Abstract
Lymphomas of the ocular adnexa are a heterogeneous group of malignancies, composing approximately 1% to 2% of non-Hodgkin lymphomas (NHLs) and 8% of extranodal lymphomas. The most common subtype, accounting for up to 80% of cases of primary ocular adnexal lymphoma, is marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) type. In the recent past, there have been significant advances in our understanding of the clinical characteristics, morphology and phenotype, etiology, pathogenesis, diagnosis, natural history, treatment approaches, outcome, and prognostic factors of this disease entity. Novel immunologic and molecular techniques have aided in the distinction between MALT lymphoma and other lymphoproliferative disorders and led to the identification of tissue markers of prognostic significance. Modern imaging modalities provide invaluable tools for accurate staging and treatment planning. Besides radiotherapy and chemotherapy, a variety of new treatment options have emerged in the management of patients with ocular adnexal MALT lymphoma, especially monoclonal antibody therapy and antibiotic therapy against Chlamydia psittaci, which has been associated with the pathogenesis of ocular adnexal lymphomas in some parts of the world. In this review, we present a state-of-the-art summary of ocular adnexal MALT lymphomas.
Introduction
Lymphomas of the ocular adnexa are a heterogeneous group of malignancies, accounting for approximately 1% to 2% of non-Hodgkin lymphomas (NHLs) and 8% of extranodal lymphomas.1 According to Surveillance, Epidemiology, and End Results data, the incidence of ocular adnexal lymphoma (OAL) has risen steadily between 1975 and 2001, with an annual increase of 6.3%.2 Lymphomas constitute the most frequent malignant tumors of the ocular adnexa; in the Florida Cancer Registry, they represent up to 55% of all orbital tumors.3
The majority of OALs are primary extranodal neoplasms; however, 10% to 32% are secondary tumors in patients with disseminated lymphoma.4-7 More than 95% are of B-cell origin, and 80% are low-grade lymphomas. The most common subtype of primary OAL, accounting for 35% to 80% of cases, is extranodular marginal zone lymphoma (ENMZL) of mucosa-associated lymphoid tissue (MALT) type, followed by follicular lymphoma (∼ 20%), diffuse large B-cell lymphoma (∼ 8%), and less commonly mantle cell lymphoma, small lymphocytic lymphoma, and lymphoplasmacytic lymphoma.4,6,8-14 Few cases of primary T-cell lymphoma and Hodgkin lymphoma of the ocular adnexa have been reported.10 In Japan and Korea, the proportion of MALT lymphoma among primary OAL appears to be higher (80%-90%) than in Western countries.11,15-17
Secondary ocular involvement occurs in 2.4% to 5.3% of patients with advanced systemic NHL. Of note, extranodular marginal zone lymphoma with secondary ocular manifestation typically involves simultaneously other extranodal MALT sites.
In this review, we focus on primary ocular adnexal MALT lymphoma (OAML), highlighting the most recent advances in our understanding of their clinical characteristics, morphology, phenotype, etiology, pathogenesis, diagnosis, natural history, treatment approaches, outcome, and prognostic factors, discussing existing controversies and indicating areas for future research.
Review search strategy
Medline PubMed was searched for English-language articles using the terms “marginal zone lymphoma,” “MALT,” “eye,” “ocular,” “adnexal,” “orbital,” “conjunctival,” “lacrimal,” “uveal,” “etiology,” “pathogenesis,” “Chlamydia psittaci infection,” “pathology,” “staging,” “treatment,” “radiation therapy,” “surgery,” “chemotherapy,” “immunotherapy,” “outcome,” and “prognosis.” Original as well as review articles, clinical trials, case series, single case reports, and meta-analyses were identified. Studies including different subtypes of lymphoma or different sites of MALT lymphoma were considered whenever the data allowed for a selective analysis of MALT lymphoma of primary ocular sites. The bibliography of each relevant article was screened for additional references.
Histopathologic and immunophenotypic characteristics
Lymphoproliferative lesions in the ocular adnexa encompass a wide spectrum of disorders, ranging from benign reactive lymphoid hyperplasia (RLH), that is, a nonspecific orbital inflammation, to malignant lymphoma.15 Before the advent of immunophenotyping and molecular diagnostic techniques, MALT lymphomas were frequently (mis)diagnosed as RLH or “pseudotumors” because of their cellular heterogeneity and presence of reactive germinal centers. There have been case reports of ocular adnexal RLH with subsequent development of disseminated (extraocular) lymphoma, suggesting progression from benign to malignant lymphoid disease; however, the majority of these cases were described before recognition of MALT lymphoma as an entity and thus may represent misdiagnosis. Therefore, further systematic study of the natural history of orbital RLH in the current era of clinicopathologic diagnosis is needed before final conclusions can be drawn.
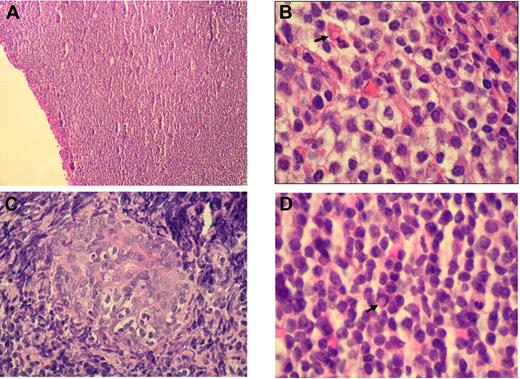
Histologically, RLH shows a dense infiltration of mature small lymphocytes with scattered histiocytes and plasma cells. In contrast, OAMLs are characterized by an expansion of a heterogeneous cell population, consisting of centrocyte-like, monocytoid, and plasmacytoid cells, with occasional blasts in the marginal zone surrounding reactive follicles (Figure 1A-B). Pathognomonic histopathologic features include “follicular colonization” (secondary infiltration of germinal centers by malignant lymphocytes) and the formation of “lymphoepithelial lesions” through invasion of neighboring epithelial structures by nests of MALT lymphoma cells (Figure 1C). “Dutcher bodies,” intranuclear pseudoinclusions of periodic acid-Schiff–positive eosinophilic cytoplasm, have been observed in low-grade lymphoid malignancies, especially in OAMLs with plasmacytoid differentiation (Figure 1D).
Histologic findings in OAML. (A) Diffuse infiltration of the conjunctiva by a dense small lymphocytic cell population (hematoxylin and eosin, original magnification ×100). (B) Monocytoid cells arranged in sheets in an orbital mass with plasma cell and a Russell body ( ; hematoxylin and eosin, original magnification ×1000). (C) Lymphoepithelial lesion in lacrimal gland (hematoxylin and eosin, original magnification ×500). (D) Centrocyte-like cells, plasma cells, and Dutcher body (
; hematoxylin and eosin, original magnification ×1000). (C) Lymphoepithelial lesion in lacrimal gland (hematoxylin and eosin, original magnification ×500). (D) Centrocyte-like cells, plasma cells, and Dutcher body ( ) beneath conjunctival epithelium (hematoxylin and eosin, original magnification ×1000). Images of immunohistochemical staining were acquired using a Nikon Eclipse E400 microscope (Nikon) and a Nikon DS-Li digital camera. Digitized images were processed using Adobe Photoshop 7 image processing and manipulation software (Adobe Systems).
) beneath conjunctival epithelium (hematoxylin and eosin, original magnification ×1000). Images of immunohistochemical staining were acquired using a Nikon Eclipse E400 microscope (Nikon) and a Nikon DS-Li digital camera. Digitized images were processed using Adobe Photoshop 7 image processing and manipulation software (Adobe Systems).
Histologic findings in OAML. (A) Diffuse infiltration of the conjunctiva by a dense small lymphocytic cell population (hematoxylin and eosin, original magnification ×100). (B) Monocytoid cells arranged in sheets in an orbital mass with plasma cell and a Russell body ( ; hematoxylin and eosin, original magnification ×1000). (C) Lymphoepithelial lesion in lacrimal gland (hematoxylin and eosin, original magnification ×500). (D) Centrocyte-like cells, plasma cells, and Dutcher body (
; hematoxylin and eosin, original magnification ×1000). (C) Lymphoepithelial lesion in lacrimal gland (hematoxylin and eosin, original magnification ×500). (D) Centrocyte-like cells, plasma cells, and Dutcher body ( ) beneath conjunctival epithelium (hematoxylin and eosin, original magnification ×1000). Images of immunohistochemical staining were acquired using a Nikon Eclipse E400 microscope (Nikon) and a Nikon DS-Li digital camera. Digitized images were processed using Adobe Photoshop 7 image processing and manipulation software (Adobe Systems).
) beneath conjunctival epithelium (hematoxylin and eosin, original magnification ×1000). Images of immunohistochemical staining were acquired using a Nikon Eclipse E400 microscope (Nikon) and a Nikon DS-Li digital camera. Digitized images were processed using Adobe Photoshop 7 image processing and manipulation software (Adobe Systems).
Immunophenotypically, OAMLs have a characteristic profile, which allows their differentiation from benign lymphoproliferative disorders and other small B-cell lymphomas. They are characterized by dense, CD20+, CD10−, CD23−, BCL-6− B-cell lymphocytic infiltrates, with few interspersed CD3+ T lymphocytes. OAMLs are almost always (95%) negative for CD5, distinguishing them from mantle cell lymphoma and small lymphocytic lymphoma/chronic lymphocytic leukemia. Few cases of OAMLs with CD5 immunoreactivity have been reported, posing intriguing differential diagnostic difficulties. In this setting, careful morphologic evaluation, combined with immunohistochemical assessment of cyclin D1 expression and fluorescence in situ hybridization analysis for translocation t(11;14)(q13;q32), is helpful in establishing the correct diagnosis. B-cell monoclonality can be demonstrated by molecular studies for immunoglobulin heavy chain gene rearrangements, using either Southern blot hybridization or polymerase chain reaction (PCR), and can aid in the distinction from RLH, which shows polyclonal lymphoid proliferation. Our diagnostic approach to suspicious ocular adnexal biopsies includes immunohistochemical staining for CD20, CD79a, CD10, CD5, CD23, BCL-2, BCL-6, and PCR for immunoglobulin heavy and light chain gene rearrangements, which allows a correct diagnosis in the majority of cases.
Molecular and cytogenetic features
Several genetic aberrations have been described in OAML. Karyotypic alterations include trisomy of chromosomes 3 and 18 in up to 68% and 57% of MALT lymphoma patients, respectively.18-21 Gains of chromosome 3 have been most frequently observed in orbital (81%), in contrast to conjunctival and lacrimal gland OAML.22 An association between plasmacytic differentiation and the presence of +3 or +18q gains was noted. Trisomy 18 is found predominantly in young females and in tumors of conjunctival origin and is associated with prominent lymphoepithelial lesions and disease recurrence.20
The most frequent translocation in OAML, observed in 15% to 40% of patients,23 is t(11;18)(q21;q21), juxtaposing the API2 (apoptosis inhibitor 2) gene on chromosome 11 and the MALT1 gene on chromosome 18, creating the API2-MALT1 fusion protein. The t(14;18)(q32;q21) translocation, found in up to 38% of patients,24 juxtaposes the MALT1 gene and the IgH promoter on chromosome 14, inducing constitutive expression of the MALT1 gene. The t(1;14)(p22;q34) translocation, present in less than 5% of patients, induces juxtaposition of the BCL-10 gene on chromosome 1 and the IgH promoter, leading to constitutive expression of the BCL-10 gene. The t(3;14)(p14;q32) translocation, found in 20% of patients,25 juxtaposes the FOXP1 gene on chromosome 3 and the IgH promoter. This translocation is frequently accompanied by other cytogenetic abnormalities, especially trisomy 3, but none of the other previously described balanced translocations. All chromosomal alterations affect a common signaling pathway, resulting in activation of the nuclear factor-κB complex, leading to transcription of several genes contributing to lymphomatous transformation, cell proliferation, and survival.
Although the study of cytogenetic abnormalities has led to a better understanding of the pathogenesis of OAML, in our practice, they are not used for routine diagnosis and therapeutic decisions.
Etiologic factors and pathogenesis
MALT lymphomas typically arise in tissues or organs that are normally devoid of any organized lymphoid tissue, such as the orbital region, but acquire reactive lymphoid tissue in response to persistent antigenic stimulation, as a result of chronic inflammatory or autoimmune disorders. Analyses of somatic mutations in the variable (V) region of the immunoglobulin (Ig) and heavy (H) chain gene segment have suggested a role of chronic antigen stimulation in the pathogenesis of OAML.26 This process, initially dependent on ongoing antigenic stimulation, eventually becomes autonomous. Chronic antigenic stimulation may eventually progress to genetic instabilities with successive chromosomal abnormalities, causing transformation of a clone of normal lymphoid cells to MALT lymphoma. Additional genetic abnormalities, including p53 or p16 mutation/deletion, may ultimately result in progression to a more aggressive lymphoma (diffuse large B-cell lymphoma) in less than 10% of cases.
The nature of the underlying inflammatory processes in patients with OAML remains unknown in the majority of cases. A large cohort study from the United Kingdom, including 369 patients with OAML, showed a significant (5%) prevalence of autoimmune thyrotoxicosis with thyroid orbitopathy, preceding the diagnosis of OAML by a median of 17.5 years.27 In a prospective case-control study from Italy, a significant association was demonstrated between rural residence, exposure to household animals, and history of chronic conjunctivitis in patients with OAML.28 In our experience of 93 recently treated patients, only 1 patient had a history of autoimmune thyroid disease and none had chronic conjunctivitis (I.S.L., unpublished observation, 2009), suggesting variable association of predisposing factors in different geographic areas.
Microbial pathogens that underlie chronic inflammatory processes and eventually lead to the acquisition of MALT may also play a pivotal role in both malignant transformation and subsequent clonal expansion of the lymphoma. Among patients with primary OAML, single case reports have shown an association with Helicobacter pylori and Chlamydia pneumoniae DNA.29 In another study from Korea, H pylori DNA was detected in all 15 analyzed cases of conjunctival MALT lymphoma.30 In contrast, Sjo et al31 failed to identify H pylori DNA in any of the 13 analyzed cases of conjunctival MALT lymphoma.
Association with C psittaci infection
Recently, Ferreri et al32 demonstrated an association between OAML and infection with C psittaci (Cp) in Italian patients. Cp-DNA was detected by immunohistochemistry (using a monoclonal antibody against Cp lipopolysaccharide) and PCR analysis in 80% of 40 lymphoma samples, compared with 12% of RLH samples. Moreover, bacterial DNA was found in 43% of peripheral blood mononuclear cells of patients, but not in healthy donors. Similar findings were reported from Korea, where Cp-DNA was detected in 79% of patients.33 Clinicopathologic characteristics, recurrence rate, progression-free survival, and overall survival did not differ in patients with or without Cp infection. Yeung et al34 reported on the coincidence of Cp-positive adult inclusion conjunctivitis with giant follicles and conjunctival MALT lymphoma in a single case. In a large study of 142 cases, Chanudet et al35 described an overall prevalence of Cp infection in OAML in 22%, but marked geographic variation, with highest incidences in Germany (47%), the East Coast of the United States (35%), and The Netherlands (29%).
However, several subsequent studies from different countries have failed to show an association between Cp infection and OAML.22,36-41 In a clinicopathologic study of 57 patients from our institution, Cp-DNA was not detected in any of the cases, indicating that, in South Florida, OAMLs are not associated with Cp infection.42 A meta-analysis43 has attempted to shed light on the association between Cp and OAML across geographic regions and between different studies. Among 11 studies with 458 cases of OAML from 10 different countries, Cp was identified in 25% of MALT lymphoma specimens. Moreover, the analysis showed that 90% of the Cp-positive OAML specimens came from 3 of the 11 studies, suggesting a striking variability in the association between Cp and OAML across geographic regions and even between studies from the same geographic regions. Of note, similar discrepant findings have also been reported regarding the association between cutaneous MALT lymphoma and infection with Borrelia burgdorferi in studies from Europe, Asia, and the United States.
The question has been raised whether other microbial species may be involved in the pathogenesis of OAML. At our institution, analysis of 49 OAML samples using 2 distinct PCR techniques based on universal bacterial primers failed to detect bacterial DNA in any of the cases.44 Overall, the studies published to date suggest variable associations between Cp and OAML across geographic regions worldwide. However, differences in PCR methodology may also contribute to some of the differences observed in these studies. Side-by-side comparison of the PCR methodologies is required. In addition, further studies evaluating viral infections in the etiology of OAML are needed.
Clinical presentation
OAMLs are mostly seen in the 5th to 7th decade of life (median age, ∼ 65 years), with female predominance (male/female = 1:1.5/2). In contrast, studies in Korean populations reveal a significantly younger age (median, ∼ 46 years) at the time of diagnosis, with male rather than female predominance.11,16,17 Most frequent site of origin is the orbit (∼ 40%), followed by conjunctiva (35%-40%), lacrimal gland (10%-15%), and eyelid (∼ 10%).2 Bilaterality occurs in 10% to 15% of cases (80% simultaneous, 20% sequential events). Table 1 summarizes information on OAML patient characteristics, primary sites, and initial disease stage.6,7,10-12,15,17,42,45-54
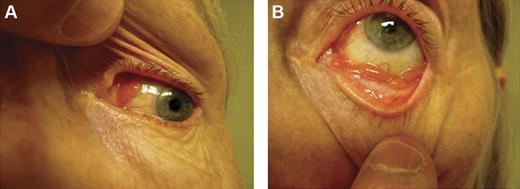
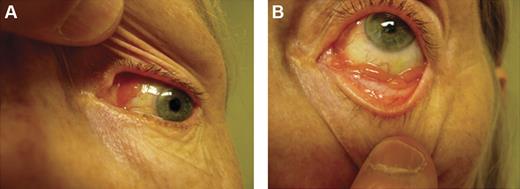
Conjunctival lesions typically present as mobile pink infiltrates in the substantia propria (“salmon-pink patch”), causing conjunctival swelling, redness, and irritation (Figure 2). Orbital lymphoid proliferations are characterized by a palpable, firm or rubbery mass causing insidious, progressive proptosis, occasionally associated with periorbital edema, decreased visual acuity, motility disturbances, and diplopia. Lacrimal gland lymphomas originate in the superior anterior orbit, leading to inferior and nasal displacement of the globe. Lymphomatous lesions of the eyelids are typically found in the dermis or orbicularis muscle of the superior eyelid and may cause ptosis.
Clinical presentations of OAML. Conjunctival MALT lymphoma with involvement of the nasal bulbar conjunctiva (A) and inferior fornix (B).
Clinical presentations of OAML. Conjunctival MALT lymphoma with involvement of the nasal bulbar conjunctiva (A) and inferior fornix (B).
The median interval between onset of symptoms and time of diagnosis is variable, ranging from 1 month to 10 years (median, 7 months). This delay can be attributed to the slow evolution of symptoms, especially in conjunctival lymphomas, which may masquerade as chronic conjunctivitis and frequently show an impressive initial response to topical steroids. In our experience, steroids may mask the clinical presentation and render pathologic diagnosis more difficult, frequently resulting in a need for repeat biopsy to establish the diagnosis.
The majority (85%-90%) of patients with OAML present with localized disease (stage I). Nodal involvement is reported in approximately 5% of patients. In various case series, 10% to 15% of patients have disseminated disease (stage IV) at initial presentation, including bone marrow involvement in approximately 5%. In our published series of 62 patients with OAML, initial bone marrow involvement was observed in 8% of patients.42 Few patients present with unfavorable prognostic features, such as B symptoms, performance status of more than 1, elevated serum lactate dehydrogenase (LDH), or β2-microglobulin levels.
Diagnostic and staging procedures
The initial evaluation of patients with OAL requires careful ophthalmologic examination and adequate tissue sampling for histopathologic diagnosis. Further assessment for accurate staging and therapy planning includes a complete history and physical examination, routine laboratory studies, serum protein electrophoresis, serum LDH, β2-microglobulin, chest x-ray, computed tomography (CT) of chest, abdomen, and pelvis, and bone marrow biopsy. The latter is considered controversial by some physicians but is routinely done at our institution.
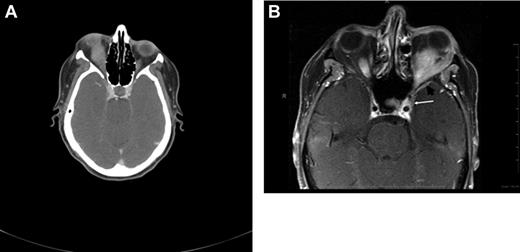
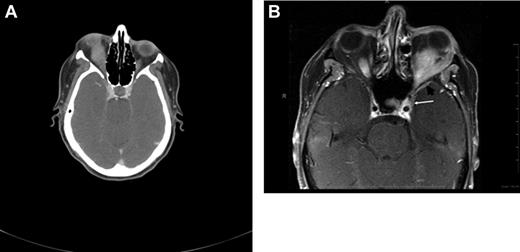
CT and magnetic resonance tomography (MRI) with contrast enhancement are the primary radiographic imaging tools in the evaluation of ocular adnexal proliferations. They aid in the assessment of location, size, and degree of infiltration; however, they cannot reliably distinguish between benign and malignant processes. Cuts of 1 to 3 mm are performed in axial and coronal planes, including the cavernous sinus and parasellar area to determine extraorbital extension. Typical imaging appearance of a lymphoid lesion is that of a unifocal, homogeneous, well-circumscribed lesion of isodensity to slight hyperdensity, with mild to moderate contrast enhancement, and smooth, distinct borders, molding into adjacent tissues and displacing rather than infiltrating orbital structures (Figure 3).55,56 Infiltration into the globe or bony erosions are unusual features of MALT lymphoma and should raise suspicion for high-grade transformation with aggressive clinical behavior.
Radiographic presentations of OAML. (A) CT image of right orbital MALT lymphoma involving the inferior rectus muscle, causing mild proptosis. (B) MRI of left orbital MALT lymphoma involving the lateral and inferior rectus muscles, causing medial deviation of the optic nerve, with involvement of the cavernous sinus (white arrow) and mild dural enhancement in the medial anterior cranial fossa (black arrow). Analysis of the cerebrospinal fluid was positive for lymphoma. This case represents an unusual extension of OAML into the central nervous system leading to lymphomatous meningitis.
Radiographic presentations of OAML. (A) CT image of right orbital MALT lymphoma involving the inferior rectus muscle, causing mild proptosis. (B) MRI of left orbital MALT lymphoma involving the lateral and inferior rectus muscles, causing medial deviation of the optic nerve, with involvement of the cavernous sinus (white arrow) and mild dural enhancement in the medial anterior cranial fossa (black arrow). Analysis of the cerebrospinal fluid was positive for lymphoma. This case represents an unusual extension of OAML into the central nervous system leading to lymphomatous meningitis.
Positron emission tomography (PET) imaging may represent a valuable addition in the evaluation of OAL, despite its low sensitivity (27%) in detecting orbital lesions.57 Several studies have indicated that PET has a higher sensitivity than CT scan in the detection of distant disease (86% vs 72%) in patients with OAL.55 In 71% of cases, the addition of PET imaging to CT and MRI has provided additional, clinically meaningful information that led to “upstaging” and change in patient management.55,57,58 However, overall FDG uptake is reported in only 50% of cases of MALT lymphomas in other locations; thus, further studies are needed to justify its routine use in OAML. At our institution, PET imaging is not routinely performed in the evaluation of patients with OAML. Additional studies examining the contribution of PET imaging in upstaging of patients with OAML and consequent change of the therapeutic approaches as well as on their cost-effectiveness are needed before this imaging modality should be routinely used in the management of OAML patients.
Based on different patterns of expression of somatostatin receptors by gastric (−) and extragastric (+) MALT lymphoma, somatostatin-receptor scintigraphy using 111In-octreotide is a useful tool in the initial staging and posttreatment evaluation in patients with nongastric MALT lymphoma. Raderer et al59 demonstrated not only reliable distinction between gastric and extragastric origin of disseminated MALT lymphoma, irrespective of the site of presentation, but also accurate identification of residual disease or early relapse after completion of treatment, which, in cases of OAML, preceded detection by other radiographic imaging techniques. We have reported on our experience using somatostatin-receptor scintigraphy for initial staging and evaluation of response to treatment in a patient with MALT lymphoma of the lacrimal gland.60 Given the frequent lack of PET avidity of MALT lymphoma, somatostatin-receptor scintigraphy may be helpful in the staging and response evaluation of selected patients with complex clinical scenarios.
Treatment
Various treatment modalities are available for the management of patients with OAML, including surgical resection, radiotherapy, single-agent or combination chemotherapy, and immunotherapy with monoclonal antibodies. However, no prospective clinical trials have been conducted to evaluate these therapeutic options or define the optimal treatment approach for these patients. Recently, antichlamydial antibiotic therapy was also proposed as a novel treatment option.
All therapeutic strategies are associated with unique short- and long-term efficacy and toxicities, which need to be carefully weighed. The final treatment decision requires a multidisciplinary approach, taking into account the extent of the disease, the impact of the lymphoma on the eye and visual function, and finally patient- and disease-related prognostic factors.
Surgical resection
Encapsulated tumors, such as conjunctival and lacrimal gland tumors, may be completely removed by surgical resection. However, because of tumor microinfiltration into surrounding tissue, the risk of recurrence is relatively high if no adjuvant chemotherapy or radiotherapy is administered. Several case series have included patients who did not undergo any additional treatment after surgical excision.4,11-13,48,61-65 Although several patients may remain free of recurrence during the subsequent surveillance period, we, like most authors, conclude that this approach is inferior.
Expectant observation
Tanimoto et al66 evaluated a “watch and wait” policy in 36 asymptomatic patients (median age, 63 years) with localized OAML. After a median follow-up of 7.1 years, 69% did not require treatment, 47% progressed, and 6% died because of progressive lymphoma. In another retrospective analysis, Mannami et al15 observed 12 patients with stage I OAML for a median duration of 50 months. None of the patients progressed during this time period. In our opinion, this strategy remains controversial but may be appropriate in frail elderly patients with asymptomatic disease or in the setting of severe comorbidities that preclude an aggressive therapeutic approach. However, even in this patient population, local radiation therapy can usually be given.
Radiation therapy
Radiation therapy is the treatment of choice for the majority of patients with localized OAML. Radiotherapy techniques typically vary according to disease location in the orbit: Tumors confined to superficial structures, such as conjunctiva, eyelid, and lacrimal gland, are usually approached with a direct electron beam using a lens-sparing device to avoid cataract formation, whereas retrobulbar tumors are treated with photon beam radiation.
Several retrospective studies, most of which include a variety of lymphoma subtypes, have documented the short- and long-term efficacy and side effects of this therapeutic modality. Table 2 summarizes the available data for OAML.5,46-48,51,52,54,67,68 Overall, radiotherapy leads to very high local control rates of 85% to 100%. These outstanding results have to be balanced against frequent treatment-related toxicities and a substantial risk of distant recurrence (10%-25%) over at least 10 years after treatment.
There is no universally accepted radiation schedule for patients with OAML, and controversy exists regarding the optimal radiation dose and fractionation. Several studies indicate that tumor doses of at least 25 Gy are required to provide optimal local control and minimize the rate of local failures in OAML,47,51,68 whereas doses of 25 Gy or less may be sufficient in the management of benign RLH.69 Fung et al5 have reported 5-year local control rates of 81% for doses of less than 30 Gy and 100% for doses of more than or equal to 30 Gy, suggesting an optimal radiation dose for OAML of 30.6 to 32.4 Gy in daily fractions of 1.8 Gy, based on their dose-response data. Le et al67 found no differences in local or distal recurrence and survival after radiation with less than or equal to 34 Gy compared with higher doses; however, significant ophthalmologic toxicity with vision loss was observed after doses of more than or equal to 34 Gy. In our study, including 46 patients with stage I OAML, radiation therapy of 30 to 36 Gy (45 Gy in 2 patients) led to durable local control in 100% of cases.42
Current National Cancer Center Network guidelines recommend radiotherapy of 20 to 30 Gy for initial treatment of early-stage nongastric ENMZL of all sites and reirradiation for locally recurrent disease. Based on our experience and current evidence from the literature, we consider 30 Gy the optimal radiation dose for local disease control in patients with OAML. Reirradiation in the setting of relapsed disease exceeds the maximum tolerable dose of the eye and should be avoided.
Immediate toxicity resulting from radiotherapy consists of mild to moderate cutaneous or conjunctival reactions. Long-term complications are observed in up to 50% of patients, including cataract formation (30%-50%) and xerophthalmia (20%-40%). Radiation doses of more than or equal to 36 Gy may result in deleterious ophthalmologic toxicity, such as ischemic retinopathy, optic atrophy, corneal ulceration, and neovascular glaucoma, associated with significant vision loss.70
Chemotherapy
There are limited data on chemotherapy for patients with OAML. A small number of single-center, retrospective case series have included patients treated with different chemotherapy regimens, such as COP/CVP (cyclophosphamide, vincristine, prednisone), CHOP (cyclophosphamide, adriamycin, vincristine, prednisone), C-MOPP (cyclophosphamide, vincristine, procarbazine, prednisone), and chlorambucil, either alone or in combination with other modalities,6,42,71,72 but only few reports contain detailed response rates and outcomes.49,50,53,54,71,73 It is very difficult to draw conclusions and make practical recommendations based on the published data. Chlorambucil, given in a variety of doses, schedules, and combinations, is the most frequently used chemotherapy agent and has a highly favorable toxicity profile, making it a suitable agent for the treatment of frail, elderly patients. Irrespective of chemotherapy regimens, complete responses are observed in 67% to 100% of patients; however, long-term outcome data suggest that local recurrence is the predominant cause of treatment failure, occurring in up to 29% of patients. Several authors report successful salvage with radiotherapy in patients who experience local failure after initial treatment with chemotherapy.53,71 Our personal experience confirms this observation; however, distant relapses may also occur.
Restrepo et al74 conducted a retrospective analysis exploring the need for central nervous system (CNS) prophylaxis in patients with OAL. In their study of 71 patients, including 54 with OAML, CNS prophylaxis was not administered, and none of the recurrences (15% after a median follow-up of 20 months) occurred in the CNS. The authors conclude that CNS prophylaxis in patients with OAL is unnecessary. However, very rare cases of CNS involvement can be observed (Figure 3B).
Immunotherapy
There is a limited body of evidence regarding the efficacy of rituximab, a monoclonal chimeric anti-CD20 antibody, in patients with MALT lymphoma arising in different organs. Phase 2 data by Conconi et al,75 Raderer et al,76 and from our institution77 using single-agent rituximab in previously untreated patients demonstrated overall response rates between 50% and 87%, but median time to disease progression was less than 1 year.
Only a few case reports using single-agent rituximab in patients with OAML have been published.60,75,78-81 They confirm the high activity of rituximab in both newly diagnosed and relapsed disease, but early recurrence is common, particularly in pretreated patients.79 Further investigations with longer follow-up will be needed to better define the role of rituximab in the management of OAML.
Radioimmunotherapy
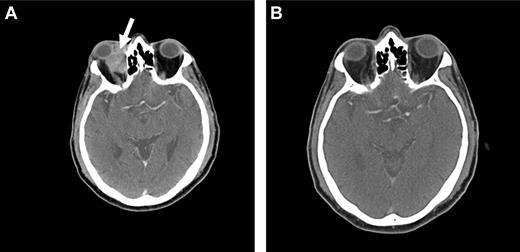
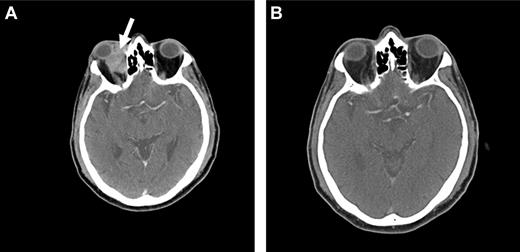
Because OAML is highly sensitive to radiotherapy, it is probable that radioimmunotherapy may be effective in controlling local disease as well as preventing distant relapse. At our institution, we are currently conducting a prospective phase 2 study using Y-90 ibritumomab tiuxetan (Zevalin) as first-line therapy in patients with newly diagnosed marginal zone lymphoma of all sites. Initial data suggest that this agent may be highly active and a useful approach in the treatment of OAML, with a favorable side effect profile (Figure 4).
Radioimmunotherapy with Y-90 ibritumomab tiuxetan (Zevalin). Pretreatment (A) and posttreatment (B) CT imaging of a patient with OAML involving the right orbit (arrow), demonstrating a complete response 12 weeks after radioimmunotherapy.
Radioimmunotherapy with Y-90 ibritumomab tiuxetan (Zevalin). Pretreatment (A) and posttreatment (B) CT imaging of a patient with OAML involving the right orbit (arrow), demonstrating a complete response 12 weeks after radioimmunotherapy.
Antibiotic therapy against Cp infection
Based on the association between Cp infection and OAML, Cp-eradicating antibiotic therapy has received attention as a novel treatment approach for this disease. After encouraging results of a small pilot study,82 Ferreri et al conducted a prospective phase 2 clinical trial of 27 patients (15 newly diagnosed and 12 relapsed) with OAML, using doxycycline 100 mg orally twice daily for 3 weeks.83 Cp infection was confirmed in 11 patients. Partial or complete lymphoma regression after antibiotic therapy was observed in 7 of 11 Cp-positive and 6 of 16 Cp-negative patients, with an overall response rate of 48%. The 2-year failure-free survival rate among patients treated with doxycycline was 66%. Failure to detect Cp resulting from lack of sensitivity of PCR technique, the existence of different bacterial strains, or previous antibiotic therapy could, according to the authors, explain responses to doxycycline in Cp-negative patients. Abramson et al84 treated 3 patients with biopsy-proven conjunctival MALT lymphoma with antibiotic therapy, resulting in 2 complete remissions and 1 partial response.
Given the variable prevalence of Cp infection in patients with OAML, empiric antibiotic treatment without prior testing for chlamydial infection cannot be generally recommended, as elaborated by Grünberger et al.85 To shed more light on the efficacy of antibiotics in the treatment of patients with OAML, Husain et al43 conducted a meta-analysis, identifying 4 studies with a total of 42 patients who had been treated with oral doxycycline. Objective documentation of response was available for only 3 of 20 patients who reportedly achieved some response, whereas another 20 patients had stable disease and 2 progressed during antibiotic therapy. After antibiotic therapy, 7 patients developed disease recurrence, 6 of them within 12 months of follow-up. Prospective trials with standardized objective response criteria and longer follow-up will be necessary to further evaluate the role of antibiotics in the treatment of OAML in different geographic regions.
Outcome and prognosis
The prognosis of patients with OAML is generally favorable, with a high proportion of localized disease, indolent clinical course, prolonged disease-free intervals, and low lymphoma-related mortality rates, as illustrated in Table 3.4,6,7,9,11,12,17,42,45,54,68,71,86,87 Because of the heterogeneity of the data with regard to patient populations, disease characteristics, management approaches, and duration of follow-up, the results of these mostly retrospective analyses are difficult to compare. In many case series, nonconjunctival primary site, advanced disease stage, nodal involvement, age older than 60 years, presence of B symptoms, and elevated serum LDH levels have been identified as negative prognostic factors in patients with OAML.5,6,9,11-13,42,45,70,72,88
The primary site of presentation of OAML appears to correlate with the risk for systemic involvement. In general, conjunctival primaries are associated with the lowest (∼20%), orbital with intermediate (∼ 35%), and eyelid with the highest (∼ 65%) risk of disseminated disease.89-91 Several small case series have suggested that lacrimal gland primaries may present with higher rates of bilateral disease and extraorbital involvement and have a relatively poor prognosis, especially in patients with advanced disease.9,92 However, other studies did not confirm this observation.93
There are conflicting data regarding the prognostic impact of disease stage at diagnosis: Up to one-third of patients with OAML present with or subsequently develop disseminated disease.9,14,94 Whereas some authors have reported an association between disseminated disease and increased risk of disease progression and/or lymphoma-related death, others did not demonstrate an inferior outcome. Similarly, the presence of bilateral adnexal disease is not invariably associated with increased risk of systemic involvement and/or lower disease-specific survival.14,89 Based on these conflicting data, the role of extensive staging evaluation at the time of diagnosis is an area of controversy. Most authors agree, however, that confirmation of limited (stage I) disease by routine staging examinations remains an essential prerequisite for therapeutic decision-making because the majority of these patients can be rendered disease-free with radiotherapy alone.
Several histopathologic and immunophenotypic features have been studied as potential predictors of outcome in patients with OAML. Plasmacellular differentiation, although associated with more disseminated disease at diagnosis, could not be statistically linked with an inferior clinical outcome.7 The expression of CD595,96 or CD4397 by a small proportion of patients with OAML may be associated with an unfavorable clinical course and outcome. Coupland et al identified the cell-cycle markers, p53 and BCL-6, as potential negative prognostic indicators in patients with OAML.64 Using tissue microarray analysis, Franco et al98 correlated aberrant nuclear expression of BCL-10 with statistically significant shorter failure-free survival, whereas other investigators did not confirm this association.63,99 At present, there are no reproducible or validated biomarkers that can be routinely used for outcome prediction in patients with OAML. Additional studies, involving large number of patients with OAML, are needed to identify robust prognostic biomarkers.
Summary and future outlook
There has been a significant rise in the incidence of OAML worldwide over the last several decades. On the basis of our institutional experience, we are advocating an interdisciplinary approach in the diagnosis and treatment of these patients, involving ophthalmology, medical oncology, and radiation oncology. Systemic staging is fundamental for optimal treatment planning and outcome because approximately 15% of patients present with disseminated disease. Standards of care at our institution include radiation therapy (30 Gy) for localized disease with excellent local control and combination chemotherapy/immunotherapy for disseminated disease.
Future studies should focus on the etiology, pathogenesis, and treatment of OAML. Current data suggest significant geographic variation of the association between Cp and OAML; however, these differences may be the result of methodologic rather than true geographic variability. Collaborative international studies with analysis of identical samples in different laboratories across the Atlantic are needed to exclude this possibility. Additional investigations should explore a possible viral etiology of OAML. Because MALT lymphomas frequently arise in the setting of chronic antigenic stimulation, more extensive analysis of IgV genes, their mutation status, and CDR sequences may lead to discovery of a common antigen. Application of novel molecular techniques, such as paraffin-based real-time PCR and gene expression arrays, may further elucidate the pathophysiology of OAML and identify specific molecular targets. As long as the role of bacterial antigens in the etiology of OAML remains poorly defined, the use of antibiotics should be limited to patients in clinical trials. Prospective collaborative studies enrolling sufficient numbers of patients are needed to compare different chemotherapy regimens and determine the optimal approach in patients with disseminated or relapsed disease.
Acknowledgments
The authors thank Dr David Tse from the Bascom Palmer Eye Institute and Dr Gerald Byrne from the Department of Hematopathology for their contribution of clinical and pathology images to this manuscript.
This work was supported by the United States Public Health Service, National Institutes of Health (grants R01-CA109335 and R01-CA122105), and the Dwoskin Family Foundation.
National Institutes of Health
Authorship
Contribution: A.S. and I.S.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Izidore S. Lossos, Department of Hematology and Oncology, Sylvester Comprehensive Cancer Center, University of Miami, 1475 NW 12th Ave (D8-4), Miami, FL 33136; e-mail: Ilossos@med.miami.edu.



 ; hematoxylin and eosin, original magnification ×1000). (C) Lymphoepithelial lesion in lacrimal gland (hematoxylin and eosin, original magnification ×500). (D) Centrocyte-like cells, plasma cells, and Dutcher body (
; hematoxylin and eosin, original magnification ×1000). (C) Lymphoepithelial lesion in lacrimal gland (hematoxylin and eosin, original magnification ×500). (D) Centrocyte-like cells, plasma cells, and Dutcher body (