self-targets. This observation generates a model that needs a distinct study design for further exploration of the above possibilities. It is however of note that MICA polymorphism was found to be associated with relapse rate independently of the disease-risk status. Nevertheless, we cannot exclude the possibility that the MICA-129 dimorphism behaves as a closely linked genetic marker to a yet-to-be-defined causative locus or as an SNP tag accounting for some functionally relevant variations lying within or outside the MICA gene. Because recent studies highlight the particular functional characteristics of the common MICA*008 variant allele, including raised levels of sMICA secretion,42 we tested the potential relationship between the HLA-B and MICA-129 genotypes in patients having or not having experienced cGVHD. Focused analysis of HLA-B*07, -B*08, and -B*44 (all linked to MICA*008 with MICA-129 val and with MICA A5.1 STR variation)43 failed to show any association with cGVHD, rendering MICA*008 unlikely to be the marker for susceptibility to cGVHD. The question however merits in its own right a detailed study of MICA polymorphism in its entirety in the context of HSCT.
Concerning sMICA, the sera of patients before transplantation (86% of them with malignant disorders) had higher levels of sMICA than sera of healthy donors, consistent with the reported elevated levels in a variety of cancers, including leukemia.44,45 Further increase of sMICA in posttransplantation conditions in our patients is associated with a higher incidence of cGVHD independent of preexisting history of aGVHD as well as MICA-129 genotype. Albeit several works analyzed the relationship between sMICA and diseases, in settings such as cancer, autoimmunity, infection, or organ transplantation, there is a paucity of information in HSCT. Overall, MICA shedding by means of cellular isomerases and proteinase enzymes46,47 with consequent release of the soluble forms result both in the reduction of MICA cell-surface density and systemic NKG2D down-regulation on NK and T cells, 2 mechanisms thought to subvert NKG2D-mediated immune surveillance. This broadly admitted mechanism seems to have different functional consequences, depending on the underlying pathology. Although the above-mentioned pathway promotes tumor evasion/progression, premalignancy transformation,48 immune deficiency in end-stage renal dysfunction,49 and acute bacterial infections,50 in heart transplantation settings, a recent study showed that patients having a high amount of sMICA molecules are protected from graft rejection.18 The investigators hypothesized that the impairment of NK/T -cell activation through NKG2D inactivation by sMICA could provoke a tolerogenic situation favoring graft maintenance. Similarly to MICA-129 genotype, the association between sMICA and cGVHD could be discussed under the conceptual framework based on the ability of such isoforms to mediate NK-cell inactivation, through NKG2D down-regulation. Impaired effector cell activation could lead here again to a TH1/TH2 imbalance and an autoimmune-like situation. Because we did not find significant correlation between levels of sMICA before and after transplantation, it is likely that the observed increase could be related to the transplantation event per se. Nevertheless, we cannot exclude fully that the observed raised levels of sMICA may merely reflect the inflammatory status common in clinical situations such as cGVHD and thus may behave as a generic marker of inflammation (not strictly specific to these complication).
Because MICA molecules may act as an alloantigen capable of eliciting alloantibody response, we checked the presence of MICA Abs before and after HSCT and found significant levels in both periods. Interestingly, we found also that the presence of Abs before transplantation correlates with low levels of sMICA after transplantation and consequently with a low incidence of cGVHD, strongly suggesting a neutralizing effect of MICA Abs on sMICA. Our data are consistent with a study showing that patients with monoclonal gammopathy of undetermined significance with high MICA Abs were those with the lower level of sMICA as well as with a relatively low tendency to evolve toward multiple myeloma.51 It has also been shown that patients with melanoma who respond to antibody blockade of cytotoxic T lymphocyte-associated antigen 4 or to vaccination with autologous irradiated tumor cells produced high amounts of MICA Abs and had low circulating sMICA accompanied by an increase in NK and CD8+ T cell–mediated cytotoxicity. The same study showed the capacity of MICA Abs to opsonize efficiently the cancer cells and to participate in the complement-mediated lysis.52 These data perfectly fit with the trend we observed in that MICA Abs confers protection against relapse.
To our knowledge, this study provides the first observation about MICA-related features in human HSCT and highlights their potential use as predictive biomarkers for cGVHD. If confirmed in a larger patient cohort, our data may open up prophylactic immunotherapeutic strategies against cGVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by research grants from the ATC Inserm Medicament et Vectorization (A04166HS) and institutional funding to AP-HP and Inserm UMRS 940.
Authorship
Contribution: W.B. designed the research, analyzed and interpreted data, and wrote the manuscript; M.B. collected data, performed statistical analysis, and analyzed and interpreted data; R.P.d.L. and V.R. collected clinical data; C. Suberbielle, D.B., N.D., P.H., and C. Scieux were involved in phenotype analysis; H.A. performed the research; E.G. critically reviewed the manuscript; R.K. designed the research and critically reviewed the manuscript; A.T. participated in the research design and critically reviewed the manuscript; D.C. critically reviewed the manuscript; G.S. analyzed and interpreted data and critically reviewed the manuscript; and R.T. designed the research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ryad Tamouza, Laboratoire d'Immunologie et d'Histocompabilité. Centre Hayem 3ème étage, Hôpital Saint Louis, 1 avenue Claude Vellefaux, 75010, Paris, France; e-mail: ryadtamouza@yahoo.fr.
References
Author notes
W.B. and M.B. contributed equally to this study.