Abstract
Although the combination of lenalidomide and dexamethasone is effective therapy for patients with relapsed/refractory multiple myeloma, the influence of high-risk cytogenetic abnormalities on outcomes is unknown. This subanalysis of a large, open-label study investigated the effects of the most common unfavorable cytogenetic abnormalities detected by fluorescence in situ hybridization, del(13q), t(4;14), and del(17p13), in 130 evaluable patients treated with this regimen. Whereas patients with either del(13q) or t(4;14) experienced a median time to progression and overall survival comparable with those without these cytogenetic abnormalities, patients with del(17p13) had a significantly worse outcome, with a median time to progression of 2.22 months (hazard ratio, 2.82; P < .001) and median overall survival of 4.67 months (hazard ratio, 3.23; P < .001). Improved therapeutic strategies are required for this subgroup of patients. This study was registered at www.ClinicalTrials.gov as #NCT00179647.
Introduction
The introduction of novel therapeutic agents has significantly improved the survival in multiple myeloma (MM).1 Nevertheless, survival rates vary considerably among MM subgroups2-4 ; in particular, patients with deletion of 17p132,5 or t(4;14),2,4 as detected by fluorescent in situ hybridization (FISH), retain their poor prognosis despite the use of single or tandem autologous stem cell transplantation2,3 and maintenance thalidomide.6,7 Whereas bortezomib was recently demonstrated to overcome the negative prognostic impact of t(4;14),8-10 limited data are available on the role of lenalidomide in patients harboring “high-risk” cytogenetic abnormalities.11-16 Therefore, we undertook a post hoc subanalysis of the Expanded Access Program database (MM-016 study)17 to investigate the effect of cytogenetic aberrations on outcomes in MM patients treated with lenalidomide and dexamethasone.
Methods
We evaluated 130 patients treated in 3 Canadian centers participating in the Expanded Access Program,17 in whom FISH studies for del(13q), t(4;14), and del(17p13) were available. The inclusion/exclusion criteria for the study and treatment schedules were the same as for the phase 3 studies, except that patients previously resistant to dexamethasone were eligible.18,19 The institutional review boards or ethics committees at each participating center approved the study protocol, and all patients provided written informed consent in accordance with the Declaration of Helsinki. Responses were evaluated using the European Group for Blood and Marrow Transplant criteria,20 with the addition of near-complete response describing negative serum and urine electrophoresis but positive immunofixation.
FISH studies and analysis
Immunofluorescent detection of clonotypic plasma cells was performed as previously described.21 del(13q) abnormality was analyzed with one of the following probes specific for the 13q14.3 locus (LSI D13S319, Abbott Laboratories; or LSI 13q14.3 probe, Cytocell Technologies) and the 13q34 locus (LSI 13q34, Abbott Laboratories). del(17p13) was assessed using a commercial probe specific for the 17p13.1 locus (LSI p53, Abbott Laboratories). The LSI IGH/FGFR3 dual-color probe (Abbott Laboratories) was used to detect t(4;14). Patients were evaluated only if 200 or more scorable cells per sample were available. The background cutoff level for these probes was 10%.
Statistical analyses
The primary outcome measure (time to progression [TTP]) and the secondary outcome measure (overall survival [OS]) were defined according to the International uniform response criteria.22 For both, data were censored at the time of the patient's last visit. Time-to-event variables with censoring were estimated by Kaplan-Meier methods. Two-sided log rank tests were used to compare survival between treatment groups. Cox proportional hazards regression was used to examine the relationship between patients' baseline factors and outcomes. Pearson χ2 tests were used to compare response rates.
Results and discussion
Between August 2005 and July 2007, of 159 patients enrolled on the MM-016 study, 130 (81.8%) had FISH study results for all 3 chromosomal abnormalities and were included in this analysis. Patient characteristics are summarized in Table 1. Overall, 83.1% responded to treatment (> minimal response), with 13.1% obtaining more than or equal to near-complete response. The presence of del(13q) or del(17p13), but not t(4;14) abnormality, resulted in a significantly lower response rate, with responses seen in 77.8% (P = .007), 58.3% (P < .001), and 78.5% (P = .06) respectively.
With a median follow-up of 19.7 months, 92 (70.8%) had progressed with a median TTP of 7.1 months (95% confidence interval, 5.0-11.1 months); median OS was 22.7 months (95% confidence interval, 13.4 months to not reached).
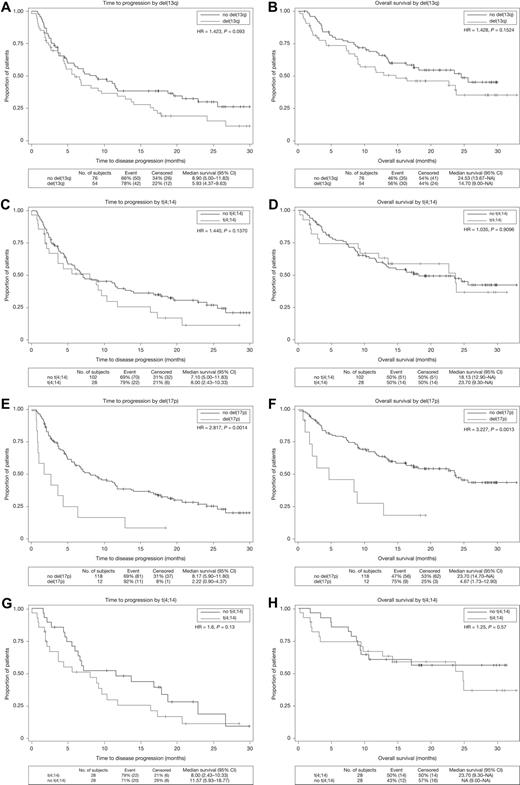
The median TTP for patients with del(13q) or t(4;14) abnormality was 5.9 months (hazard ratio [HR], 1.42; P = .09) and 8.0 months (HR, 1.44; P = .14), respectively (Figure 1A,C). Median OS was also similar to patients without these cytogenetic abnormalities [del(13q): HR, 1.43; P = .15; and t(4;14): HR, 1.04; P = .91; Figure 1B,D]. However, del(17p13) resulted in significantly shorter survivals, with a median TTP and OS of 2.2 and 4.7 months, respectively (TTP: HR, 2.82; P < .001; and OS: HR, 3.23; P < .001; Figure 1E-F). In multivariate analysis, the presence of del(17p13) (HR, 2.44; P = .007), prior thalidomide (HR, 2.49; P < .001), or bortezomib exposure (HR, 1.57; P = .036), and an elevated serum creatinine (HR, 2.56; P = .016) were identified as significant adverse risk factors for TTP, whereas longer time from initial diagnosis was a favorable risk factor (HR, 0.854; P = .007). Of note, t(4;14), del(13q), prior autologous stem cell transplantation, advanced International Staging System stage, or age more than or equal to 65 years did not significantly affect TTP. Adjusting for the number of prior therapies in the regression model did not alter these results. Similarly, only del(17p13) (HR, 3.16; P = .002), prior thalidomide or bortezomib exposure, older age (≥ 65 years), serum creatinine level more than 176 μM, or low albumin level (< 35 g/L) significantly affected OS.
Kaplan-Meier curves for TTP and OS in the cytogenetic abnormality groups. The vertical axis represents the percentage of patients with disease progression (A,C,E,G) or the percentage of patients surviving (B,D,F,H). (A-B) TTP and OS for patients with or without del(13q). (C-D) TTP and OS for patients with or without t(4;14). (E-F) TTP and OS for patients with or without del(17p13). (G-H) TTP and OS for patients with or without t(4;14) matched pairs. CI indicates confidence interval; and HR, hazard ratio.
Kaplan-Meier curves for TTP and OS in the cytogenetic abnormality groups. The vertical axis represents the percentage of patients with disease progression (A,C,E,G) or the percentage of patients surviving (B,D,F,H). (A-B) TTP and OS for patients with or without del(13q). (C-D) TTP and OS for patients with or without t(4;14). (E-F) TTP and OS for patients with or without del(17p13). (G-H) TTP and OS for patients with or without t(4;14) matched pairs. CI indicates confidence interval; and HR, hazard ratio.
The TTP of 7.1 months in this analysis was shorter than that reported in the phase 3 trials comparing lenalidomide and dexamethasone with dexamethasone alone. This is probably the result of the high proportion of heavily pretreated patients (83.85% with ≥ 2 prior therapies) and those previously given thalidomide (53.8%).18,19 Indeed, the TTP we observed is similar to that of 8.4 months recently described in thalidomide-exposed patients.23
In the current study, the median TTP of 8.0 months in patients with t(4;14) is encouraging and compares favorably with that previously reported for treatment with thalidomide or bortezomib in relapsed t(4;14) MM.9,24 Nevertheless, these results should be interpreted with caution because of the relatively short follow-up and the inherent imbalance in the clinical characteristics among patients with and without t(4;14) (Table 1). To try to address this issue, we performed a matched-pair analysis in which patients with and without t(4;14) were matched 1:1 for number of prior therapies, time from diagnosis, serum albumin, and β2-microglobulin (Table 1). The median TTP and OS for patients with or without t(4;14) were similar in this analysis (Figure 1G-H). Furthermore, the presence of t(4;14) did not independently impact survival outcomes in a multivariate analysis accounting for the patients characteristics listed in Table 1 as covariates. The effectiveness of lenalidomide in patients with t(4;14) was also observed in several small series of patients with newly diagnosed and relapsed MM.11,12,14 However, these results are to be contrasted, in part, with a recent report by the Intergroupe Francophone du Myélome in which t(4;14) had a negative impact on survival in a univariate, but not multivariate, modeling.15 Lastly, the prognosis of t(4;14) patients might be different at relapse compared with diagnosis9 partially because “high-risk” t(4;14) patients may not survive long enough to accrue to relapsed/refractory myeloma trials. Therefore, our results are only applicable in the relapsed setting, and a prospective trial is still needed to fully address the impact of lenalidomide in newly diagnosed t(4;14) MM.
Patients with a del(17p13) faired very poorly in our analysis, with a lower response rate (58.3%) and a significantly shorter survival, suggesting relative resistance to lenalidomide. Similar results were recently reported among relapsed del(17p13) patients treated with lenalidomide in combination with adriamycin and dexamethasone.14 The loss of chromosome 17p13.1-17p12, where the TP53 gene resides, has also conferred an inferior outcome with other antimyeloma therapies.2,3,5 Specifically, low TP53 expression by gene expression profiling resulted in a lower 2-year probability of survival even among patients treated aggressively with the bortezomib-containing regimen in total therapy 3.25
In conclusion, the combination of lenalidomide and dexamethasone is an effective therapy for relapsed/refractory myeloma patients and induces durable responses among relapsed t(4;14) disease. However, lenalidomide appears to be ineffective in patients with del(17p13), emphasizing the need for novel therapeutic approaches for this highly refractory subgroup of patients.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors received editorial support in the preparation of Figure 1 of this manuscript, funded by Celgene Corporation. However, the authors are fully responsible for the writing, content, and editorial decisions for this manuscript.
Authorship
Contribution: H.C., B.R., H.B., and D.E.H. performed and interpreted FISH studies; T.F. and N.J.B. analyzed results and performed statistical analysis; N.J.B., D.R., K.W.S., D.A.S., A.M., C.C., E.M.-K., and Y.T. provided the care for the patients on this study and collected the data; and N.J.B., D.R., and K.W.S. designed the research. All authors participated in the writing of the manuscript.
Conflict-of-interest disclosure: D.R., K.W.S., C.C., and N.J.B. declare research support from Celgene and have received honoraria for lectures and consulting work. T.F. is an employee of Celgene and receives salary support from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Nizar Jacques Bahlis, University of Calgary, Division of Hematology and Bone Marrow Transplant, 1403 29th St NW, Rm 681, Calgary, AB T2N 2T9, Canada; e-mail: nbahlis@ucalgary.ca.